Abstract
Mortality and morbidity in patients with ST elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI) are still high [1]. A huge amount of the myocardial damage is related to the mitochondrial events happening during reperfusion [2]. Several drugs directly and indirectly targeting mitochondria have been administered at the time of the PCI and their effect on fatal (all-cause mortality, cardiovascular (CV) death) and non fatal (hospital readmission for heart failure (HF)) outcomes have been tested showing conflicting results [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Data from 15 trials have been pooled with the aim to analyze the effect of drug administration versus placebo on outcome [17]. Subgroup analysis are here analyzed: considering only randomized clinical trial (RCT) on cyclosporine or nicorandil [3], [4], [5], [9], [10], [11], excluding a trial on metoprolol [12] and comparing trial with follow-up length <12 months versus those with longer follow-up [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. This article describes data related article titled “Clinical Benefit of Drugs Targeting Mitochondrial Function as an Adjunct to Reperfusion in ST-segment Elevation Myocardial Infarction: a Meta-Analysis of Randomized Clinical Trials” [17].
Keywords: Reperfusion injury, Myocardial infarction, PCI, Cyclosporin, Nicorandil, Follow-up
Specifications Table
| Subject area | Clinical research; meta-analysis |
| More specific subject area | Medicine; Cardiology; Reperfusion injury |
| Type of data | Figure |
| How data was acquired | Meta-analysis |
| Data format | Analyzed |
| Experimental factors | Ciclosporin or nicorandil, exclusion of metoprolol and follow-up length for reperfusion in ST elevation myocardial elevation treated with primary coronary intervention. |
| Experimental features | 15 studies focused on drugs targeting mitochondrial function vs. placebo in patients undergoing primary PCI for STEMI, of which 3 with cyclosporine, 2 with nicorandil, only one study with metoprolol were retrieved from MEDLINE, Cochrane Library, Google Scholar and Biomed Central |
| Data source location | Italy, USA, Israel, Japan, Denmark, UK, France, Norway, Spain. |
| Data accessibility | Data is with this article |
Value of the data
-
•
The use of cyclosporine or nicorandil at the time of primary percutaneous coronary angioplasty (PCI) on fatal (all-cause mortality, cardiovascular (CV) death) and non fatal (hospital readmission for heart failure (HF)) outcomes, show the absence of any potential benefit.
-
•
Excluding a trial on metoprolol [12], which has a complex mechanism of action, not targeting only mitochondrial function, the pooled analysis on fatal and non fatal outcomes of the 14 studies did not changed.
-
•
The analysis on follow-up length shows effects on hospital readmission for HF for trials with longer follow-up.
-
•
These additional analyses should be the basis to plan further randomized clinical trials (RCTs) on reperfusion injury in ST elevation myocardial infarction (STEMI) patients undergoing PCI, focusing attention on other molecular mitochondrial targets.
-
•
New RCTs on reperfusion injury should have a longer follow-up analysis.
1. Data
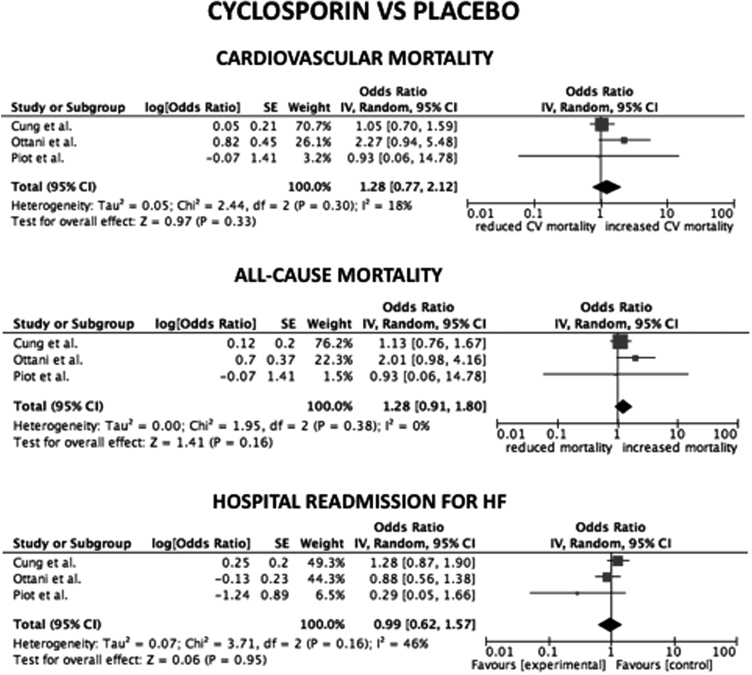
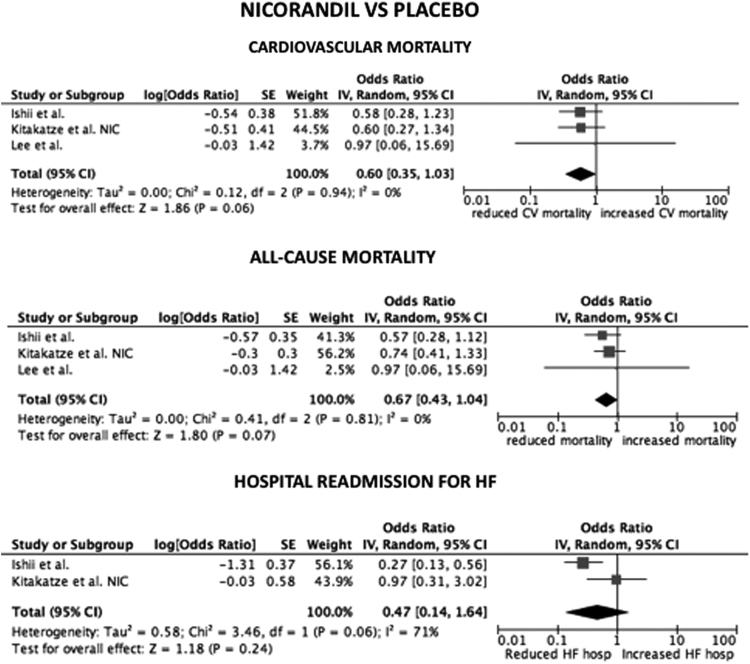
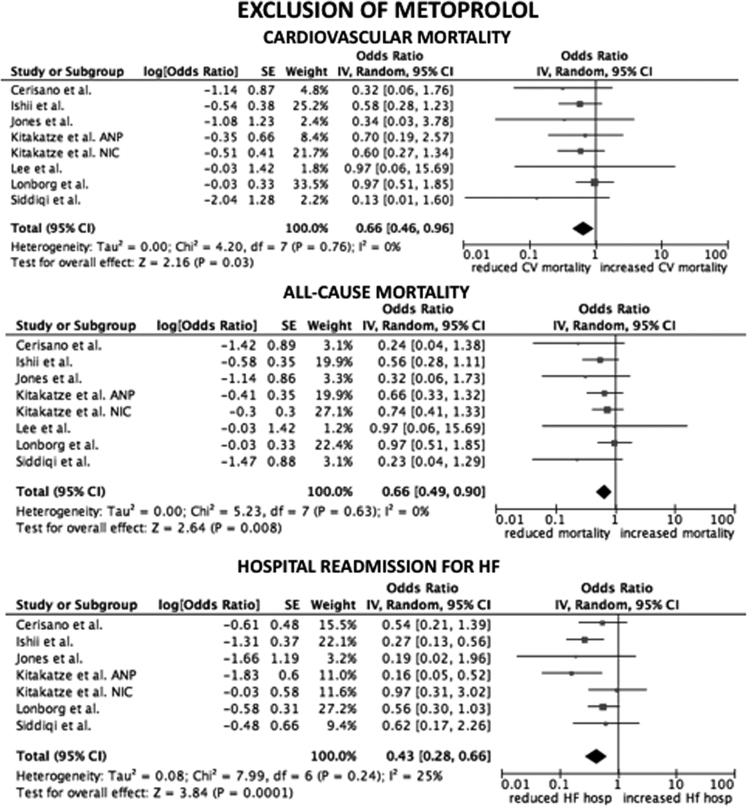
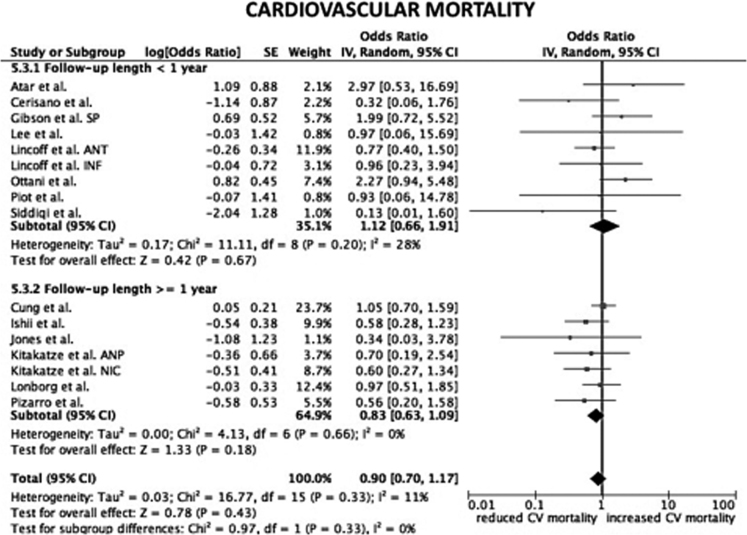
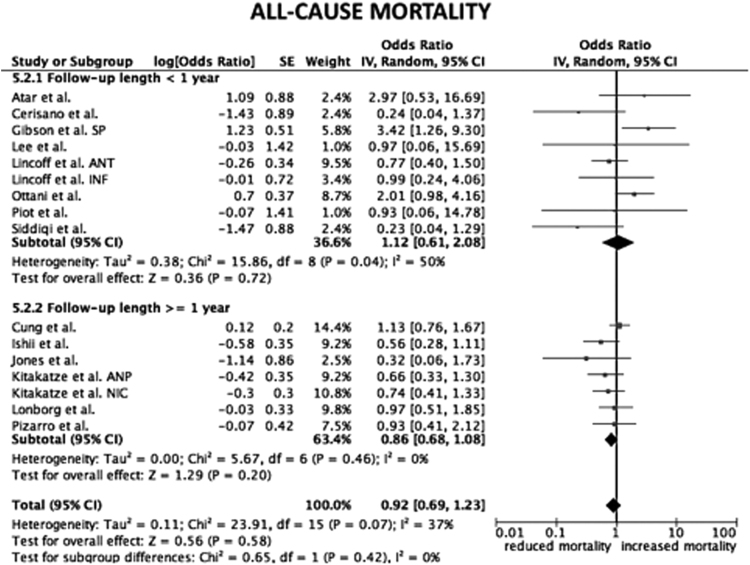
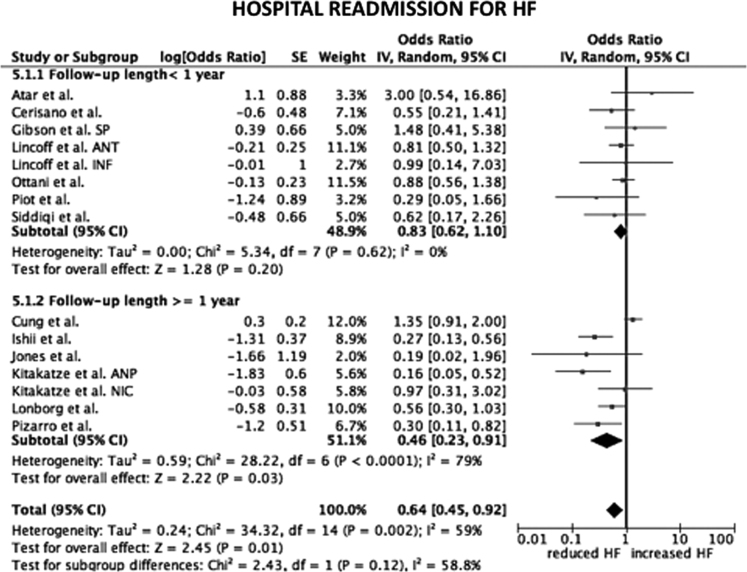
Considering only trial focused on cyclosporine versus placebo, the HR for CV mortality, all-cause mortality and hospital readmission for HF were not statistical significant (p=0.33; p=0.16; p=0.95, respectively) (Fig. 1). The same data are obtained considering only trials on nicorandil (p=0.06 for CV mortality; p=0.07 for all-cause death; p=0.2 for hospital readmission for HF) (Fig. 2). After the exclusion of the study on metoprolol from pooled analysis on trials with indirect/unspecific mechanism of action against mitochondrial component/pathway, the HR for CV death, all-cause death and hospital readmission for HF were significantly reduced (p=0.03; p=0.008; p=0.0001, respectively) (Fig. 3). Finally, the analysis on follow-up on all the studies included in the meta-analysis showed a reduction in hospital readmission for HF in studies with follow-up length ≥12 months (HR 0.46; 95% CI 0.45–0.92, p=0.03) (Fig. 4, Fig. 5, Fig. 6).
Fig. 1.
Forest plots on cardiovascular mortality, all-cause mortality and hospital readmission for HF in studies randomizing to cyclosporine vs. placebo. CV: cardiovascular.
Fig. 2.
Forest plots on cardiovascular mortality, all-cause mortality and hospital readmission for HF in studies randomizing to nicorandil vs. placebo. CV: cardiovascular.
Fig. 3.
Forest plots on cardiovascular mortality, all-cause mortality and hospital readmission for HF in studies randomizing indirect/unspecific mechanism of action against mitochondrial component/pathway vs. placebo, excluding the study on metoprolol [12]. ANP: atrial natriuretic peptide. NIC: nicorandil. CV: cardiovascular. HF: heart failure. hosp: hospitalization.
Fig. 4.
Forest plot on cardiovascular mortality after stratification of studies according to follow-up length. SP: safety population. ANT: anterior cohort. INF: inferior cohort. ANP: atrial natriuretic peptide. NIC: nicorandil. CV: cardiovascular.
Fig. 5.
Forest plot on all-cause mortality after stratification of studies according follow-up length. SP: safety population. ANT: anterior cohort. INF: inferior cohort. ANP: atrial natriuretic peptide. NIC: nicorandil.
Fig. 6.
Forest plot on hospital readmission for heart failure after stratification of studies according follow-up length. SP: safety population. ANT: anterior cohort. INF: inferior cohort. ANP: atrial natriuretic peptide. NIC: nicorandil. HF: heart failure.
2. Experimental design, materials and methods
2.1. Search strategy
A systematic review and meta-analysis was performed following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria [18], [19], [20], [21]. The protocol of this study was published on PROSPERO (CRD42016033085).
Papers were retrieved in MEDLINE, Cochrane Library, Google Scholar and Biomed Central. The terms searched were: (reperfusion injury) AND ((PCI) OR (percutaneous coronary intervention) OR (ST elevation myocardial infarction) OR (STEMI) OR (myocardial infarction)) [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16].
2.2. Selection criteria
Detailed description of selection criteria of the papers is described elsewhere [17]. In particular, we focused on i) RCTs ii) enrolling STEMI patients; with iii) reperfusion strategy by primary PCI; iv) comparison of agent/drug against RI vs. placebo/gold standard treatment.
2.3. Data abstraction, endpoints, contact with authors
We performed a pre-hoc stratification of studies according to mechanism of action targeting a mitochondrial component/pathway (direct/selective vs. indirect/unspecific) according to a recent overview [22]. The analyses were performed according to the following criteria: i) administration of cyclosporine, ii) administration of nicorandil, iii) follow-up length <12 vs. ≥12 months iv) indirect/unspecific drugs after exclusion of the study of Pizarro et al. [12]. The primary endpoint of the analysis was the incidence of cardiovascular death. Secondary endpoints were: all-cause death, hospital readmission for heart failure (HF).
2.4. Data analysis and synthesis
The endpoints were expressed as odds ratio (OR). Point estimates and standard errors were calculated and combined by the generic inverse variance method [23], computing risk estimates with 95% confidence intervals according to logarithmic transformation of the OR. A random effect model was used. Statistical heterogeneity was assessed with the Cochran's Q test and the I2 statistic [24]. To test the difference between sub-group analyses the Chi2 test has been used. Prometa (Internovi, Cesena, Italy) and RevMan 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) software were used for statistical analyses.
Acknowledgements
Conflict of interest: Lincoff receives research support from Kai Pharmaceuticals; Gibson receives research support from Stealth pharmaceuticals; other authors do not declare conflict of interest.
Funding: none.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2017.07.033.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J., vol. 35, 2014, pp. 2541–2619. [DOI] [PubMed]
- 2.Morciano G., Giorgi C., Bonora M. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Piot C., Croisille P., Staat P. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 4.Cung T.T., Morel O., Cayla G. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 5.Ottani F., Latini R., Staszewsky L., CYCLE investigators Cyclosporine A in reperfused myocardial infarction: the multicenter, controlled, open-label CYCLE trial. J. Am. Coll. Cardiol. 2016;67:365–374. doi: 10.1016/j.jacc.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 6.Lincoff A.M., Roe M., Aylward P., PROTECTION AMI investigators Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI randomized controlled trial. Eur. Heart J. 2014;35:2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- 7.Atar D., Arheden H., Berdeaux A. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: mitocare study results. Eur. Heart J. 2015;36:112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- 8.Gibson C.M., Giugliano R.P., Kloner R.A. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur. Heart J. 2015 doi: 10.1093/eurheartj/ehv597. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Ishii H., Ichimiya S., Kanashiro M. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005;112:1284–1288. doi: 10.1161/CIRCULATIONAHA.104.530329. [DOI] [PubMed] [Google Scholar]
- 10.Kitakaze M., Asakura M., Kim J., J-WIND investigators Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.C., An S.G., Choi J.H. Effect of intra-coronary nicorandil administration prior to reperfusion in acute ST segment elevation myocardial infarction. Circ. J. 2008;72:1425–1429. doi: 10.1253/circj.cj-08-0212. [DOI] [PubMed] [Google Scholar]
- 12.Pizarro G., Fernández-Friera L., Fuster V. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) J Am. Coll. Cardiol. 2014;63:2356–2362. doi: 10.1016/j.jacc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Jones D.A., Pellaton C., Velmurugan S. Randomized phase 2 trial of intracoronary nitrite during acute myocardial infarction. Circ. Res. 2015;116:437–447. doi: 10.1161/CIRCRESAHA.116.305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lønborg J., Vejlstrup N., Kelbæk H. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqi N., Neil C., Bruce M., NIAMI investigators Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur. Heart J. 2014;35:1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerisano G., Buonamici P., Valenti R. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur. Heart J. 2014;35:184–191. doi: 10.1093/eurheartj/eht420. [DOI] [PubMed] [Google Scholar]
- 17.Campo G., Pavasini R., Morciano G. Clinical benefit of drugs targeting mitochondrial function as an adjunct to reperfusion in ST-segment elevation myocardial infarction: a meta-analysis of randomized clinical trials. Int. J. Cardiol. 2017 doi: 10.1016/j.ijcard.2017.06.040. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Cook D.J., Eastwood S., Olkin I., Rennie D., Stroup D.F. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.J. Higgins, S. Green, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, The Cochrane Collaboration, 2009. 〈http://handbook.cochrane.org〉. (2011, Accessed 28 December, 2015).
- 21.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulluck H., Yellon D.M., Hausenloy D.J. Reducing myocardial infarct size: challenges and future opportunities. Heart. 2016;102:341–348. doi: 10.1136/heartjnl-2015-307855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trial. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material