Highlights
-
•
A subtle case report of primary Hodgkin’s disease of the breast.
-
•
Definition and staging of primary lymphomas of the breast.
-
•
A comprehensive review of the Literature about this kind of disease.
Keywords: Hodgkin’s kymphoma, Breast cancer, Primary kymphoma of the breast
Abstract
Introduction
According to literature, primary Hodgkin’s lymphomas of the breast represent one of the rarer entity in the primary breast lymphoma (PBL) scenario.
This is the reason why these tumors are insidious in mammary oncology.
Presentation of the case
We report a case of HL primitive breast in an elderly patient in whom radiology suspected an advanced breast cancer with ipsilateral axillary involvement and in which the fine-needle aspiration came back not significant.
Discussion
Eighteen cases of primary Hodgkin’s lymphoma of the breast has been described in Literature in a very large period of time: from 1928 to 2016.
The nodular sclerosis type is the most frequent histological variant.
Conclusion
Their rarity together with the fact that radiological investigations are not significant for the purpose of an exact diagnosis, make these lesions extremely difficult to identify.
1. Introduction
According to literature, primary Hodgkin’s lymphomas of the breast represent one of the rarer entity in the primary breast lymphoma (PBL) scenario.
According to the first description made by Wiseman in 1972, a lymphoma of the breast needs to satisfy several prerequisites to be considered as a primary lesion.
These prerequisites include: adequate pathological specimen, lymphomatous infiltrate and mammary tissue should be in close association and no preceding or concurrent diagnosis of widespread lymphoma.
Rarity is the reason why these tumors are insidious in mammary oncology.
We report a case of HL primitive breast in an elderly patient in whom radiology suspected an advanced breast cancer with ipsilateral axillary involvement and in which the fine-needle aspiration came back not significant.
In support of our case report, we performed also an extensive review of the literature about primary Hodgkin’s lymphomas of the breast previously described.
The work has been reported in line with the SCARE criteria [30].
2. Case report
A 79-year-old woman was referred to our unit with a single palpable painless mass in the superior outer quadrant of the right breast and many palpable lymph nodes in the right axilla.
The patient said that at the beginning she had noticed a little asymptomatic lump which rapidly increased in terms of dimensions in only 2 months.
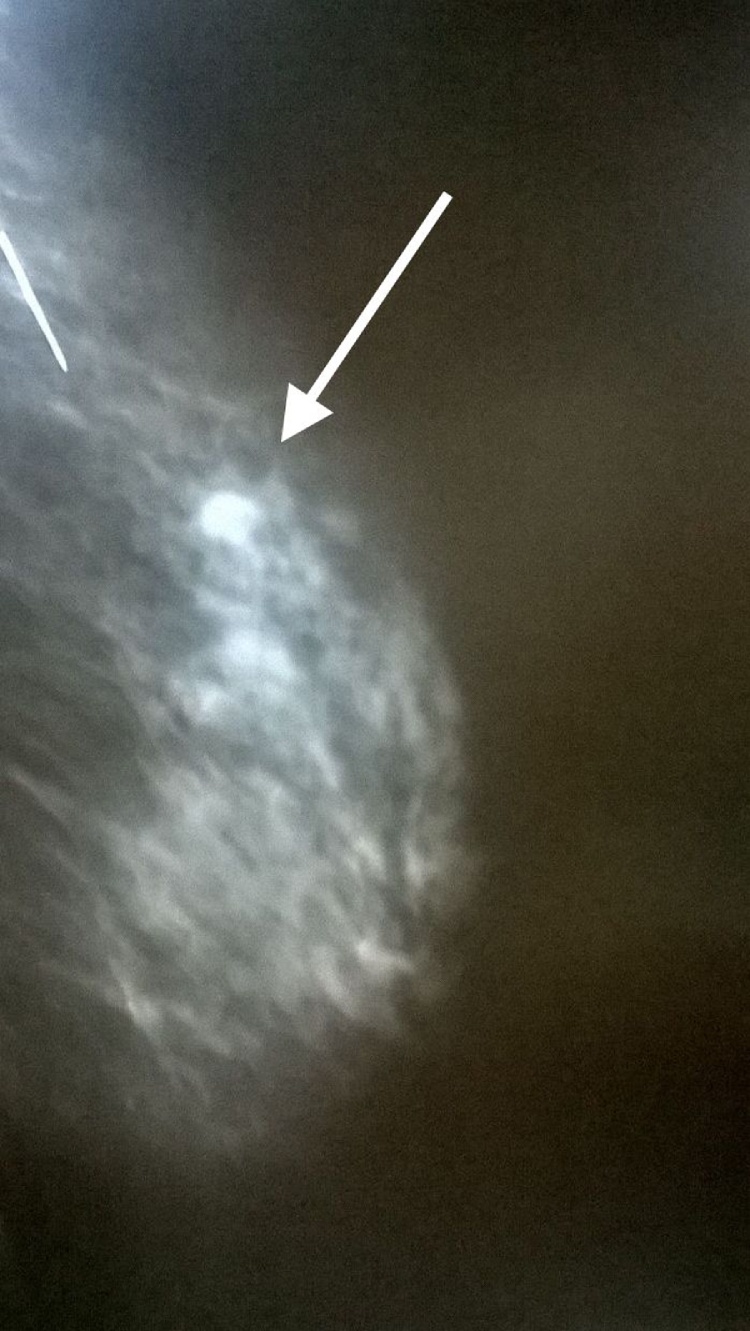
A mammogram was performed and showed a 2,5 cm single mass with well defined contour at the junction between the superior outer quadrant and the axillary tail of the right breast (Fig. 1).
Fig. 1.
The mammogram showed a 2,5 cm single mass with well defined contour at junction bet- ween the superior outer quadrant and the axillary tail of the right breast.
Lesion size was calculated by measuring the maximum diameter on the mammogram. An ultrasonography was then performed in order to study both the breast lump and the axillary involvement.
This procedure confirmed a grossly involvement of the lymph nodes of the right axilla that were confluent in consistent lumps wrapped around the axillary vein.
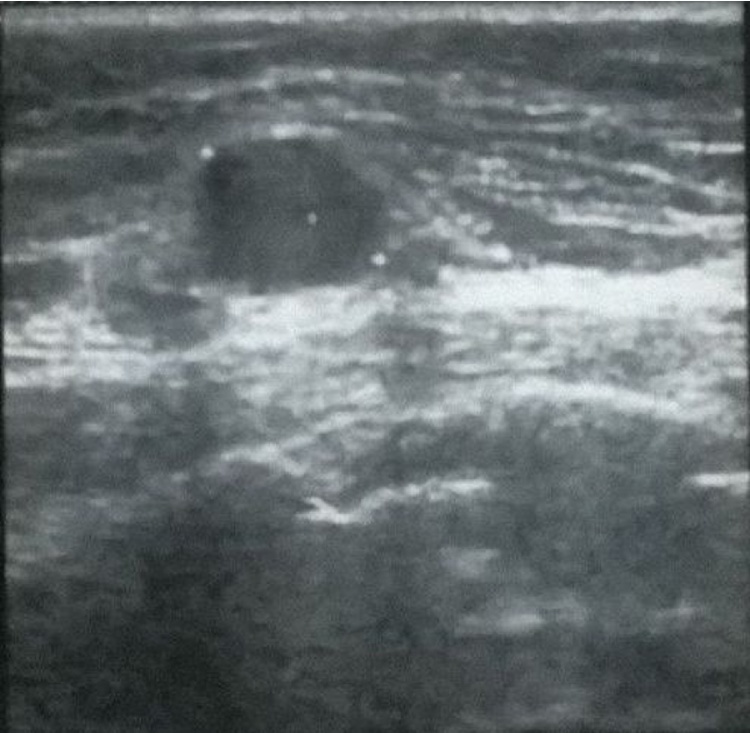
For what concerned the breast mass, the ultrasound findings showed a homogeneously hypoecoic lump with an oval shape and well-defined margins, no vascularity was seen at Color Doppler (Fig. 2).
Fig. 2.
The ultrasound findings showed a homogeneously hypoecoic lump with an oval shape and well-defined margins, no vascularity was seen at Color Doppler.
The patient underwent a fine-needle aspiration biopsy (FNAB) but the pathology report was not significant.
Therefore, we decided to proceed with a lumpectomy, in order to better asses the nature of the tumor.
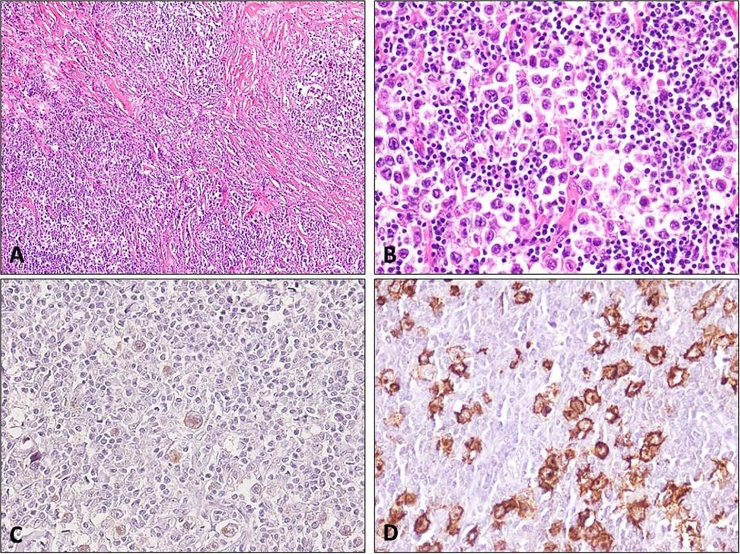
Microscopic examination in hematoxylin and eosin sections showed nodules of polymorphous inflammatory cells surrounded by broad fibrous bands extending from a thick capsule. The cellular nodules contained small lymphocytes, monocytes, neutrophils, eosinophils and scattered Reed-Sternberg cells with lacunar features. In some fields Reed-Sternberg cells formed aggregates. Immunohistochemical studies were performed on formalin-fixed and paraffin embedded tissue. Reed-Sternberg cells were negative for CD20 and showed intense positivity for CD15 with a membranous staining pattern and slightly positivity for CD30 with a dot-like staining pattern. A diagnosis of nodular sclerosis variant of classical Hodgkin disease with syncytial features was rendered (Fig. 3).
Fig. 3.
(A) (H&E, 10×): microscopic examination showed fibrous bands circumscribing lymphoid nodules. (B) (H&E, 40×): numerous large cells with retraction of the cytoplasm (lacunar cells) within small lymphocytes. (C) (CD30 staining, 40×): Reed-Sternberg cells showed dot-like positivity for CD30. (D) (CD15 staining, 40×): Reed-Sternberg cells showed intense membranous positivity for CD15.
The patient was then referred to the Oncology Department, where the neoplasm was staged as level II A\E (right breast primary with ipsilateral axillary nodal involvement).
The patient underwent systemic treatment with chemotherapy, consisting in a 50% reduced ABVD (Doxorubicin, Bleomicyn, Vinblastine, Dacarbazine) scheme.
We observed a complete response to the treatment and the patient was relapse-free after one year follow-up.
3. Discussion
Lymphomas of the breast are rare entities in oncology and they were first described by Wiseman and Liao in 1972 [1].
The lymphatic involvement of the breast parenchyma can be expression either of a primary disease or of a secondary disease, in which the infiltration of the breast is due to a systemic disease or a disease recurrence.
Diagnostic criteria for primary lymphoma of the breast are several: the presence of adequate tissue for pathological evaluation, close association between breast tissue and lymphomatous infiltrate and no evidence of concurrent widespread disease or preceding extramammary lymphoma.
The presence of ipsilateral axillary nodal involvement does not constitute ground for rejection only if both lesions developed simultaneously.
According to the Literature, primary breast lymphomas (PBL) represent 0,5% of primary malignant tumor of the breast and between 1,7% and 2,2% of extranodal lymphomas [2], [3], [4], [5], [6]. More than 95% of primary breast lymphomas are represented by B-type non-Hodgkin lymphoma (NHL), the remaining 5% is represented by T-cell lymphoma.
For what concerns primary T-cell lymphoma of the breast, the 80% is represented by Ana- plastic Large Cell Lymphoma (ALLC), which could also be caused by breast implant in breast reconstruction [7], [8], and the remaining 20% by Hodgkin Lymphoma (LH) [9], [10].
The age distribution of primary breast lymphomas is difficult to determine because of their rarity.
The staging of primary breast lymphomas is performed according to the Ann Arbor Classification, from stage one to stage four.
In stage one the tumor is limited to the breast, in stage two the tumor is limited to the breast and the ipsilateral axillary nodes, in stage three the tumor is limited to the breast and metastasizes from both sides of the diaphragm and in stage four the tumor is limited to the breast and metastasizes to extranodal lymphoid tissue.
Another classification is based on symptoms: type A is asymptomatic and type B is symptomatic (pain, fever, weight loss, sweat).
The first case in literature of primary HL of the breast was described by Kueckens [11] in 1928, then in a time lapse of 32 years (from 1928 to 1960) only other six cases were reported [12], [13], [14], [15], [16], [17].
In 1966 Lawler et al. [18] reported two cases of primary LH of the breast: one rich in lymphocytes type and the other one nodular sclerosis type.
In 1971 the Ann Arbor Classification for Hodgkin’s disease was published and this is probably the reason why the incidence of this kind of neoplasm has been underestimated be- fore that date.
In 1973 Wood and Coltman [19] presented the first review of the literature regarding primary extranodal Hodgkin’s disease, in which no new case of primary HL of the breast was described.
In 1981 Schouten et al. [20] reported a retrospective review of 13 patients over a 10-years period of time.
The histologic findings in two patients were positive for primary HL of the breast, both of them nodular sclerosis type.
In 1985 Shehata et al. [21] published a new case (rich in lymphocytes type) and the first literature review regarding primary HL of the breast.
In 1987 Dixon et al. [22] identified 15 patients with primary lymphoma of the breast over a 30-years period of time, one patient had a HL but no information were given about its histological type.
In 1990 Hugh et al. [23] reported 20 cases of primary breast lymphomas recorded at the Alberta Cancer Register over the last 23 years, no HL were found.
In 1995 Ariad et al. [24] published a 10-years retrospective review in which they found 16 patients affected by breast lymphoma, seven patients out of sixteen fulfilled the criteria for primary breast lymphoma. In this group one case of primary HL of the breast was found. In 2002 Sabatè et al. [25] performed a retrospective study on 28 female patients affected by breast lymphomas, which were referred in a period of time of 30 years.
According to this paper, 12 patients were affected by primary breast lymphomas and 16 by secondary breast lymphomas.
In the first group no HL were reported, whereas in the other group one case of HL was reported (nodular sclerosis type).
In the same year Domcheck et al. [26] published a retrospective review on 86 patients over an 11-years period. In this large series two patients affected by Hl were identified: one with mixed cellularity HL that recurred to the breast after chemotherapy and the other with nodular sclerosis HL involving the breast tissue as part of a disseminated disease.
We do not take none of these cases into account because they are not primary breast disease.
In 2005 Cox et al. [10] presented a retrospective review over a 13-years period of time on 32 female patients affected by lymphomas of the breast (12) and the axilla (20).
In the first group, concerning primary breast lesion, no HL were found.
In the second group, concerning the axillary lymphomas, one case of HL was found (classification not described).
In 2008 Talwalkar et al. [27] identified 116 patients affected by lymphomas involving the breast over a 21-years period. These neoplasms were divided into two groups based on extent of disease at initial diagnosis: localized disease (n = 50) and disseminated disease (n = 56).
In the first group one HL was found, whereas in the other group three HL were found, each neoplasm had a nodular sclerosis pattern.
In this large series, only the HL of the localized disease group has been taken into account in our review because we can not consider it as primary Hodgkin’s lymphoma of the breast.
In 2012 Surov et al. [28] published a retrospective study over a 12-years period on 36 patients with breast lymphoma (22 primary and 14 secondary).
Among this group, only two patients were affected by T-cell Lymphoma, but in the paper is not mentioned whether these neoplasms were primary or secondary, and no histopathological description is reported about them.
In 2015 Zarnescu et al. [29] presented a case of primary HL of the breast, nodular sclerosis variant.
In 2016 Perez et al. [9] conducted a retrospective study over a 27-years period on 55 patients affected by primary breast lymphoma, only two cases of primary Hodgkin’s Lymphoma were found: one sclerosing nodular type and the other rich in lymphocytes type. Eighteen cases of primary Hodgkin’s lymphoma of the breast has been described in Literature in a very large period of time: from 1928 to 2016. (Table 1)
Table 1.
Primary Hodgkin’s lymphomas of the breast described in Literature.
| Year | Author | Number of Primary HL of the Breast | Histologic Features |
|---|---|---|---|
| 1928 | Kueckens [11] | 1 | unknown |
| 1930 | Petrignani [12] | 1 | unknown |
| 1939 | Gendreav [13] | 1 | unknown |
| 1943 | Wray [14] | 1 | unknown |
| 1945 | Adair [15] | 1 | unknown |
| 1945 | Randall [16] | 1 | unknown |
| 1960 | McGregor [17] | 1 | unknown |
| 1966 | Lawler [18] | 2 | nodular sclerosis and rich in lymphocyte |
| 1981 | Schouten [20] | 2 | both nodular sclerosis |
| 1985 | Shehata [21] | 1 | rich in lymphocyte |
| 1987 | Dixon [22] | 1 | unknown |
| 1995 | Ariad [24] | 1 | nodular sclerosis |
| 2008 | Talwalkar [27] | 1 | nodular sclerosis |
| 2015 | Zarnescu [29] | 1 | nodular sclerosis |
| 2016 | Perez [9] | 2 | nodular sclerosis and rich in lymphocyte |
The nodular sclerosis type is the most frequent histological variant.
Their rarity together with the fact that radiological investigations are not significant for the purpose of an exact diagnosis, make these lesions extremely difficult to identify.
The paper written by Zarnescu [29] also reported a poor efficacy of Fine Needle Aspiration Biopsy (FNAB) procedure.
Despite all these drawbacks, the literature shows that primary Hodgkin’s lymphomas of the breast have a good response to oncological treatment and their prognosis is excellent.
In this scenario, we reported the nineteenth case of a primary Hodgkin’s lymphoma (HL) of the breast, the first described in Italy, in a 79-year-old woman who was referred to us with diagnosis of advanced breast cancer.
The lymphoma of the breast are rare, as mentioned above, and their clinical features do not distinguish them from other breast malignancy, their diagnosis is based only on core biopsy, which is the gold standard in breast cancer.
In this case report, FNAB was not significant probably due to the cellular architecture of the lymphoma, which presented nodular sclerosis.
For that reason an excision biopsy of the lesion was performed under local anesthesia and the pathology report came back positive for Hodgkin’s lymphoma, nodular sclerosis type.
According to the histological findings, the patient was referred to the Oncology Department of our University and underwent a targeted therapy.
Conflicts of interest
No conflicts of interest.
Funding
None.
Ethical approval
Not given.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Mario Faenza: writing the paper Andrea Ronchi: pathology report.
Corrado Rubino : data analysis.
Antonio Santoriello: surgical operator of the patient.
Gorizio Pieretti: data collection.
Antonio Guastafierro: data collection.
Giuseppe Andrea Ferraro: study concept.
Giovanni Francesco Nicoletti: study concept.
Guarantor
Mario Faenza.
References
- 1.Wiseman C., Liao K. Primary lymphoma of the breast. Cancer. 1972;29:1705–1712. doi: 10.1002/1097-0142(197206)29:6<1705::aid-cncr2820290640>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Fruchart C., Denoux Y., Chasle J. High grade primary breast lymphoma: is it a different clinical entity? Breast Cancer Res. Treat. 2005;93:191–198. doi: 10.1007/s10549-005-5088-8. [DOI] [PubMed] [Google Scholar]
- 3.Valdire P., Capovilla C., Asselain B. Primary breast non-Hodgkin’s lymphoma: a large single center study of initial characteristics, natural history and prognostic factors. Am. J. Hematol. 2009;84:133–139. doi: 10.1002/ajh.21353. [DOI] [PubMed] [Google Scholar]
- 4.Domcheck S.M., Hecht J.L., Fleming M.D., Pinkus G.S., Canellos G.P. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002;94:6–13. doi: 10.1002/cncr.10163. [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Lang R., Fu L. Primary breast lymphoma (PBL): a literature review. Clin. Oncol. Cancer Res. 2011;8:128–132. [Google Scholar]
- 6.Cheah C.Y., Campbell B., Seymour J. Primary breast lymphoma. Cancer Treat. Rev. 2014;40:900–908. doi: 10.1016/j.ctrv.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Kim B., Roth C., Young V.L., Chung K.C., van Busum K., Schnyer C., Mattke S. Anaplastic large cell lymphoma and breast implants: results from a structured expert consultation process. Plast. Reconstr. Surg. 2011 Sep;128(3):629–639. doi: 10.1097/PRS.0b013e31821f9f23. [DOI] [PubMed] [Google Scholar]
- 8.Farace F., Bulla A., Marongiu F., Campus G.V., Tanda F., Lissia A., Cossu A., Fozza C. Rubi- no C. anaplastic large cell lymphoma of the breast arising around mammary implant capsule: an Italian report. Aesthetic Plast. Surg. 2013;37(June (3)):567–571. doi: 10.1007/s00266-013-0120-6. [DOI] [PubMed] [Google Scholar]
- 9.Franco Pérez F., Lavernia J., Aguiar-Bujanda D., Miramón J., Gumá J., Álvarez R., Gómez- Codina J., Arroyo F.G., Llanos M., Marin M., Alfaro J., Quero C., Delgado M., Nogales E., Menarguez F., Martinez N., Torrente M., Royuela A., Abreu D., Provencio M. Primary breast lymphoma: analysis of 55 cases of the Spanish lymphoma oncology group. Clin. Lymphoma Myeloma Leuk. 2016;(September):30560–30562. doi: 10.1016/j.clml.2016.09.004. pii: S2152-2650(16)30560-2. [DOI] [PubMed] [Google Scholar]
- 10.Cox J., Lunt L., McLean L. Hematological cancers in the breast and axilla: a drop in an ocean of breast malignancy. Breast. 2005;14:51–56. doi: 10.1016/j.breast.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Kueckens H. Ein lokales Lynphogranulom der brust in furm eines mammatumors. Beitr. Pathol. 1928;80:135–137. [Google Scholar]
- 12.Petrignani R. Lymphogranulomatose maligne primitive du sein. Ann. Anat. Pathol. (Paris) 1930;7:360–364. [Google Scholar]
- 13.Gendreav J.E., Pinsonneault G. Un cas de lymphogranulomatose maligne du sein. Union Med. Can. 1939;68:161–163. [Google Scholar]
- 14.Wray S. Hodgkin’s Disease of the Breast. J. Path Bact. 1943;55:75. [Google Scholar]
- 15.Adair F.E., Carver L.F., Hermann J.B. Hodgkin’s disease of the breast. Surg. Gynecol. Obstet. 1945;80:205–210. [Google Scholar]
- 16.Randall K.J., Spalding J.E. Primary Hodgkin’s disease of the breast: report of a case and commentary. Guy’s Hosp. Rep. 1945;94:137–141. [Google Scholar]
- 17.McGregor J.K. Hodgkin’s disease of the breast. Am. J. Surg. 1960;99:348–351. [Google Scholar]
- 18.Lawler M.R., Jr., Riddel D.H. Hodgkin’s disease of the breast. Arch. Surg. 1966;93:331–334. doi: 10.1001/archsurg.1966.01330020123021. [DOI] [PubMed] [Google Scholar]
- 19.Wood N.L., Coltman C.A., Jr. Localized primary extranodal Hodgkin’s disease. Ann. Int. Med. 1973;78:113–118. doi: 10.7326/0003-4819-78-1-113. [DOI] [PubMed] [Google Scholar]
- 20.Schouten J.T., Weese J.L., Carbone P.P. Lymphoma of the breast. Ann. Sure. 1981;194:749–753. doi: 10.1097/00000658-198112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shehata W.L., Pauke T.W., Schleuter J.A. Hodgkin disease of the breast: case report and review of the literature. Breast. 1985;11:19–21. [Google Scholar]
- 22.Dixon J.M., Lumsden A.B., Krajewsky A. Primary lymphoma of the breast. Br. J. Surg. 1987;74:214–216. doi: 10.1002/bjs.1800740322. [DOI] [PubMed] [Google Scholar]
- 23.Hugh J.C., Jackson F.I., Hanson J., Poppema S. Primary breast lymphoma: an immunohistologic study of 20 new cases. Cancer. 1990;66:2602–2610. doi: 10.1002/1097-0142(19901215)66:12<2602::aid-cncr2820661224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Ariad S., Lewis D., Cohen R., Bezwoda W.R. Breast lymphoma. A clinical and pathological review and 10-year treatment results. S. Afr. Med. J. 1995;85(2):85–89. [PubMed] [Google Scholar]
- 25.Sabatè J.M., Gomez A., Torrubia S., Camins A., Roson N., De Las Heras P., Villalba-Nuno V. Lymphoma of the breast: clinical and radiological features with pathologic correlation in 28 patients. Breast J. 2002;8:294–304. doi: 10.1046/j.1524-4741.2002.08509.x. [DOI] [PubMed] [Google Scholar]
- 26.Domcheck S.M., Hecht J.L., Fleming M.D., Pinkus G.S., Canellos G.P. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002;94:6–13. doi: 10.1002/cncr.10163. [DOI] [PubMed] [Google Scholar]
- 27.Talwalkar S.S., Miranda R.N., Valbuena J.R., Routbort M.J., Martin A.W., Medeiros L.J. Lymphoma involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am. J. Surg. Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 28.Surov A., Holzhausen H.J., Wienke A., Schmidt J., Thomssen C., Arnold D., Ruschke K., Spielmann R.P. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br. J. Radiol. 2012;85:e195–e205. doi: 10.1259/bjr/78413721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarnescu N.O., Iliesu A., Procop A., Tampa M., Matei C., Sajin M., Costache M., Dumitru A., Lazaroiu A.M. A challenging case of primary breast Hodgkin’s lymphoma. Maedica (Buchar) 2015;10(1):44–47. [PMC free article] [PubMed] [Google Scholar]
- 30.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., The SCARE Group The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 Oct;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]