Highlights
-
•
There is aortic adaptation to resistance training in an experimental animal model.
-
•
Resistance training promoted left ventricle concentric hypertrophy and improved aortic wall structure by increasing the density of elastic fibers and collagen fibers and increasing the thickness of collagen fibrils.
-
•
Resistance training rats displayed aortic remodeling.
Keywords: Anaerobic training, Great vessels, Morphometry, Movement
Abstract
Background
Little information is available on the effects of resistance training on the aortic wall.
Objective
This study aimed to quantify the effects of a resistance-training program on blood pressure and aortic wall structural components.
Methods
Rats (aged three months) were randomized into sedentary group (control group, CG; n = 10) or trained group (TG; n = 10). The TG rats performed resistance training by climbing a 1.1-m vertical ladder (80° incline) five times a week for 12 weeks, and the CG remained sedentary. The rats were sacrificed and 5 mm of the ascending aorta was submitted to histological sections, which were stained with hematoxylin–eosin, Picrosirius red, and Verhoeff's elastin, and used for morphometric studies. Left ventricle (LV) hypertrophy was determined by measuring LV wall thickness and LV internal diameter.
Results
The rats had similar repetition maximum before the resistance training. At the end of the resistance training period, the repetition maximum of the TG was 3.04-fold greater than the body weight. In the twelfth month, the left ventricular weight was 15.3% larger in the TG than in the CG, and the left ventricular internal diameter was reduced by 10% in the TG. Rats exposed to resistance training had a significant increase in aortic wall thickness, in both elastic lamina and collagen fibers, and in the thickness of collagen fibrils.
Conclusion
Resistance training induces the development of concentric cardiac hypertrophy and improves the aortic wall components by producing a morphological expression pattern distinct from aortic pathological adaptation.
Introduction
Many researchers who studied the adaptation of aortic structure to chronic exercise training have used laboratory rats,1, 2, 3, 4, 5, 6, 7, 8 and important information has emerged from these studies. The presence of smooth muscle, collagen, and elastic fibers in the aortic wall1, 2, 3, 4, 5, 6 gives greater plasticity to the vessel wall, revealed by its capacity to produce a structural response to different conditions. One such condition is physical training that promotes morphological changes to its structure.2, 3, 7, 8
Resistance training involves contractions of specific muscle groups against external resistance to increase muscle strength and muscle power. The skeletal and cardiac muscles adapt in response to resistance training.9 When performed with appropriate execution velocity, range of movement, volume, and intensity,10 resistance training promotes cardiac hypertrophy that is also known as concentric hypertrophy. Concentric hypertrophy is characterized by increases in the left ventricular wall thickness and changes in the diameter of the left ventricle cavity in diastole.9, 11 However, there are few observations related to the aortic wall when comparing resistance-trained rats with sedentary controls. The purpose of this study was to analyze the effects of resistance training on the aortic wall of trained rats when compared to sedentary ones. Our hypothesis was that RT could modify the structure of the aortic wall.
Methods
Sample and procedures
The animals used in this study were male Wistar rats (aged three months). They were randomly allocated into two groups: control group (CG, n = 10) and resistance trained group (TG, n = 10). The rats in both groups were sacrificed at six months of age. Three rats were housed in each cage, provided with standard laboratory chow and water ad libitum throughout the study. Rats were weighed every week. The animal room was maintained at 21 ± 1 °C with artificial 12:12 light and dark cycle.
Resistance training
After a period of adaptation (one week) to the device, rats in the TG were trained to climb a 1.1-m vertical ladder (80° incline) with weights tied to their tails.12, 13 The rats were trained once a day, five days per week, for 12 weeks. Each training session consisted of six climbs requiring 8–12 dynamic movements per climb.13
Body weight (BW) was measured at the beginning of each week of the experiment, and the new weight to be carried by the animals was adjusted according to their body weight during that week.13 After measurement of the maximum weight lifted (one repetition maximum-1RM), the training load was set at 60% 1RM. The animals did not require any external stimulus to conduct the training. Animals of the CG were trained once a day for five days a week during 12 weeks to undergo the same procedures as the trained rats and standardize the sample, but each training session consisted of just one climb. Blood pressure was measured at the end of the experiment through the tail-cuff plethysmography. The Research Ethics Committee of the Universidade São Judas Tadeu (USJT), São Paulo, SP, Brazil, approved the animals’ handling in compliance with the International Guiding Principles for Biomedical Research involving Animals (Protocol number: 015/2006).
Quantitative study
At the end of the experiment (24 h after the last RT session), each animal was anesthetized with Pentobarbital sodium (3 mg/100 g body weight, intra peritoneal) and killed by exsanguination. The rat was weighed, the heart removed and weighed, and the left ventricle (LV) dissected and weighed. The ascending aorta was excised after thoracotomy, and an arterial ring (5 mm long) was isolated and cleaned from adherent adipose and connective tissues.
Ten aortas from each group were immersed in the fixative (freshly prepared) 4% (w/v) formaldehyde in 0.1 M phosphate buffer, pH 7.2 for 48 h.14 The arterial rings were sectioned according to the vertical section method,15 processed according to the routine histological procedures, and embedded in Paraplast plus (Sigma Chemical Co., St Louis, USA).
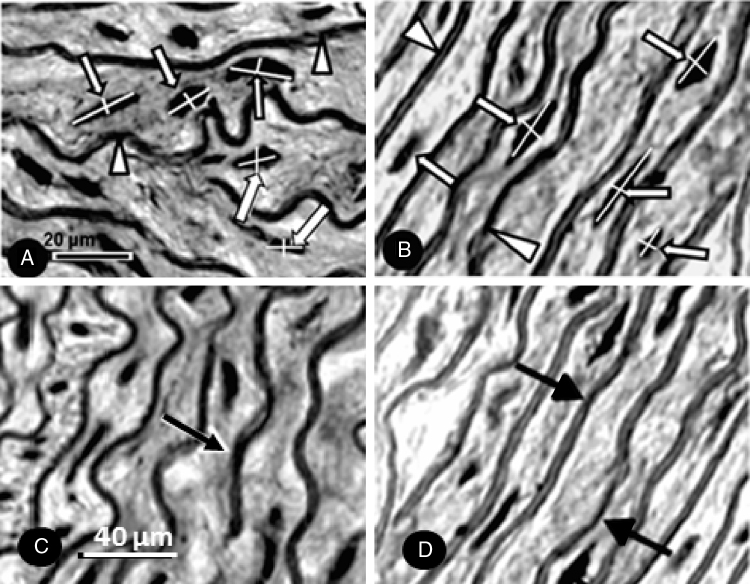
The volume of smooth muscle cell nuclei (V[smc], μm3) was obtained by measuring the major and minor diameter of the myocyte nuclei in four non-consecutive histological sections per animal. The following formula was used to calculate the nuclear volume: V = a2.b/1.91, where V is the volume; a is the smaller axial diameter; b is the greater axial diameter; 1.91 is constant.16 The final value was the average of the measurements (Fig. 1A and B).
Figure 1.
Histological sections (Verhoeff staining) of the ascending aorta of the CG (A) and the TG (B) rats used for measurement of the major and minor diameter of smooth muscle cell nucleus (arrows). Arrowheads – elastic fibers. (C and D) Verhoeff stained histological sections of the ascending aorta to verify the number of elastic lamellae (arrows) of the CG (C) and TG (D) rats.
We obtained the number of elastic lamina (N[lamina]) by counting their number at four different points of the aortic wall in three non-consecutive histological sections per animal. The final value was the average of the counts (Fig. 1C and D).
The elastic lamina surface density (Sv[lamina]) was quantified according to the stereological method described by Marques et al.17 We put a 16 cycloid arc test system on the monitor screen, calibrated it (Zeiss micrometer 1 mm/100), and arranged the minor axes of the cycloids in parallel with the defined vertical axis. The number of elastic lamina intersections with the cycloid arcs (I[lamina]) was counted, allowing estimation of elastic lamina surface density (Sv[lamina]) according to the formula: (Sv[lamina]) = 2 × I[lamina]/LT (LT is the test line length based on the system calibration).
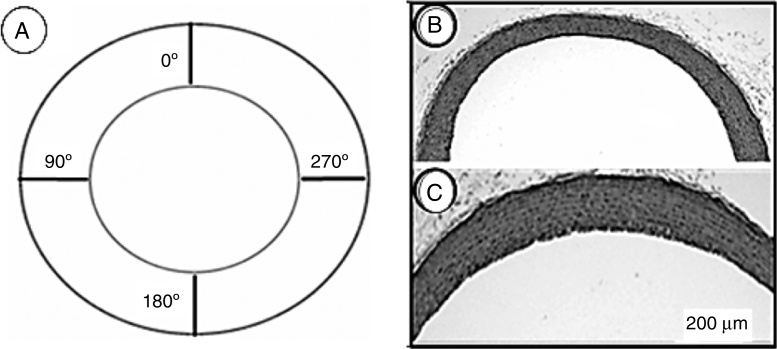
The collagen content was determined in four fields of four histological non-serial sections of five μm stained with the Picrosirius technique and examined under polarized light. When studied with this method, tissues containing collagen fibers show birefringent thick and thin fibers intensely.18 We entered histological sections into a KS-400 digital analyzing computer program (Zeiss, Germany) to determine the collagen content range in the two groups, and it quantified the area percentage (12,000 μm2) of collagen fibers.19 The sections were analyzed using a microscope equipped with appropriate polarization lenses. In each section, we selected four randomized fields and quantified the area percentage of collagen fibers in each field. The aortic wall thickness was measured in four non-consecutive sections stained with hematoxylin and eosin in four fields located at 0°, 90°, 180°, and 270° (Fig. 2A).
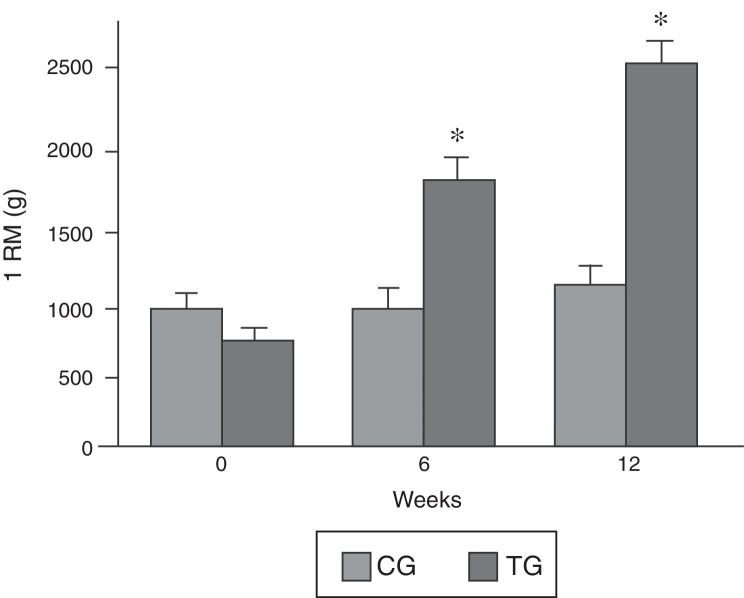
Figure 2.
Absolute values for 1RM test. Results are mean ± SEM. *p < 0.05, compared with the previous test. To compare the means of the groups, we used the t test.
The maximum and minimum diameters of collagen fibrils were determined in ten electron micrographs of each aorta at a final magnification of 130,000× obtained from regions where the fibrils were transversely sectioned. We used the image analyzer program to measure the diameters of each fibril. By commencing at a corner of each field and radiating outward in an arc, the diameters of the fibrils present in the field were measured.
Statistical analysis
We used InStat for data analysis and the mean ± Standard Error of Mean (SEM, when not specified) for descriptive statistics. The independent Student's t test was used to compare the aortic parameters of the two groups of rats. Two-way ANOVA (group vs. time) was performed to evaluate changes in 1RM. The level of significance was set at p < 0.05.
Results
One-repetition maximum
Fig. 2B shows the progressive increase in the weight lifted by the rats of the TG obtained during the repetition maximum test. The CG and TG had similar values for repetition maximum at the beginning of the experiment. After 6 weeks and 12 weeks, the load lifted by the animals in the CG group was similar to that lifted in the first test. At the end of 6 weeks, the TG lifted 1580 (SD = 92 g), and 2405 (SD = 140 g) at the end of 12 weeks, which represents 1.6-fold and 3.04-fold the body weight for 6 and 12 weeks, respectively.
Effects of exercise on body weight and cardiac growth
After 12 weeks of resistance training, no significant difference was observed between groups for body weight or blood pressure (Table 1A). The ratios of heart and ventricular weights to BW were higher for the TG compared to the CG, indicating that the heart grew more than the BW in the TG. The LVW (left ventricular weight), LV, and wall thickness were significantly higher in the TG rats than in the CG rats. However, LV internal diameter was significantly smaller (10%) in the TG than in the CG rats. These results may indicate the development of concentric hypertrophy in hearts from the TG rats.
Table 1.
Effects of resistance training on cardiac growth and stereological parameters of the aorta of rats after 3-month of training.
| CG | TG | p-Value | |
|---|---|---|---|
| A. Cardiac parameters | |||
| B W (g) | 388 ± 39 | 377 ± 20 | >0.05 |
| LVW (g) | 0.78 ± 0.02 | 0.90 ± 0.05* | <0.05 |
| LVW:BW (mg/g) | 2.01 ± 0.2 | 2.38 ± 0.03* | <0.05 |
| LV wall thickness (mm) | 2.09 ± 0.03 | 2.79 ± 0.2* | <0.05 |
| LV internal diameter (cm) | 0.71 ± 0.04 | 0.64 ± 0.03* | <0.05 |
| BP (mm Hg) | 109.7 ± 2.4 | 113.4 ± 1.4 | >0.05 |
| B. Stereological parameters | |||
| Thickness (μm) | 121.31 ± 1.25 | 204.94 ± 2.65* | <0.05 |
| V[smc] (μm3) | 23.1 ± 1.3 | 25.6 ± 3.1* | <0.05 |
| N[lamina] (104 mm2/mm3) | 11.71 ± 0.24 | 14.06 ± 0.24* | <0.05 |
| SV[lamina] | 16.44 ± 0.77 | 20.4 ± 0.77* | <0.05 |
| Collagen density (%) | 2.1 ± 0.5 | 3.8 ± 0.4* | <0.05 |
CG, control group; TG, trained group; LV, left ventricle; BP, mean arterial blood pressure; V[smc], smooth muscle cell nuclei volume; (N [lamina], number of elastic lamina; SV[lamina], lamina surface density. Values are means ± SD.
Significant vs. CG (p < 0.05).
Morphometry
The aortic wall thickness was larger in the TG than in the CG rats (p < 0.05) (Table 1B and Fig. 3). The volume of smooth muscle cell nucleus was larger in the TG than in the CG rats (p < 0.05) (Table 1B). Resistance training also affected aortic stereological parameters. N[lamina] and SV[lamina] were greater in the TG than in the CG rats (p < 0.05) (Table 1B).
Figure 3.
(A) Schematic representation of a cross-section of the aorta showing the locations where measurements of the wall thickness were taken. (B and C) Photomicrographs of aortic wall sections from the CG (B) and TG (C) rats stained with hematoxylin and eosin and taken with the same magnification. The wall thickness in (C) seems to be greater than in (B).
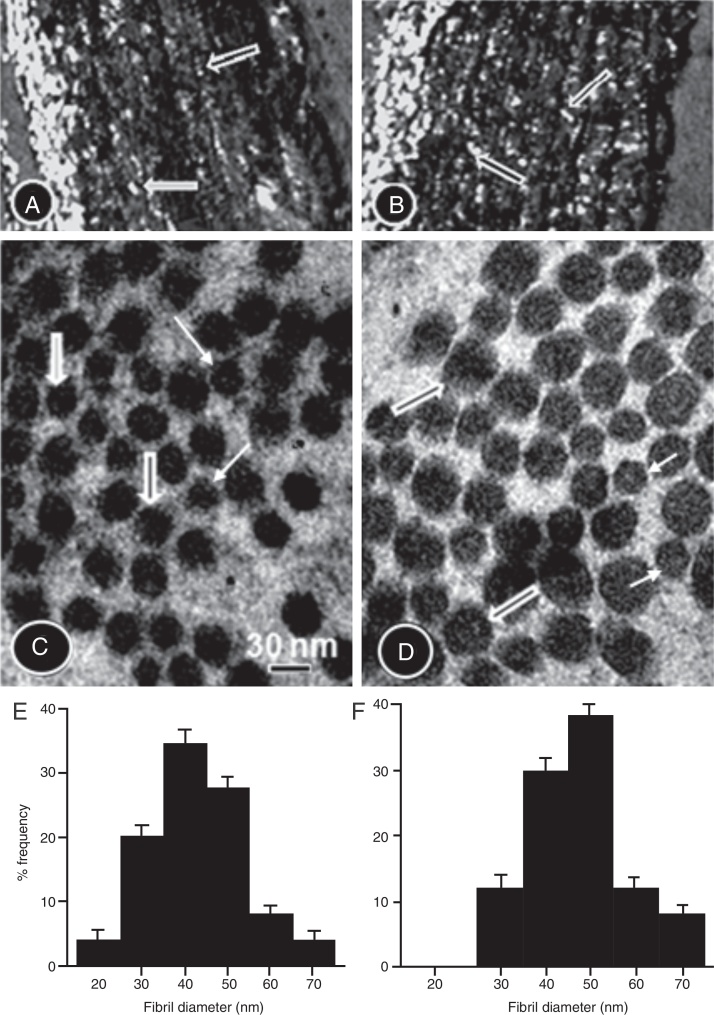
The collagen content was higher in the TG compared to the CG (Fig. 4A and B and Table 1B). In the CG rats (Fig. 4C and D), the collagen fibers were composed mainly of small diameter fibrils. Collagen fibrils of small and large diameter formed the aortic wall in the TG rats (Fig. 4C and D), with predominance of large diameter fibrils. Fig. 4E and F is histograms showing the distribution of collagen fibril diameter in the CG and TG aortas, respectively.
Figure 4.
Picrosirius red-stained aorta cross-sections of the CG (A) and TG (B) rats viewed under polarized light to show the collagen fibers. The number of collagen fibers in A seems to be greater than in B. High-power electron micrographs of transverse sectioned collagen fibers from the aorta wall of the CG (C) and TG (D) groups of rats. The electron micrographs are viewed with the same magnification showing collagen fibrils of large (thick arrows) and short diameter (thin arrows). The number of large fibers seems to be greater in the TG than in the CG. (E and F) Histograms showing the distribution of collagen fibril diameter in CG and TG aortas, respectively.
Discussion
There are two major findings in our study. Firstly, rats submitted to resistance training for 12 weeks exhibited a significant increase in aortic wall thickness compared to sedentary controls, indicating that training improves aortic wall structural components. Secondly, structural components (collagen, elastin, and muscle cell) in the aortic wall were significantly increased in resistance-trained animals compared with sedentary controls. This resistance training protocol has proved to be successful in producing positive exercise responses in the heart of rats.13, 20 The present results also showed that resistance training has proved to be successful in producing positive exercise responses in the aorta.
In this work, rats performing resistance training exhibited a significant enhancement in aortic wall thickness compared to sedentary controls. This change is known as arteriogenesis, and its occurrence requires remodeling with the participation of endothelial cells, smooth muscle cells, and fibroblasts (chronic physical exercise can promote it).21, 22, 23 The mechanisms involved in the process of increasing artery wall thickness are well known.24 In addition, resistance training can favor shear stress because the mechanical stimulus triggers the downstream signaling of nitric oxide, prostacyclin, and hyperpolarizing factor, which regulate the function of vascular smooth muscle cells.24
The present study demonstrated a significant increase in the aortic wall components by resistance training. The volume of smooth muscle cell nucleus, the tunica media thickness, the number and density of elastic lamina and collagen content, and diameter of collagen fibrils in the aortic wall increased with resistance training. This is consistent with the finding that the incremental load in the aortic wall promoted by exercise reflects a bend of pressure and volume overload, producing vascular remodeling.21, 23 Our finding is not in agreement with the observation that there is no significant increase in aortic parameters after resistance training in rats.25 This discrepancy may be mainly due to the different protocol used by these authors. The training was not adjusted according to the body weight of the animals, and there was no information on whether the training load was set at 60% of 1RM.
This study showed the modifications of smooth muscle cells and their products, collagen, and elastic laminae after the 12-week resistance training. This is consistent with studies demonstrating that the adaptation to physiological loading could be associated with the molecular phenotype of aortic muscle cell gene expression.26 However, there is little information about smooth muscle cell expression of the genes after chronic physiological loads.
In the present study, the number and density of elastic lamina increased significantly with training. The increase with training could be due to the continued stimulus produced by the pressure increase in the artery wall during training. This increase in wall elasticity would be a protective mechanism to prevent wall damage by making it less rigid and more resilient.
We demonstrated that the aortic collagen increased with training. This result implies increased collagen turnover with training, with degradation rates falling more slowly than the simultaneous increase in synthesis rate. This is consistent with the finding that arterial stiffness increases during training27 and is inversely correlated with training performance.28 To date, there is no explanation for the collagen degradation process and its regulation during resistance training. The increase in systolic blood pressure during training is a possible explanation. Then, the increase in aortic collagen may represent a response to increased loading conditions imposed by stress training in the aortic wall. Alternatively, it is possible that TGF-1β increases with pressure overload, which could explain the increase in collagen transcription in the exercised aorta. Apparently, resistance training triggers opposite effects in the aortic wall: it increases elastic lamellae along with increases in collagen content. Further studies are necessary to elucidate these results.
The LVW and LV wall thickness of the TG rats was significantly higher than that of the CG rats, but the LV internal diameter was not. These results indicate the development of concentric hypertrophy in the heart of the TG rats,29 providing consistent evidence of myocardial tissue increase associated with resistance training. The improvement in cardiac structure was associated with the increase in aortic resistance promoted by training. Resistance training promoted the change in the mechanical properties of the aortic wall related to the elastic/collagen changes.30, 31
In summary, according to the results of the present study, resistance training displayed improved aortic remodeling in rats. The aortic adaptation to resistance training was associated with an increase in smooth cell size and collagen and an increase in elastin in the aortic wall.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kojimahara M. Age-induced changes in the aortas of rats. Exp Pathol. 1985;28(4):191–195. doi: 10.1016/s0232-1513(85)80008-6. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda M., Nosaka T., Sato M., Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol. 1993;66(2):122–126. doi: 10.1007/BF01427052. [DOI] [PubMed] [Google Scholar]
- 3.Coura M., Pacheco M., Simões H., Moraes J., Campbell C. Morphoquantitative study of the aorta's wall in aerobically trained elderly wistar rats. Motricidade. 2012;8(4):71–79. [Google Scholar]
- 4.Lima N., Lopes I., Ervilha U.F. Effects of resistance exercise on ascendent aorta on ovariectomized elderly rats. Braz J Morphol Sci. 2012;29(4):248–252. [Google Scholar]
- 5.Schriefl A.J., Zeindlinger G., Pierce D.M., Regitnig P., Holzapfel G.A. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface. 2012;9(71):1275–1286. doi: 10.1098/rsif.2011.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsamis A., Krawiec J.T., Vorp D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10(83):20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor C.A., Hughes T.J., Zarins C.K. Effect of exercise on hemodynamic conditions in the abdominal aorta. J Vasc Surg. 1999;29(6):1077–1089. doi: 10.1016/s0741-5214(99)70249-1. [DOI] [PubMed] [Google Scholar]
- 8.de Andrade Moraes-Teixeira J., Félix A., Fernandes-Santos C., Moura A.S., Mandarim-de-Lacerda C.A., de Carvalho J.J. Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. Exp Mol Pathol. 2010;89(3):351–357. doi: 10.1016/j.yexmp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Barauna V.G., Rosa K.T., Irigoyen M.C., de Oliveira E.M. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin Med Res. 2007;5(2):114–120. doi: 10.3121/cmr.2007.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beniamini Y., Rubenstein J.J., Zaichkowsky L.D., Crim M.C. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol. 1997;80(7):841–846. doi: 10.1016/s0002-9149(97)00533-x. [DOI] [PubMed] [Google Scholar]
- 11.Longhurst J.C., Kelly A.R., Gonyea W.J., Mitchell J.H. Echocardiographic left ventricular masses in distance runners and weight lifters. J Appl Physiol. 1980;48(1):154–162. doi: 10.1152/jappl.1980.48.1.154. [DOI] [PubMed] [Google Scholar]
- 12.Heyward V.H. Designing resistance training programs. In: Heyward V.H., editor. Advanced Fitness Assessment & Exercise Prescription. 3rd ed. Human Kinetics; Champaign (IL): 1998. pp. 121–144. [Google Scholar]
- 13.Hornberger T.A., Jr., Farrar R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 14.Carson F., Martin J., Lynn J. Formalin fixation for electron microscopy: a re-evaluation. Am J Clin Pathol. 1973;59(3):365. doi: 10.1093/ajcp/59.3.365. [DOI] [PubMed] [Google Scholar]
- 15.Baddeley A., Gundersen H.-J.G., Cruz-Orive L.M. Estimation of surface area from vertical sections. J Microsc. 1986;142(3):259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 16.Salvatore C. A cytological examination of uterine growth during the estrous cycle and artifically induced estrus. Rev Bras Biol. 1948;8:505–523. [Google Scholar]
- 17.Marques C.M., Nascimento F.A., Mandarim-de-Lacerda C.A., Aguila M.B. Exercise training attenuates cardiovascular adverse remodeling in adult ovariectomized spontaneously hypertensive rats. Menopause. 2006;13(1):87–95. doi: 10.1097/01.gme.0000191209.13115.46. [DOI] [PubMed] [Google Scholar]
- 18.Junqueira L.C.U., Bignolas G., Brentani R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 19.Mendes A., Ferro M., Rodrigues B., de Souza M.R., Araujo R.C., de Souza R.R. Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (B Aires) 2012;72(72):216–220. [PubMed] [Google Scholar]
- 20.Lee S., Barton E.R., Sweeney H.L., Farrar R.P. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J App Physiol. 2004;96(3):1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lehoux S., Tronc F., Tedgui A. Mechanisms of blood flow-induced vascular enlargement. Biorheology. 2001;39(3–4):319–324. [PubMed] [Google Scholar]
- 22.Resnick N., Einav S., Chen-Konak L., Zilberman M., Yahav H., Shay-Salit A. Hemodynamic forces as a stimulus for arteriogenesis. Endothelium. 2003;10(4–5):197–206. doi: 10.1080/10623320390246289. [DOI] [PubMed] [Google Scholar]
- 23.Prior B.M., Yang H., Terjung R.L. What makes vessels grow with exercise training? J Appl Physiol. 2004;97(3):1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 24.Mombouli J., Vanhoutte P.M. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35(1):679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- 25.Li M., Li W., Yoon J.H., Jeon B.H., Lee S.K. Resistance exercise training increase activation of AKT-eNOS and Ref-1 expression by FOXO-1 activation in aorta of F344 rats. J Exerc Nutr Biochem. 2015;19(3):165–171. doi: 10.5717/jenb.2015.15071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rensen S., Doevendans P., Van Eys G. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15(3):100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borlaug B.A., Olson T.P., Lam C.S. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertovic D.A., Waddell T.K., Gatzka C.D., Cameron J.D., Dart A.M., Kingwell B.A. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension. 1999;33(6):1385–1391. doi: 10.1161/01.hyp.33.6.1385. [DOI] [PubMed] [Google Scholar]
- 29.Pluim B.M., Zwinderman A.H., van der Laarse A., van der Wall E.E. The athlete's heart a meta-analysis of cardiac structure and function. Circulation. 2000;101(3):336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 30.Lévy B. Aging of the arterial system. Presse Mèd. 1992;21(26):1200–1203. [PubMed] [Google Scholar]
- 31.Qiu H., Depre C., Ghosh K. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116(6):669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]