Highlights
-
•
FES is widely used in patients with chronic heart failure.
-
•
FES increased quadriceps muscle strength after eight weeks of training.
-
•
FES increased lower limb muscle endurance after eight weeks of training.
-
•
FES was unable to modify functional capacity after eight weeks of training.
Keywords: Electric stimulation, Thoracic surgery, Rehabilitation
Abstract
Background
Functional electrical stimulation (FES) has shown benefits in patients with chronic heart failure. Therefore, it is possible that FES can benefit patients similarly after cardiac surgery.
Objective
This randomized placebo-controlled trial aimed to evaluate the effects of FES on lower limb functional capacity, strength, endurance, and muscle mass after discharge from cardiac surgery.
Methods
Twenty patients were allocated (1:1) to the group receiving FES to the quadriceps (FESG) or FES placebo (FESPG). FES was applied at a frequency of 15 Hz, with 0.5 ms pulse width, 5 s contraction time, and 10 s resting time, twice a week for 40 min over a period of eight weeks. Functional capacity was assessed using the six-minute walk test (6MWT), lower limb muscle strength using the one repetition maximum test (1RM), endurance using the sit-and-stand test (SST), and muscle using the perimeter of the thighs.
Results
Both groups increased the distance covered in the 6MWT (FESG: 49.6 m, 95% CI 15.9–83.3; FESPG: 41.5 m, 95% CI 7.8–75.2), but without a difference between groups. There were significant between-group differences for quadriceps muscle strength (7.2 kg, 95% CI 0.2–14.2) and muscle endurance (2.2 repetitions, 95% CI 1.0–3.4) in favor of the FESG.
Conclusion
FES improves lower limb muscle strength and endurance in patients after cardiac surgery. Larger trials are needed to confirm our findings.
Introduction
Cardiac rehabilitation (CR) aims to optimize physical and psychosocial recovery after a cardiac event and to reduce the risk of a subsequent event through changes in lifestyle for these patients.1, 2 Physical training is an important part of the CR program and aims to improve exercise capacity and physical functioning regarding limitations to individual physical activities.3 However, some patients do not tolerate even low levels of exercise and therefore tend not to engage in conventional physical training programs.4
In this sense, functional electrical stimulation (FES) can be an alternative within the CR programs to improve the fitness of an individual who is less motivated.5 Furthermore, FES generates less hemodynamic risk when compared to conventional exercise training, consisting in a safe and low risk intervention for adverse events.4, 6
When applied to individuals with chronic heart failure (CHF), FES was able to increase quadriceps muscle strength by 25%.7 In addition, the cross-sectional area and resistance to muscle fatigue also improved.8 In fact, FES and conventional aerobic exercise have similar effects on lower limb muscle strength and on the distance walked in the six-minute walk test (6MWT) in this population. However, conventional aerobic exercise is superior to FES for increasing peak oxygen consumption (VO2peak).5 On the other hand, FES promotes greater benefits in VO2peak and distance covered in the 6MWT (6MWD) when compared to the control group.5 FES also has positive effects on aspects of quality of life in patients with moderate to severe heart failure.4 Therefore, taking into account the findings of Sbruzzi et al.5 and Smart et al.,4 FES may be an alternative to conventional aerobic exercise for patients with chronic heart failure and for those who are unable to perform this kind of exercise.
Despite quite a large number of studies reporting the benefits of FES in patients with CHF, systematic reviews published to date point to the need for a greater number of randomized controlled trials. Patients undergoing coronary artery bypass graft (CABG) or Heart Valve Surgery (HVS) after discharge enter phases II and III of cardiac rehabilitation. This period may be marked by initial physical inactivity, fear of activities of daily living, and difficulty in adapting to conventional physical training. Thus, FES has been used by different protocols and remains as a potential research tool in the processes of CR. We hypothesized that FES increases lower limb muscle mass, strength, and endurance, thus improving the functional capacity of patients in CR. Therefore, this study aimed to evaluate the effects of FES on lower limb functional capacity, muscle mass, strength, and endurance after discharge from cardiac surgery.
Methods
This is a randomized, placebo-controlled trial with a 1:1 allocation ratio. The project was approved by the Research Ethics Committee of Universidade Federal de Santa Maria (UFSM; CAAE 0187.0.243.000-10), recorded in the protocol system ClinicalTrials.gov (NCT 02088138 identifier) and informed consent was obtained from all patients. After consulting the database of the cardiology department of Hospital Universitário de Santa Maria (HUSM), the invitation was extended to the patients by telephone. The inclusion criteria were patients of both sexes who underwent CABG or HVS and who had already been discharged from hospital. All patients were submitted to phase I of cardiac rehabilitation postoperatively, but were sedentary on admission to the study. Patients with cognitive impairment that prevents performing evaluations, as well as those who were unable to understand the informed consent form were excluded. Patients were also excluded if they presented with peripheral vascular changes in the lower limbs, such as deep vein thrombosis or thromboangiitis obliterans, chronic obstructive pulmonary disease, cerebrovascular disease, musculoskeletal disease, or who presented the following contraindications to the use of FES: epidermal lesions at the site of application, intolerance to electrical stimulation, any change in skin sensitivity, or use of pacemaker.
Randomization
Eligible patients were randomized to the FES placebo group (FESPG) or the intervention group (FESG). After the first evaluation, randomization was generated by the www.random.org online software. The sequence of numbers was generated by researchers blinded to the study after the selection of patients and only disclosed prior to the start of the intervention program to the physical therapist responsible for applying FES or FES placebo, in order to ensure concealment of the allocation sequence and to keep patients blinded to the type of intervention. Furthermore, the sessions were performed individually and at different times to avoid contact between groups.
Evaluations
The electronic medical records of patients were consulted and an interview was performed for data collection identification, type and date of surgery, medications and comorbidities. The evaluations were made in the outpatient physical therapy and cardiology service of HUSM before and after the intervention period and by the same evaluator, who was kept blind to the allocation of patients.
The 6MWD9 was defined as the primary outcome. Secondary outcomes were lower limb strength, endurance, and muscle mass. Assessments of outcomes were done using the following tests respectively: six-minute walk test (6MWT), one repetition maximum test (1RM), sit-and-stand test (SST), and perimeter of the thighs.
Functional capacity evaluation
The 6MWT was conducted to assess functional capacity in a rectangular, flat, 30-meter long hallway as recommended by the American Thoracic Society.10 Patients were instructed to walk as far as possible, without running for six minutes. Every minute of the test, the subject was verbally encouraged through standardized phrases. Heart rate, blood pressure, oxygen saturation, and perceived exertion scale for dyspnea and lower limb fatigue were recorded prior to the start and immediately after the test.10
Muscle strength evaluation
The dynamic strength of the quadriceps muscle was assessed using the 1RM test on a device called the extension table (Kikos, São Paulo, SP, Brazil). In the sitting position, with the hips and knees at 90° of flexion, the patient was instructed to extend both knees against the resistance placed in the anterior region of the ankles up to the maximum range of motion. If two repetitions were completed, the load was increased until the patient was able to perform a single maximum repetition throughout the range of motion without postural compensations.11
Muscle endurance evaluation
The SST used to evaluate lower limb function and endurance was performed with the subject seated on a 43-cm high armless chair with arms crossed in front of the chest. The subject had to get up and return to the sitting position as many times as possible in 30 s.12
Muscle mass evaluation
Perimeter evaluation was the method used to measure lower limb muscle mass. The circumference of the thighs was measured at five locations of 5–20 cm from the upper edge of the patella in the proximal direction. The measurement was performed with the patient in the supine position and with the muscle in isometric contraction.13
Intervention
Interventions were performed in the pediatric neurology clinic of the Department of Physiotherapy and Rehabilitation of UFSM. On the first day of intervention with FES, the motor areas of the vastus medialis and lateralis muscles of both thighs were determined through a 3 cm-diameter electrode (ValuTrode, CF3200 model, São Paulo, SP, Brazil), and another 5 × 5 cm (ValuTrode, CF5050 model, São Paulo, SP, Brazil) in size to close the circuit, the latter being positioned near the inguinal fold. Later mapping motor areas, they were copied on a plastic sheet so that the other interventions could determine the precise position to place the electrodes. Before and after each session, we measured heart rate, respiratory rate, oxygen saturation, blood pressure, and level of dyspnea using the Borg Scale.
The FES and FES placebo sessions occurred in the supine position with the legs on the quadriceps board (Arktus, São Paulo, SP, Brazil) and 60° angle of knee flexion.14 A properly calibrated FES device (Endophasys NMS 0501 model, KLD-biosystems, Amparo, SP, Brazil) was used. The rectangular symmetrical biphasic pulsed electric current was applied through adhesive and hypoallergenic electrodes (ValuTrode, CF5050/CF5090 models, São Paulo, SP, Brazil), size 5 × 5 cm or 5 × 9 cm (according to the motor area of the patient) placed on the vastus medialis and lateralis of both thighs and two other electrodes (size 5 × 9 cm) positioned near the inguinal fold to also close the circuit in both thighs. They were changed when the electrical resistance increased.
FES was applied at a frequency of 15 Hz, with 0.5 ms pulse width, 5 s contraction time, and 10 s resting time (totaling 4 contractions per minute),8 twice a week for eight weeks, totaling 16 sessions of 40 min each. The adopted intensity was the same for both legs as the 1RM test was bilateral and simultaneous. Still, the intensity was adjusted at each session according to patient tolerance in the FESG and only at the sensory threshold level in the FESPG. The FESG underwent intervention with FES so that muscle contraction was visible and, when the knee extension occurred, an overload of weight between 25% and 30% of the 1RM test8 was added at the ankles of patients by means of leggings so that the subject could maintain knee extension. The percentage did not exceed 30% of 1RM.
Statistical analysis
The sample size calculation was performed using Gpower software® and it was based on rates of 5% alpha error and 80% power for a magnitude of effect of 1.181.7 To calculate the sample size, the mean difference between groups of 61 ± 51.5 m covered in the 6MWT was used, as reported by Banerjee et al.7 According to the calculation performed, a sample of 10 subjects was required in each group.
For data analysis, SPSS version 13.0 was used. Data distribution was assessed using the Kolmogorov–Smirnov test. Data are presented as mean ± standard deviation and 95% confidence interval (95% CI). The comparison within and between groups was performed using two-way ANOVA with repeated measures and the results were analyzed for time, group, and interaction effects, followed by the Tukey post hoc test. The analysis was performed per-protocol on the patients included in the study. The significance level was set at 5% (p < 0.05).
Results
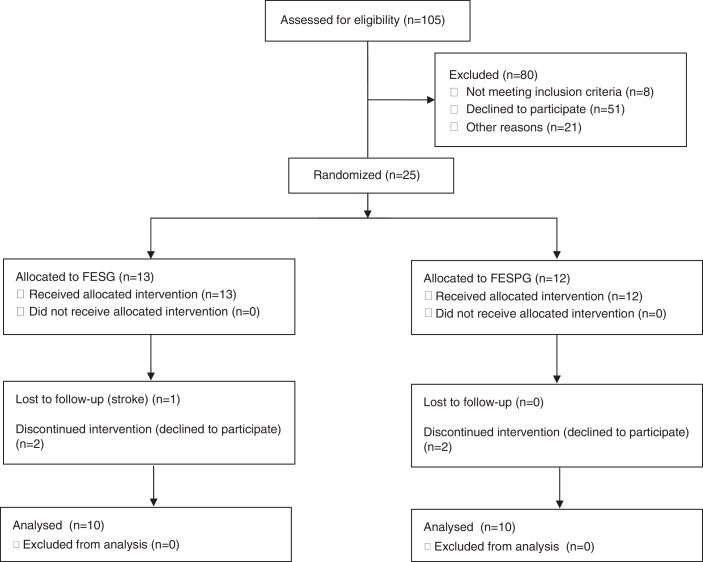
One hundred and five patients undergoing coronary artery bypass grafting (CABG) or heart valvesurgery (HVS) from March 2014 to September 2015 were selected. Fig. 1 shows the flowchart of the trial.
Figure 1.
Flowchart for admitting patients and groups composition.
Table 1 shows that the groups are similar as to demographic, anthropometric, and clinical characteristics, ejection fraction, vital signs, and peripheral capillary oxygen saturation. The left ventricular ejection fraction was preserved in both groups. The intensity of electrical stimulation, as expected, had higher results for FESG.
Table 1.
Characteristics of the groups on admission to the study.
| FESG (n = 10) | FESPG (n = 10) | |
|---|---|---|
| Age (years) | 60.0 (7.3) | 63.5 (5.0) |
| Sex (F/M) | 3/7 | 3/7 |
| BMI (kg/m2) | 27.3 (3.1) | 29.1 (6.2) |
| Type of surgery (CABG/ HVS) | 8/2 | 9/1 |
| PO (days) | 240.3 (129.8) | 233.4 (120.6) |
| LVEF (%) | 60.2 (5.1) | 57.9 (5.3) |
| Heart rate (bpm) | 65.6 (9.5) | 67.7 (7.1) |
| Respiratory rate (cpm) | 17.9 (3.2) | 19.1 (3.8) |
| BP systolic (mmHg) | 131.0 (19.1) | 130.0 (11.5) |
| BP diastolic (mmHg) | 86.0 (9.7) | 84.0 (5.2) |
| SpO2 (%) | 97.2 (1.8) | 96.8 (1.6) |
FESG, functional electrical stimulation group; FESPG, placebo group; F, female; M, male; BMI, body mass index; CABG, coronary artery bypass grafting; HVS, heart valve surgery; PO, post-operative; LVEF, left ventricular ejection fraction; SpO2, peripheral capillary oxygen saturation. Values are shown as mean (standard deviation) or number of subjects.
The distance walked in the 6MWT increased (time effect; p < 0.01) in the FESPG by 10.4% (41.5 m, 95% CI 7.8–75.2) and in the FESG by 11.0% (49.6 m, 95% CI 15.9–83.3). As shown in Table 2, there were no significant between-group differences for 6MWD. The groups were similar at baseline for 6MWD (p > 0.05).
Table 2.
Mean (SD) of groups, mean (SD) difference within groups, and mean (95% CI) difference between groups for all outcomes.
| Outcome | Groups |
Difference within groups | Difference between groups | ||||
|---|---|---|---|---|---|---|---|
| Before |
After |
After minus before |
After minus before | ||||
| FESG | FESPG | FESG | FESPG | FESG | FESPG | FESG minus FESPG | |
| Functional capacity | |||||||
| 6MWT (m) | 452.1 (83.8) | 397.0 (72.3) | 501.7 (85.0) | 438.5 (49.3) | 49.6 (41.2)* | 41.5 (45.9)* | 8.1 (−30.1 to 46.3) |
| Muscle strength | |||||||
| 1RM (kg) | 36.0 (13.2) | 30.3 (6.8) | 45.5 (11.6) | 32.6 (8.2) | 9.5 (9.9)* | 2.3 (5.4) | 7.2 (0.2–14.2)* |
| Muscle endurance | |||||||
| SST (repetitions) | 10.1 (3.3) | 9.1 (2.1) | 13.0 (2.7) | 9.8 (1.8) | 2.9 (1.4)* | 0.7 (1.4) | 2.2 (1.0–3.4)* |
| Muscle mass above the patella | |||||||
| Right thigh | |||||||
| 10 cm | 44.2 (4.1) | 45.8 (4.8) | 44.7 (2.9) | 45.5 (4.3) | 0.5 (2.6) | −0.4 (1.2) | 0.9 (−0.9 to 2.7) |
| 20 cm | 51.1 (3.8) | 52.7 (5.0) | 52.0 (3.3) | 52.6 (4.6) | 0.9 (2.2) | −0.1 (1.9) | 1.0 (−0.8 to 2.8) |
| Left thigh | |||||||
| 10 cm | 43.5 (3.0) | 45.9 (4.9) | 44.9 (2.3) | 45.9 (5.1) | 1.4 (1.6) | 0.0 (2.1) | 1.4 (−0.2 to 3.0) |
| 20 cm | 51.3 (3.4) | 54.4 (5.0) | 52.2 (2.5) | 53.7 (5.1) | 0.9 (1.8) | −0.7 (1.8) | 1.6 (0.0–3.2) |
FESG, functional electrical stimulation group; FESPG, placebo group; 6MWT, six-minute walk test; 1RM, one repetition maximum test; SST, sit-and-stand test.
Significant difference (p < 0.05).
In time effect analysis, quadriceps muscle strength increased (p < 0.01) by 26.4% in the FESG (9.5 kg, 95% CI 3.4–15.7), but did not differ in the FESPG (2.3 kg, 95% CI −3.8 to 8.4). Muscle endurance also increased (p < 0.01) in the FESG at 28.7% (2.9 repetitions, 95% CI 1.8–4.0) and it was not changed in the FESPG (0.7 repetitions, 95% CI −0.4 to 1.8). There were significant between-group differences for quadriceps muscle strength and muscle endurance (Table 2). The groups were similar at baseline for lower limb muscle strength and endurance.
There were no significant changes in thigh muscle mass or differences between groups, regardless of the segment and level of circumference evaluated (Table 2).
Concerning the safety of this therapeutic modality, no significant changes were observed in the vital signs of patients during the intervention with FES nor adverse effects or complications related to the electrical current after the stimulation session.
Discussion
The main findings of this study were an increase in lower limb strength and endurance in the FESG and a similar increase in functional capacity in both groups. Our results also showed a significant increase in the muscle strength of the knee extensor muscles in the FESG over time and a significant between-group difference. These findings corroborate those of Banerjee et al.,7 who studied 10 patients with CHF using a protocol of 60 min daily, five times a week for eight weeks and found that electrical stimulation increased the maximum lower limb strength. However, in our study only two weekly sessions of FES were performed, demonstrating that similar effects can be achieved with a lower frequency of treatment and with just 16 sessions of FES.
The study results also demonstrated that lower limb muscle endurance was improved in the FESG, with a significant between-group difference. This finding indicates that electrical currents applied at frequencies up to 20 Hz can recruit tonic/slow-twitch muscle fibers with high fatigue resistance. As suggested by Sbruzzi et al.,15 the recruitment of certain types of muscle fibers appears to be related to the frequency of electrical stimulation. With a frequency below 20 Hz, the work is directed predominantly at type I fibers,8 which feature fatigue resistant muscle contractions being performed at low metabolic cost.16 With an electrical stimulation frequency between 35 and 70 Hz, type II fibers predominate.16 This offers the possibility of working strength gain using selective muscle stimulation,17 which can be advantageous when the aim is to recruit specific muscle fibers, such as fibers with a high oxidative potential that is decreased in heart failure patients.18, 19
In a study by our group, Sbruzzi et al.15 compared the acute effect of functional electrical stimulation with frequencies of 15 and 50 Hz on the strength of quadriceps and found that FES with 50 Hz determines higher peak torque muscle than FES with 15 Hz. The study attributed this to the fact that muscle strength is proportional to the frequency of stimulation and the number of motor units recruited. Thus, the higher the frequency, the greater the motor recruitment producing greater muscle force.20 In this context, assuming that muscle strength and tropism pose a direct proportional relationship, the absence of increased muscle mass for both groups after the end of the 16 sessions seems to be related to the stimulation of tonic muscle fibers, since we use a frequency of 15 Hz, aiming at muscle endurance.
Nuhr et al.8 applied FES with a frequency of 15 Hz for 4 h/day, overload 20–30% of maximum strength for 10 weeks in patients with heart failure and reported increased lower limb muscle mass, especially type I fibers (+20%), with a reduction in type II fibers (−20%). Thus, the lack of change in muscular mass reported in our study may be further attributed to the shorter application period (8 weeks) as well as to shorter sessions (40 min). Other authors also mention the use of different FES parameters that can justify our findings: 60 min daily/5 days per week for 5 weeks,20, 21 60 min daily/5 days per week for 8 weeks,7 30 min a day/5 days per week for 6 weeks.22
In the analysis of functional capacity, both groups traveled greater distances in the 6MWT after intervention with functional electrical stimulation; however, it was not possible to identify differences between groups. This significant increase in functional capacity, even in subjects who received FES placebo, may be related to the natural recovery process after surgery and the stimulus provided by the attention given to patients by rehabilitation. The patients were instructed not to do any other form of exercise during the treatment with FES, but it is not possible to control the activities undertaken by patients in their everyday life, which may have affected the results for this variable. In contrast, in a study by Karavidas et al.,22 an increase in the 6MWD was only observed in the group treated with FES. Applying FES for a longer period of time and with a greater frequency of weekly sessions could offer more appropriate therapy to increase functional capacity. In addition, another proposed intervention based on an aerobic exercise program could offer greater specificity to change the 6MWD.
Overall, the results demonstrate a positive effect on the cardiac rehabilitation process of patients after cardiac surgery and encourage further research regarding FES as part of treatment programs offered to these patients. However, new tests should be conducted on this topic with a longer follow-up and with the inclusion of a larger number of subjects as well as variables that assess a broader dimension to the functional aspects in response to FES. Moreover, biochemical and morphological analyses of the quadriceps femoris muscle could promote a better understanding of the mechanisms involved in our findings.
It is noteworthy that the protocol used and described in detail in this study can be applied in patients undergoing cardiac surgery after hospital discharge safely and effectively; however, although FES is an interesting alternative of treatment, it does not replace conventional rehabilitation.
Among the main limitations, this was a small study in which an ideal intention to treat analysis was not fulfilled because the data of patients with loss of follow-up were incomplete. Thus, further studies are needed to confirm our findings.
Conclusion
This randomized controlled trial helped to expand the level of scientific evidence regarding the use of FES as an alternative and valuable resource, since even a short period of application and low weekly frequency increased lower limb muscle strength and endurance in patients after cardiac surgery.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Thomas R.J., King M., Lui K. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;50(14):1400–1433. doi: 10.1016/j.jacc.2007.04.033. PubMed PMID: 17903645. [DOI] [PubMed] [Google Scholar]
- 2.Santos A.A., Silva A.K., Vanderlei F.M., Christofaro D.G., Gonçalves A.F., Vanderlei L.C. Analysis of agreement between cardiac risk stratification protocols applied to participants of a center for cardiac rehabilitation. Braz J Phys Ther. 2016 Apr doi: 10.1590/bjpt-rbf.2014.0159. PubMed PMID: 27049503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie C.J., Thomas R.J., Squires R.W., Allison T.G., Milani R.V. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. PubMed PMID: 19339657. PMCID: PMC2665984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smart N.A., Dieberg G., Giallauria F. Functional electrical stimulation for chronic heart failure: a meta-analysis. Int J Cardiol. 2013 Jul;167(1):80–86. doi: 10.1016/j.ijcard.2011.12.019. PubMed PMID: 22236510. [DOI] [PubMed] [Google Scholar]
- 5.Sbruzzi G., Ribeiro R.A., Schaan B.D. Functional electrical stimulation in the treatment of patients with chronic heart failure: a meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2010 Jun;17(3):254–260. doi: 10.1097/HJR.0b013e328339b5a2. PubMed PMID: 20560163. [DOI] [PubMed] [Google Scholar]
- 6.Dobsak P., Homolka P., Svojanovsky J. Intra-dialytic electrostimulation of leg extensors may improve exercise tolerance and quality of life in hemodialyzed patients. Artif Organs. 2012;36(1):71–78. doi: 10.1111/j.1525-1594.2011.01302.x. PubMed PMID: 21848929. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee P., Caulfield B., Crowe L., Clark A.L. Prolonged electrical muscle stimulation exercise improves strength, peak VO2, and exercise capacity in patients with stable chronic heart failure. J Card Fail. 2009;15(4):319–326. doi: 10.1016/j.cardfail.2008.11.005. PubMed PMID: 19398080. [DOI] [PubMed] [Google Scholar]
- 8.Nuhr M.J., Pette D., Berger R. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J. 2004;25(2):136–143. doi: 10.1016/j.ehj.2003.09.027. PubMed PMID: 14720530. [DOI] [PubMed] [Google Scholar]
- 9.Santos B.F., Souza H.C., Miranda A.P., Cipriano F.G., Gastaldi A.C. Performance in the 6-minute walk test and postoperative pulmonary complications in pulmonary surgery: an observational study. Braz J Phys Ther. 2016;20(1):66–72. doi: 10.1590/bjpt-rbf.2014.0119. PubMed PMID: 26786074. PMCID: PMC4835166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks D., Solway S., Gibbons W.J. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167(9):1287. doi: 10.1164/ajrccm.167.9.950. PubMed PMID: 12714344. [DOI] [PubMed] [Google Scholar]
- 11.Clarke D.H. Adaptations in strength and muscular endurance resulting from exercise. Exerc Sport Sci Rev. 1973;1:73–102. PubMed PMID: 4806385. [PubMed] [Google Scholar]
- 12.Jones C.J., Rikli R.E., Beam W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. PubMed PMID: 10380242. [DOI] [PubMed] [Google Scholar]
- 13.Harrelson GL, Andrews JR, Wilk KE. Reabilitação Física das Lesões Desportivas. 2ª ed. 2000. 516 p.
- 14.Hoy M.G., Zajac F.E., Gordon M.E. A musculoskeletal model of the human lower extremity: the effect of muscle, tendon, and moment arm on the moment-angle relationship of musculotendon actuators at the hip, knee, and ankle. J Biomech. 1990;23(2):157–169. doi: 10.1016/0021-9290(90)90349-8. PubMed PMID: 2312520. [DOI] [PubMed] [Google Scholar]
- 15.Sbruzzi G., Schaan B.D., Pimentel G.L. Effects of low frequency functional electrical stimulation with 15 and 50 Hz on muscle strength in heart failure patients. Disabil Rehabil. 2011;33(6):486–493. doi: 10.3109/09638288.2010.498551. PubMed PMID: 20597628. [DOI] [PubMed] [Google Scholar]
- 16.Celichowski J. Mechanisms underlying the regulation of motor unit contraction in the skeletal muscle. J Physiol Pharmacol. 2000;51(1):17–33. PubMed PMID: 10768848. [PubMed] [Google Scholar]
- 17.Quittan M., Wiesinger G.F., Sturm B. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2001;80(3):206–214. doi: 10.1097/00002060-200103000-00011. quiz 15-6, 24. PubMed PMID: 11237275. [DOI] [PubMed] [Google Scholar]
- 18.Harris S., LeMaitre J.P., Mackenzie G., Fox K.A., Denvir M.A. A randomised study of home-based electrical stimulation of the legs and conventional bicycle exercise training for patients with chronic heart failure. Eur Heart J. 2003;24(9):871–878. doi: 10.1016/s0195-668x(02)00822-9. PubMed PMID: 12727155. [DOI] [PubMed] [Google Scholar]
- 19.Larsen A.I., Lindal S., Aukrust P., Toft I., Aarsland T., Dickstein K. Effect of exercise training on skeletal muscle fibre characteristics in men with chronic heart failure. Correlation between skeletal muscle alterations, cytokines and exercise capacity. Int J Cardiol. 2002;83(1):25–32. doi: 10.1016/s0167-5273(02)00014-1. PubMed PMID: 11959380. [DOI] [PubMed] [Google Scholar]
- 20.Casillas J.M., Gremeaux V., Labrunee M. Low-frequency electromyostimulation and chronic heart failure. Ann Readapt Med Phys. 2008;51(6):461–472. doi: 10.1016/j.annrmp.2008.04.006. PubMed PMID: 18550196. [DOI] [PubMed] [Google Scholar]
- 21.Deley G., Eicher J.C., Verges B., Wolf J.E., Casillas J.M. Do low-frequency electrical myostimulation and aerobic training similarly improve performance in chronic heart failure patients with different exercise capacities? J Rehabil Med. 2008;40(3):219–224. doi: 10.2340/16501977-0153. PubMed PMID: 18292925. [DOI] [PubMed] [Google Scholar]
- 22.Karavidas A., Driva M., Parissis J.T. Functional electrical stimulation of peripheral muscles improves endothelial function and clinical and emotional status in heart failure patients with preserved left ventricular ejection fraction. Am Heart J. 2013;166(4):760–767. doi: 10.1016/j.ahj.2013.06.021. PubMed PMID: 24093858. [DOI] [PubMed] [Google Scholar]