Abstract
Background:
Rurality and distance from cancer treatment centres have been shown to negatively impact cancer outcomes, but the mechanisms remain obscure.
Methods:
We analysed the impact of travel time to key healthcare facilities and mainland/island residency on the cancer diagnostic pathway (treatment within 62 days of referral, and within 31 days of diagnosis) and 1-year mortality using a data-linkage study with 12 339 patients.
Results:
After controlling for important confounders, mainland patients with more than 60 min of travelling time to their cancer treatment centre ((OR 1.42; 95% CI 1.25–1.61) and island dwellers (OR 1.32; 95% CI 1.09–1.59) were more likely to commence cancer treatment within 62 days of general practitioner (GP) referral and within 31 days of their cancer diagnosis compared with those living within 15 min. Island-dweller patients were more likely to have their diagnosis and treatment started on the same or next day (OR 1.72; 95% CI 1.31–2.25). Increased travelling time to a cancer treatment centre was associated with increased mortality to 1 year (30–59 min (HR 1.21; 95% CI 1.05–1.41), >60 min (HR 1.18; 95% CI 1.03–1.36), island dweller (HR 1.17; 95% CI 0.97–1.41).
Conclusions:
Island dwelling and greater mainland travel burden was associated with more rapid cancer diagnosis and treatment following GP referral even after adjustment for advanced disease; however, these patients also experienced a survival disadvantage compared with those living nearer. Cancer services may need to be better configured to suit the different needs of dispersed populations.
Keywords: diagnosis, treatment, travel, mortality, healthcare facilities
Short diagnostic and treatment intervals are perceived as critical to optimal cancer outcomes. This has been reflected within the past 10 years in the UK National Health Service (NHS) through the development of referral guidelines and strict targets for the timeliness of referral, investigation and management of suspected and confirmed cancer. This has corresponded to the centralisation of cancer services at high-volume hospitals, and the formation of cancer networks (Department of Health England, 2007; The Scottish Government, 2008; NHS Scotland, 2016; NICE, 2016). Primary care general practitioners (GPs) continue to be gatekeepers to secondary care within the UK NHS and represent the first of three discrete types of healthcare facility that are key touchpoints on the cancer care pathway in the United Kingdom: GP practice, cancer diagnosis centre and cancer treatment centre.
Rurality and the burden of travel (distance and/or time) from patients’ residence to key healthcare facilities have been associated with negative impacts on cancer outcomes in studies from the United Kingdom and elsewhere (Campbell et al, 2000; O’Brien et al, 2000; Coory et al, 2006; Underhill et al, 2006; Westeel et al, 2007; Jones et al, 2008a; Coughlin et al, 2008; Pozet et al, 2008). Although this association is now well described, the underlying mechanisms remain obscure. It could be that increased burden of travel restricts access to key healthcare facilities, for which some evidence already exists (Campbell et al, 2002; Jones et al, 2008b; Lin et al, 2015; Murage et al, 2016). People with colorectal cancer living further away from cancer centres are less likely to receive chemotherapy or radiotherapy compared with those living closer (Campbell et al, 2002; Lin et al, 2015). Lung cancer patients living further from a hospital were less likely to receive surgery, and both lung and rectal cancer patients were less likely to receive chemotherapy if they lived distant from these services (Jones et al, 2008b). This could lead, in turn, to under or later utilisation of diagnostic and treatment resources by more remote populations. However, if restricted access were a truly impactful mechanism, it would likely be reflected by longer delays to be diagnosed with and treated for cancer for those patients living more remotely. Longer delays ultimately could result in later-stage diagnosis and later treatment with subsequent implications for the likelihood of curative treatment and prolonged disease-free survival. Such a mechanism could be consistent with evidence reported from across a range of other health conditions, where greater distance and/or greater travel time have been linked to negative impacts on the process of care, particularly reduced engagement with preventive care, delayed diagnosis and treatment and poorer health outcomes (Chan et al, 2006; Probst et al, 2007; Baird et al, 2008; Baldwin et al, 2008; Onega et al, 2008; Ahamad, 2011; Chou et al, 2014). However, the impact of burden of travel on the process of cancer care, particularly the potential for delayed diagnosis and treatment in the United Kingdom, has been difficult to explore until now.
Data-linkage techniques are improving all the time and research opportunities are burgeoning (Higgs, 2009). Greater opportunities now exist to link evolving Geographic Information Systems (GIS) to improved routine administrative healthcare data and increasingly comprehensive specialist disease registries. The resultant linked data sets will enable greater in-depth study of the complex relationships between geography, service delivery, processes of care and health outcomes in real-life data (Higgs, 2009). This is certainly the case in cancer, and GIS are becoming prominent in cancer epidemiological studies (Hyndman and Holman, 2000; Najafabadi and Pourhassan, 2001; Nattinger et al, 2001; Jones et al, 2008b; Peipins et al, 2013). With respect to the process of cancer diagnosis and treatment, there are now opportunities to explore in greater detail than before whether burden of travel can impact the cancer care pathway rather than just cancer outcomes.
The Northeast Scotland health region (comprising the health boards of Grampian, Orkney and the Shetland Isles) has a single specialist cancer centre providing care to a geographically diverse population. Urban, suburban, rural and island communities are all represented. We have linked whole population health records across the cancer care pathway and identified a cohort of 12 339 people diagnosed with one of the UK eight commonest cancers between 2007 and 2014. In this paper we present a unique exploration of how burden of travel might influence both process and outcomes in cancer care in both mainland and island communities.
Materials and methods
Study design and population
In this data-linkage study we created a population-based cohort, Northeast and Aberdeen Scottish Cancer and Residence (NASCAR). The NASCAR cohort captured all patients with one of eight common cancers for a defined geographical area (approximate population=587 820) comprising a single health region and two island communities served by 112 GP practices, three cancer diagnosis centres (comprising two hospitals and the screening service) and one cancer treatment centre service on the UK mainland. Linkage and creation of NASCAR was approved by a privacy advisory committee and managed in Grampian Data Safe Haven (University of Aberdeen, 2016).
Data sources
The primary data source for this study was the Health Authority (NHS Grampian) Cancer Care Pathway (CCPd) database, an electronic clinical database maintained since 2006. The CCPd collects information from several sources to form a complete record of secondary care activity in individual cancer cases, from receipt of GP referral, for all people diagnosed with cancer. The CCPd records information about referral, diagnosis, subsequent investigations and secondary care appointments, intrasecondary care referrals, investigations, hospital admissions and discharges, operations and treatment. The accuracy of the CCPd has been validated (Murchie et al, 2013). Data were extracted from the CCPd for individuals diagnosed with eight common cancers (colorectal, lung, breast, prostate, melanoma, oesophagogastric, cervical and ovarian) from 2007 to 2014. This provided the patient population for the NASCAR cohort.
Using residential postcodes (geo-reference for postcode centroid), we assigned The Scottish Index of Multiple Deprivation (SIMD) and the Scottish Government Urban-Rural Classification to the CCPd cohort data set. The SIMD identifies small area concentrations of multiple deprivations across all of Scotland in a consistent way. The indicators that make up the SIMD are chosen because they are measures of deprivation regardless of where a person lives, and therefore the issue for rural areas is that poverty and deprivation are more spatially dispersed than in urban areas. The SIMD is measured across seven domains: current income, employment, health, education, skills and training, housing, geographic access to services (including drive and public transport times) and unemployment counts are averaged to take account of seasonal fluctuations in employment patterns (seasonality tends to affect rural areas more than urban areas((The Scottish Government, 2016). The Scottish Government Urban-Rural Classification provides a standard definition of rural areas in Scotland. This information was then used to assign each Scottish postcode to a two-fold category of urban or rural (The Scottish Government, 2014, 2016). The SIMD data come from a variety of different sources and data providers quality assure data before providing them to the Office of the Chief Statistician and Performance (OCSP). The OCSP also carries out further checks to ensure the data are fit for purpose.
The Scottish Cancer Registry records information on cancer type, date of diagnosis, stage at diagnosis, treatment received and date. Approximately 47 000 registrations are made annually in Scotland (Brewster et al, 1994, 2002). This registry provided information on stage at diagnosis and treatment received and allowed cross-reference with the primary data source for variables such as cancer type and date of diagnosis. Data quality of the Scottish Cancer Registry is monitored using routine indicators, computer validations and ad hoc studies of data accuracy and completeness of ascertainment (Brewster et al, 1994, 2002; Information Services Division, 2017a, 2017b).
Hospital episode data record all inpatient and day cases discharged from Scottish acute hospitals (Information Services Division, 2012). The data quality is regularly assessed and validated (Information Services Division, 2012). A weighted Charlson comorbidity score was calculated for each patient based on their hospital episode data (Charlson et al, 1987). The score was calculated from principal and supplementary diagnostic ICD 10 codes from hospital attendances and admissions for 10 years before cancer diagnosis for each patient and excluded cancer or metastatic cancer. This allowed for adjustment in the statistical analysis for comorbidity.
Death registry data from the National Records of Scotland (NRS) records information relating to all deaths, including principal and secondary causes of death. This information was used to determine if the principal cause of death was cancer. The data are of a high standard and are the most reliable death data set available in Scotland. Automatic quality checks are carried out at the point of entry and when the information is passed into the National Records of Scotland Vital Events statistical database (National Records of Scotland, 2017).
Postcodes for residential, GP practice, cancer diagnosis centre and cancer treatment centre were available for each individual from the CCPd. To model fastest travel times to the three key healthcare facilities used during each individuals cancer diagnostic pathway, road networks and flight routes (for island patients) from place of residence were calculated using the Network Analyst extension in ArcGIS V10.2 (ESRI: Environmental Systems Research Institute, Redlands, CA, USA).
Preliminary data by our research group showed that 67% of people diagnosed with the cancers of interest in the study area between 2006 and 2013 were treated within 62 days. Assuming a differential of 2% between each access groups (69%, 67% and 65%) we would have 80% power to detect that difference with a sample size of 2662 in each group (assuming equal numbers), and 90% power with 3493 in each group. We were able to meet this requirement.
Data linkage
Data linkage enables population follow-up without formal patient recruitment that minimises selection bias. Information Services Division (ISD) Scotland performed the linkages using the community health index (CHI), a unique identifier for all residents in Scotland. This allows all the records from multiple data sets from primary care, secondary care and specialist disease registries to be linked (ScottisH Informatics Project (SHIP), 2003). Data extraction and linkage were carried out by ISD Scotland and Grampian Data Safe Haven (DaSH). Data were pseudoanonymised before release to our research group for analysis in the secure virtual research environment in DaSH.
The linkage created a novel longitudinal data set, NASCAR, for a defined geographical population.
The NASCAR-linked data set contained information on mode and date of diagnosis, date of presentation, date of GP referral (where applicable), date of first hospital assessment, date of subsequent hospital investigations, date of treatment decision and the nature and date of definitive treatment. This information enabled calculation of defined individual diagnostic and treatment intervals (Weller et al, 2012).
Exposure: burden of travel expressed as travel times
Travel times from place of residence to the three key healthcare facilities were defined as follows: travelling time to GP: <5.0 min for mainland residents, 5.0–9.9 min for mainland residents, 10.0–14.9 min for mainland residents, >15.0 min for mainland residents and a category for all island residents; travelling time to centre of diagnosis and cancer centre: <15.0 min for mainland residents, 15.0–29.9 min for mainland residents, 30.0–59.9 min for mainland residents, >60.0 min for mainland residents and a category for all island residents.
In the analysis of travelling time to GP, we could have included the island patients by time, but for comparability with the other analyses we left this as a separate category. Of the island patients, 31.1% had a travelling time of >10 min compared with 16.3% of patients residing on the mainland. Having a distinct island category aimed to capture the complexity of additional factors that could influence accessibility. The reference category for GP practice was <5.0 min for mainland residents and for cancer diagnosis centre and cancer treatment centre was <15.0 min for mainland residents.
Outcomes
The main outcome measure was treatment started within 62 days after the GP referral date (yes or no). This categorisation was based on national government targets to receive treatment within 62 days from the date of urgent referral with suspicion of cancer. This includes referrals from national cancer screening programmes (Department of Health England, 2007; The Scottish Government, 2008).
We included the variable ‘first cancer treatment started within 31 days after diagnosis date’ as a secondary outcome to examine delay in more detail. This was based on national government target of 31 days from decision to treat until first treatment for all new cancer diagnoses, irrespective of the route of referral into the system (The Scottish Government, 2008). The impact of travelling time on treatment occurring on the same or next day as diagnosis was also investigated.
One-year mortality was chosen as it seems plausible that this would be most sensitive to differential geographic access to cancer diagnostic and treatment facilities. Arguably, focussing on longer-term mortality could be more susceptible to confounding by, for example, healthier rural lifestyles or lesser deprivation. Follow-up was from the date of GP referral to 5 December 2014. This cutoff date meant we were unable to include patients referred after 5 December 2013 (n=1198) in the mortality analysis. Those not registered as dead during follow-up were assumed to be still alive. Mortality was measured from date of GP referral rather than date of diagnosis or first treatment to overcome lead-time bias. A sensitivity analysis was conducted calculating mortality from date of diagnosis.
Covariates of interest
We reported age, sex, level of deprivation (SIMD quintiles), rurality (based on the Scottish two-fold urban rural classification ((urban/rural), Charlson score, urgency/referral status (urgent suspected cancer, urgent, routine, screening, emergency and other), cancer type (breast, ovarian, cervical, prostate, melanoma, lung, oesophagogastric or colorectal), diagnostic procedure (imaging, endoscopy/endoscopic biopsy, operative biopsy/surgery, or other) and main or first treatment type (surgery, chemotherapy/radiotherapy or other).
Sensitivity analyses were carried out to explore potential bias from differences in the diagnostic process for cancer type (melanoma and prostate) and all patients who were diagnosed through a screening services. Both melanoma and prostate tend to have less aggressive treatment than the other cancer types, and this could have an effect on the diagnosis and treatment targets in the diagnostic pathway and also on mortality outcomes.
Information on stage of cancer provided by the Scottish Cancer Registry was not complete, largely because stage was not collected for some of the included cancer sites during the time frame of this study. Metastatic cancer information taken from hospital episode data was used as a proxy for stage of cancer, as has been used previously (Parks et al, 2004). We carried out sensitivity analyses on patients who had stage recorded in the Scottish Cancer Registry.
Previous work published by our research group excluded tumour stage as a predictor variable from multivariate survival models (Murchie et al, 2015). The rationale behind this was that if time between presentation and treatment is an important factor for mortality, then its effects would be via more advanced disease stage. We have performed additional sensitivity analysis for mortality to 1 year without adjustment for either metastatic disease or stage.
Analysis
Statistical analyses were performed using SPSS V.23 (IBM, Armonk, NY, USA) and SAS V9.3 (SAS Institute, Cary, NC, USA). The data set was cleaned and missing data analysis was performed. In all, 1363 (12 339 of 13 702) (9.9%) had ⩾1 of the case-mix adjustment variables missing, and exploratory analysis was performed to assess missing data patterns. To assess whether missing case-mix variables would affect the results, we performed missing data imputation using the Markov chain Monte Carlo method with 5 iterations. The adjusted odds ratios (ORs) with 95% confidence interval (CI) for outcomes restricted to the cases with complete case-mix information were more conservative than the results for all cases with imputation of missing data. All estimates were therefore focussed on analysis of cases where all key data items were complete. Baseline characteristics (at time of referral) were summarised.
To explore the association for each of the three healthcare facilities between the binary outcomes (treatment by 62 days post referral, treatment by 31 days from diagnosis and treatment and diagnosis on the same or next day) with travelling time we used logistic regression to estimate the OR and 95% CIs. We present unadjusted and adjusted models. Adjusted models included age in years at the time of referral, gender, urban/rural, deprivation (based on postcode), referral urgency/referral status, cancer type, diagnostic procedure type, Charlson comorbidity index (CCI score), first treatment type and diagnosis of metastatic cancer as a marker of cancer stage. Selection of adjustment factors was based on clinical importance and statistical significance. We included the Scottish two-fold urban rural classification in the adjusted model in addition to travelling time as these represent different measures. An urban residence could have a longer travelling time than a rural residence because of the location of one of the key healthcare components.
The Kaplan–Meier curves were plotted for both cumulative observed mortality and cumulative cancer-specific mortality at 1 year from the date of GP referral for travelling time to cancer treatment centre. We used Cox proportional hazards model with adjustment for estimating the hazard of mortality within 1 year of GP referral date and calculating hazard ratios (HRs) in relation to travelling times to the three healthcare components.
Patient involvement
This is an unconsented data linkage study. As part of the Administrative Data Research Centre (ADRC) Scotland and Farr Institute Scotland, large public engagement exercises have been conducted to discuss the use of health and administrative data in research. The input from these events has shaped the types of data for linkage, the approach to linkages and the governance around data linkage. This project was reviewed by a Privacy and Public Benefit panel (PPBP) that includes lay representative input. No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients advised on the interpretation or writing up of results. As part of the dissemination of the results of the research we plan to prepare a #Datasaveslives case study that will be made available via the Farr Institute for Health Informatics Engagement Team.
Ethical approval
Ethical approval was not needed. Privacy Advisory Committee (PAC) was obtained from Information Services Division (ISD) of NHS National Services Scotland for accessing and linking the data sources (Reference number 0942/14).
Results
All categorisations stated in the results refer to travelling times for patients residing on the mainland. Island patients were a distinct category regardless of lengths of travelling times. The reference category for all analyses refers to the category closest to the key healthcare facilities.
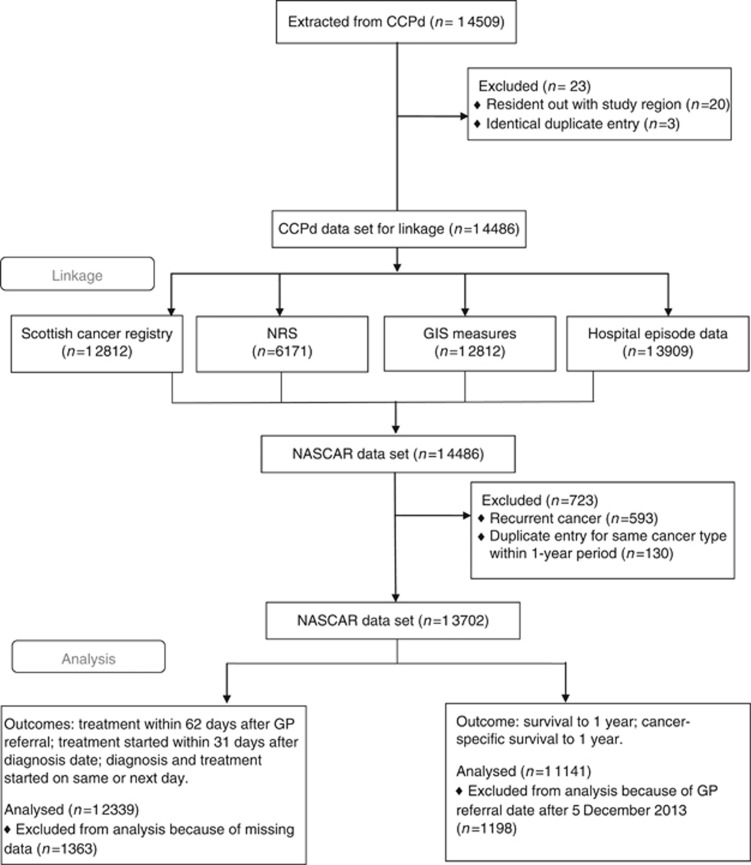
A total of 12 339 patients with one of eight common cancers were included in the study. Figure 1 presents the flow diagram detailing the numbers of individuals at each stage of the data linkage and analysis. The median (IQR) age of patients was 69 (59–77) years. Table 1 shows patient and care pathway characteristics for the cohort at the time of GP referral. Over half the cohort was female (56.1%) and over 80% were in the three least deprived quintiles. Of the cohort, 65.4% had their place of residence in an urban area; 25.9% of the cohort were referred as urgent suspected cancer (equivalent to 2-week wait referrals in England) with an additional 28.0% as urgent. Screening services identified 15.2% and emergency admissions 13.1% of the cohort.
Figure 1.
Flow diagram of study population. CCPd=Cancer Care Pathway Database (NHS Grampian); GIS=Geographic Information Systems; NASCAR=Northeast and Aberdeen Scottish Cancer and Residence; NRS=National Records of Scotland.
Table 1. Patient and pathway characteristics at the time of referral.
| Variable | N, total=12 339 | % |
|---|---|---|
|
Gender | ||
| Male | 5422 | 43.9 |
| Female | 6917 | 56.1 |
|
Deprivation (quintiles based on SIMD) | ||
| SIMD Q1 (most) | 608 | 4.9 |
| SIMD Q2 | 1522 | 12.3 |
| SIMD Q3 | 2878 | 23.3 |
| SIMD Q4 | 3675 | 29.8 |
| SIMD Q5 (least) | 3656 | 29.6 |
|
Rurality (based on Scottish two-fold URC) | ||
| Urban | 8071 | 65.4 |
| Rural | 4268 | 34.6 |
|
Urgency/referral status | ||
| Urgent suspected cancer (USC) | 3200 | 25.9 |
| Urgent | 2224 | 18.0 |
| Routine | 1414 | 11.5 |
| Screening | 1876 | 15.2 |
| Emergency | 1615 | 13.1 |
| Other | 2010 | 16.3 |
|
Cancer type | ||
| Breast | 3772 | 30.6 |
| Ovarian | 324 | 2.6 |
| Cervical | 133 | 1.1 |
| Prostate | 2007 | 16.3 |
| Melanoma | 575 | 4.7 |
| Lung | 1736 | 14.1 |
| Oesophagogastric | 1017 | 8.2 |
| Colorectal | 2775 | 22.5 |
|
Charlson comorbidity index (excluding cancer and metastatic cancer) | ||
| 0 | 8537 | 69.2 |
| 1 | 2173 | 17.6 |
| 2 | 872 | 7.1 |
| 3 | 392 | 3.2 |
| 4 | 219 | 1.8 |
| 5 | 93 | 0.7 |
| 6+ | 53 | 0.4 |
|
Charlson comorbidity index–condition | ||
| Acute MI | 757 | 6.1 |
| Cerebral vascular accident | 493 | 4.0 |
| Congestive heart failure | 428 | 3.5 |
| Connective tissue disorder | 202 | 1.6 |
| Dementia | 129 | 1.0 |
| Diabetes without long-term complications | 977 | 7.9 |
| Mild or moderate liver disease | 39 | 0.3 |
| Peptic ulcer | 289 | 2.3 |
| Peripheral vascular disease | 522 | 4.2 |
| Pulmonary disease | 1414 | 11.5 |
| Diabetes with long-term complications | 77 | 0.6 |
| Paraplegia | 61 | 0.5 |
| Renal disease | 595 | 4.8 |
| Severe liver disease | 13 | 0.1 |
| HIV | <10 | <0.1 |
|
Diagnostic procedure | ||
| Imaging | 2104 | 17.1 |
| Endoscopy/endoscopic biopsy | 5778 | 46.8 |
| Operative biopsy/surgery | 3870 | 31.4 |
| Other | 587 | 4.8 |
|
Main treatment type | ||
| Surgery | 6298 | 51.0 |
| Chemotherapy/radiotherapy | 4948 | 40.1 |
| Other | 1093 | 8.9 |
|
Metastatic cancer | ||
| Yes | 1357 | 11.0 |
| No | 10 982 | 89.0 |
Abbreviations: HIV=human immunodeficiency virus; MI=myocardial infarction; SIMD=Scottish Index of Multiple Deprivation; URC=urban rural classification.
The cancer types with the highest percentages were breast (30.6%), colorectal (22.5%), lung (14.1%) and prostate (1.3%). In addition, 69.2% of patients scored zero on the CCI at the time of cancer diagnosis.
A majority of patients were diagnosed by endoscopy or endoscopic biopsy (46.8%), operative biopsy or surgery (31.4%) or imaging (17.1%). Surgery (51.0%) was the main first treatment type.
Treatment within 62 days of referral
Table 2 presents the results of the adjusted logistic regression analysis to explore the association of treatment within 62 days of GP referral and travelling time from GP practice; cancer diagnosis centre; and cancer treatment centre.
Table 2. Patient outcomes and relationships of travelling time from home to GP, cancer diagnosis centre and cancer treatment centre.
|
Outcome=treatment within 62 days of GP referral | ||||||
| Travelling time (min) | <5.0 | 5.0–9.9 | 10.0–14.9 | >15.0 | Islands | |
| Time from home to GP practice | N | 6439 | 3123 | 1069 | 792 | 916 |
| N event (%) | 4311 (67.0) | 2019 (64.7) | 681 (63.7) | 510 (64.4) | 633 (69.1) | |
| Unadjusted OR (95% CI) | 1.00 | 0.90 (0.83–0.99) | 0.87 (0.76–0.99) | 0.89 (0.77–1.04) | 1.10 (0.95–1.28) | |
| Adjusteda OR (95% CI) | 1.00 | 0.89 (0.80–0.98) | 0.85 (0.73–0.99) | 0.84 (0.70–1.01) | 1.02 (0.86–1.21) | |
| Travelling time (min) | <15.0 | 15.0–29.9 | 30.0–59.9 | >60.0 | Islands | |
| Time from home to cancer diagnosis centre | N | 5420 | 2313 | 2418 | 1272 | 916 |
| N event (%) | 3619 (66.8) | 1540 (66.6) | 1516 (62.7) | 846 (66.5) | 633 (69.1) | |
| Unadjusted OR (95% CI) | 1.00 | 0.99 (0.89–1.10) | 0.84 (0.76–0.92) | 0.99 (0.87–1.13) | 1.11 (0.96–1.30) | |
| Adjusteda OR (95% CI) | 1.00 | 1.04 (0.93–1.17) | 0.92 (0.81–1.04) | 0.93 (0.80–1.08) | 1.07 (0.90–1.28) | |
| Time from home to cancer treatment centre | N | 4105 | 1764 | 2523 | 3031 | 916 |
| N event (%) | 2645 (64.4) | 1174 (66.6) | 1635 (64.8) | 2067 (68.2) | 633 (69.1) | |
| Unadjusted OR (95% CI) | 1.00 | 1.10 (0.98–1.24) | 1.02 (0.92–1.13) | 1.18 (1.07–1.31) | 1.24 (1.06–1.44) | |
| Adjusteda OR (95% CI) | 1.00 | 1.08 (0.94–1.24) | 1.12 (0.98–1.27) | 1.42 (1.25–1.61) | 1.32 (1.09–1.59) | |
|
Outcome=treatment within 31 days of diagnosis | ||||||
| Travelling time (min) | <5.0 | 5.0–9.9 | 10.0–14.9 | >15.0 | Islands | |
| Time from home to GP practice | N | 6439 | 3123 | 1069 | 792 | 916 |
| N event (%) | 3532 (54.9) | 1682 (53.9) | 593 (55.5) | 407 (51.4) | 546 (59.6) | |
| Unadjusted OR (95% CI) | 1.00 | 0.96 (0.88–1.05) | 1.03 (0.90–1.17) | 0.87 (0.75–1.01) | 1.22 (1.06–1.40) | |
| Adjusteda OR (95% CI) | 1.00 | 0.98 (0.89–1.07) | 1.12 (0.97–1.29) | 0.89 (0.75–1.05) | 1.21 (1.03–1.41) | |
| Travelling time (min) | <15.0 | 15.0–29.9 | 30.0–59.9 | >60.0 | Islands | |
| Time from home to cancer diagnosis centre | N | 5420 | 2313 | 2418 | 1272 | 916 |
| N event (%) | 2909 (53.7) | 1286 (55.6) | 1323 (54.7) | 696 (54.7) | 546 (59.6) | |
| Unadjusted OR (95% CI) | 1.00 | 1.08 (0.98–1.19) | 1.04 (0.95–1.15) | 1.04 (0.95–1.15) | 1.29 (1.11–1.47) | |
| Adjusteda OR (95% CI) | 1.00 | 1.06 (0.95–1.18) | 0.99 (0.88–1.11) | 0.90 (0.78–1.04) | 1.20 (1.02–1.41) | |
| Time from home to cancer treatment centre | N | 4105 | 1764 | 2523 | 3031 | 916 |
| N event (%) | 2192 (53.4) | 964 (54.7) | 1335 (52.9) | 1723 (56.9) | 546 (59.6) | |
| Unadjusted OR (95% CI) | 1.00 | 1.05 (0.94–1.18) | 0.98 (0.89–1.08) | 1.15 (1.05–1.26) | 1.29 (1.11–1.49) | |
| Adjusteda OR (95% CI) | 1.00 | 1.10 (0.97–1.25) | 1.01 (0.90–1.14) | 1.20 (1.07–1.35) | 1.33 (1.12–1.57) | |
|
Outcome=diagnosis and treatment started on same or next day | ||||||
| Travelling time (min) | <5.0 | 5.0–9.9 | 10.0–14.9 | >15.0 | Islands | |
| Time from home to GP practice | N | 6436 | 3120 | 1068 | 791 | 913 |
| N event (%) | 981 (15.2) | 468 (15.0) | 133 (12.5) | 129 (16.3) | 187 (20.5) | |
| Unadjusted OR (95% CI) | 1.00 | 0.98 (0.87–1.11) | 0.79 (0.65–0.96) | 1.08 (0.89–1.32) | 1.43 (1.20–1.71) | |
| Adjusteda OR (95% CI) | 1.00 | 1.00 (0.79–1.27) | 0.87 (0.59–1.30) | 0.92 (0.60–1.42) | 1.68 (1.14–2.47) | |
| Travelling time (min) | <15.0 | 15.0–29.9 | 30.0–59.9 | >60.0 | Islands | |
| Time from home to cancer diagnosis centre | N | 5418 | 2308 | 2417 | 1272 | 913 |
| N event (%) | 751 (13.9) | 377 (16.3) | 378 (15.6) | 205 (16.1) | 187 (20.5) | |
| Unadjusted OR (95% CI) | 1.00 | 1.21 (1.06–1.39) | 1.15 (1.01–1.32) | 1.19 (1.01–1.41) | 1.60 (1.34–1.91) | |
| Adjusteda OR (95% CI) | 1.00 | 1.09 (0.91–1.31) | 0.91 (0.75–1.11) | 0.82 (0.65–1.04) | 1.54 (1.19–1.98) | |
| Time from home to cancer treatment centre | N | 4103.0 | 1759 | 2522 | 3031 | 913 |
| N event (%) | 575 (14.0) | 271 (15.4) | 378 (15.0) | 487 (16.1) | 187 (20.5) | |
| Unadjusted OR (95% CI) | 1.0 | 1.12 (0.96–1.31) | 1.08 (0.94–1.25) | 1.18 (1.03–1.34) | 1.58 (1.32–1.90) | |
| Adjusteda OR (95% CI) | 1.0 | 1.12 (0.91–1.39) | 0.97 (0.79–1.19) | 1.16 (0.96–1.41) | 1.72 (1.31–2.25) | |
Abbreviations: 95% CI=95% confidence interval; GP=general practitioner; OR=odds ratio.
Adjusted for age, gender, urban/rural code 2, deprivation, urgency/referral status, cancer type, procedure type, Charlson comorbidity index (CCI) score, treatment type and metastatic cancer.
Patients who had between 5–9.9 and 10–14.9 min of travelling time from their residence to their GP practice were less likely to have treatment started within 62 days of referral compared with those living <5 min from their GP practice. There were no associations between travelling time from home to centre of diagnosis and treatment within 62 days of referral in the fully adjusted regression model.
Patients who had >60 min of travelling time from their residence to their cancer centre were more likely to receive treatment within 62 days of their GP referral. This was also seen for patients who resided on an island community.
Sensitivity analysis excluding melanoma and prostate cancer patients and patients picked up through screening (Supplementary Table A) and analysis on only those with a staging variable (Supplementary Table B) showed a similar pattern of results.
Treatment within 31 days of diagnosis
Table 2 presents the results of the adjusted logistic regression analysis to explore the association of treatment within 31 days of diagnosis date and travelling time from GP practice; cancer diagnosis centre; and cancer treatment centre. Travelling time to GP practice and cancer diagnosis centre for mainland patients did not show any differences in the proportion treated within 31 days from diagnosis. However, those living on islands showed an increased likelihood of treatment within 31 days from diagnosis compared with those living closer.
Patients who had >60 min of travelling time from their residence to their cancer treatment centre or those residing on the islands were more likely to start treatment within 31 days of their diagnosis date compared with those living within 15 min. Sensitivity analysis excluding melanoma and prostate cancer patients and patients picked up through screening (Supplementary Table A) and analysis on only those with a staging variable (Supplementary Table B) showed the same pattern of results.
Table 2 shows that island patients were much more likely to receive treatment the same or next day after diagnosis. Differing travelling times showed no effect here. The same pattern of results was seen for the sensitivity analysis excluding melanoma and prostate cancer patients and patients picked up through screening (Supplementary Table A) and analysis on only those with a staging variable (Supplementary Table B).
Mortality to 1 year as the outcome
A total of 11 141 patients had a GP referral date before 5 December 2013 (the cutoff date for complete 1-year follow-up) and were therefore eligible for inclusion in the mortality analysis.
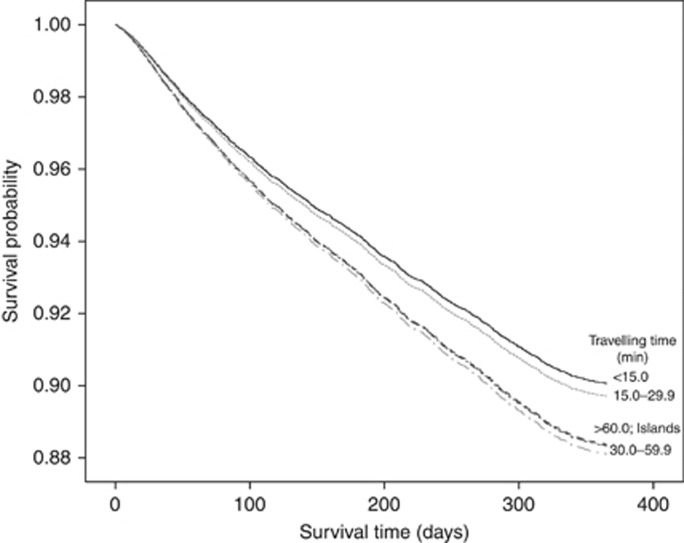
Figure 2 presents the Kaplan–Meier curve for cumulative observed mortality for 1 year following GP referral. Those residing >30 min from their cancer treatment centre and those on an island had a lower survival distribution compared with those residing <15 min from their cancer treatment centre.
Figure 2.
Survival curve according to travelling time to cancer treatment centre from place of residence.
Table 3 shows the unadjusted and adjusted HRs to explore the association between travelling time from GP practice; cancer diagnosis centre; and cancer treatment centre and mortality to 1 year from all causes of death. Patients who resided >30 min from their home to their cancer treatment centre were less likely to survive to 1 year. Living on an island community showed a similar association, although the 95% CI did not reach significance. Sensitivity analysis excluding melanoma and prostate cancer patients showed a similar pattern of results (Supplementary Table C). Analysis on only those with a staging variable (Supplementary Table D) and analysis excluding both metastatic cancer and stage (Supplementary Table E) did not alter the significance of the results for mortality to 1 year.
Table 3. Patient mortality outcomes and relationships of travelling time from home to GP, cancer diagnosis centre and cancer treatment centre.
|
Outcome=mortality to 1 year | ||||||
| Travelling time (min) | <5.0 | 5.0–9.9 | 10.0–14.9 | >15.0 | Islands | |
| Time from home to GP practice | N | 5798 | 2824 | 961 | 711 | 847 |
| N event (%) | 1308 (22.6) | 571 (20.2) | 173 (18.0) | 127 (17.9) | 187 (22.1) | |
| Unadjusted HR (95% CI) | 1.00 | 0.89 (0.80–0.98) | 0.77 (0.66–0.91) | 0.76 (0.63–0.91) | 0.98 (0.84–1.14) | |
| Adjusteda HR (95% CI) | 1.00 | 0.90 (0.81–1.01) | 0.99 (0.84–1.19) | 0.76 (0.62–0.91) | 0.98 (0.82–1.16) | |
| Travelling time (min) | <15.0 | 15.0–29.9 | 30.0–59.9 | >60.0 | Islands | |
| Time from home to cancer diagnosis centre | N | 4878 | 2094 | 2178 | 1144 | 847 |
| N event (%) | 949 (19.4) | 436 (20.8) | 488 (22.4) | 306 (26.7) | 187 (22.1) | |
| Unadjusted HR (95% CI) | 1.00 | 1.08 (0.96–1.20) | 1.16 (1.04–1.29) | 1.43 (1.26–1.63) | 1.16 (0.99–1.35) | |
| Adjusteda HR (95% CI) | 1.00 | 1.06 (0.93–1.21) | 1.20 (1.05–1.37) | 1.15 (0.98–1.35) | 1.14 (0.95–1.37) | |
| Time from home to cancer treatment centre | N | 3704 | 1605 | 2269 | 2716 | 847 |
| N event (%) | 812 (21.9) | 298 (18.6) | 456 (20.1) | 613 (22.6) | 187 (22.1) | |
| Unadjusted HR (95% CI) | 1.00 | 0.82 (0.72–0.94) | 0.90 (0.80–1.01) | 1.03 (0.93–1.15) | 1.01 (0.86–1.19) | |
| Adjusteda HR (95% CI) | 1.00 | 1.04 (0.89–1.22) | 1.21 (1.05–1.39) | 1.19 (1.04–1.36) | 1.17 (0.97–1.41) | |
|
Outcome=cancer-specific mortality to 1 year | ||||||
| Travelling time (min) | <5.0 | 5.0–9.9 | 10.0–14.9 | >15.0 | Islands | |
| Time from home to GP practice | N | 5798 | 2824 | 961 | 711 | 847 |
| N event (%) | 1175 (20.3) | 517 (18.3) | 162 (16.9) | 117 (16.5) | 164 (19.4) | |
| Unadjusted HR (95% CI) | 1.00 | 0.89 (0.81–0.99) | 0.81 (0.69–0.95) | 0.78 (0.64–0.94) | 0.96 (0.81–1.13) | |
| Adjusteda HR (95% CI) | 1.00 | 0.91 (0.81–1.02) | 1.04 (0.87–1.25) | 0.75 (0.61–0.92) | 0.94 (0.79–1.13) | |
| Travelling time (min) | <15.0 | 15.0–29.9 | 30.0–59.9 | >60.0 | Islands | |
| Time from home to cancer diagnosis centre | N | 4878 | 2094 | 2178 | 1144 | 847 |
| N event (%) | 847 (17.4) | 392 (18.7) | 447 (20.5) | 285 (24.9) | 164 (19.4) | |
| Unadjusted HR (95% CI) | 1.00 | 1.08 (0.96–1.22) | 1.19 (1.06–1.34) | 1.49 (1.31–1.71) | 1.14 (0.96–1.35) | |
| Adjusteda HR (95% CI) | 1.00 | 1.06 (0.92–1.21) | 1.22 (1.06–1.40) | 1.16 (0.99–1.37) | 1.10 (0.91–1.33) | |
| Time from home to cancer treatment centre | N | 3704 | 1605 | 2269 | 2716 | 847 |
| N event (%) | 730 (19.7) | 271 (16.9) | 416 (18.3) | 554 (20.4) | 164 (19.4) | |
| Unadjusted HR (95% CI) | 1.00 | 0.83 (0.73–0.96) | 0.91 (0.81–1.03) | 1.04 (0.93–1.16) | 0.99 (0.83–1.17) | |
| Adjusteda HR (95% CI) | 1.00 | 1.04 (0.89–1.23) | 1.21 (1.05–1.41) | 1.18 (1.03–1.36) | 1.13 (0.92–1.37) | |
Abbreviations: 95% CI=95% confidence interval; GP=general practitioner; HR=hazard ratio.
Adjusted for age, gender, urban/rural code 2, deprivation, urgency/referral status, cancer type, procedure type, Charlson comorbidity index (CCI) score, treatment type and metastatic cancer.
A sensitivity analysis was conducted measuring mortality from date of diagnosis rather than GP referral (Supplementary Table F). Date of diagnosis reduced the significance of the results seen with date of GP referral for travelling time to cancer treatment centre for all mortality.
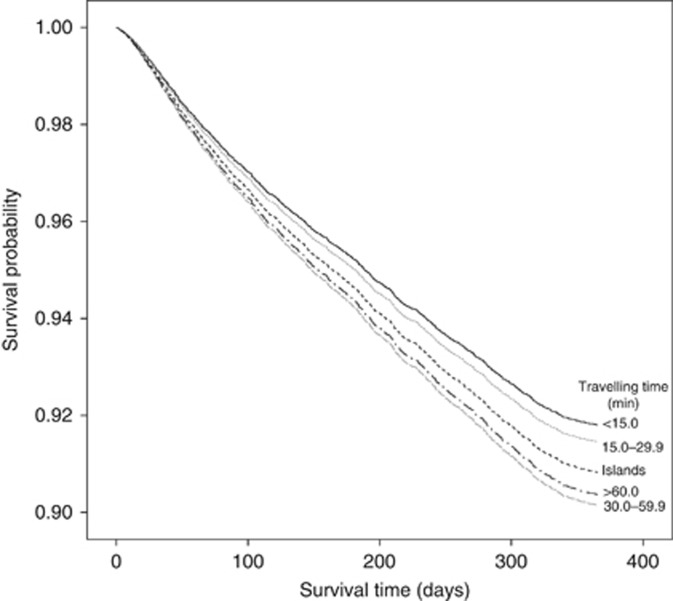
Figure 3 presents the Kaplan–Meier curve for cumulative cancer-specific mortality for 1 year following GP referral. Residents with >30 min of travelling time and island dwellers had a lower survival distribution compared with those living <15 min from their cancer treatment centre.
Figure 3.
Cancer-specific survival curve according to travelling time to cancer treatment centre from place of residence.
Table 3 also shows that patients who resided >30 min from their home to their cancer treatment centre were more likely to die from cancer at 1 year. Sensitivity analysis excluding melanoma and prostate cancer patients (Supplementary Table C) and on only those with a staging variable (Supplementary Table D) showed a similar pattern of results, although there was a reduction in the significance.
Cancer-specific mortality to 1 year from date of diagnosis reduced the significance of the results seen with date of GP referral for travelling time to cancer treatment centre (Supplementary Table F).
Discussion
Principal findings
This is the first study to our knowledge exploring the impact of burden of travel on the cancer diagnostic process. We have shown that those with an increased burden of travel to key healthcare components (GP practice, cancer diagnosis centre and cancer treatment centre) appear to be diagnosed and receive primary treatment more quickly when they develop common cancers, even when advanced disease is controlled for. Mainland patients from further away and island dwellers appear to have shorter intervals between diagnosis and treatment compared with those living closer to their cancer treatment centre. Despite this, these groups had increased mortality.
Strengths and limitations of study
We were able to explore our important research questions with greater definition than has previously been possible. The data analysed and presented were drawn from a comprehensive and detailed clinical data set. The methods used to compile the data ensure that many of the fields are automatically populated from quality electronic referral systems, enabling a high degree of completeness and accuracy (Brewster et al, 1994, 2002; Information Services Division, 2012). This was a large population-based sample and data have been included on those who died or did not otherwise receive treatment. Our study had almost complete and accurate data on rurality, deprivation and GIS calculations.
Our analysis is novel and innovative and based within a geographically diverse region of Scotland including the full range of sociodemographic and geographic concepts. Our approach and findings should now be confirmed in other areas of the United Kingdom and beyond.
A further strength is that, although we lacked complete and detailed staging data, we were able to adjust for metastatic disease at diagnosis, and conduct supplementary sensitivity analyses using the staging data that we did have (Parks et al, 2004). We also conducted a further supplementary sensitivity analysis where we included neither stage nor presence of metastatic disease at diagnosis. This is very important as it controls for the potential impact of prolonged patient delay in remote and rural areas. We can therefore reject the otherwise tempting and nihilistic conclusion that our results simply reflect rural stoicism, with longer delays and later-stage diagnoses in rural areas as a matter of course. Instead, the results suggest that potentially malleable system factors are in play and require to be urgently elucidated.
Some limitations to our data and analysis must be acknowledged. The study was observational and retrospective, and dependent on the quality of routinely collected data. Adjustment has been carried out, but there will still be some residual confounding that could lead to either an overestimation or underestimation of risk. In particular, this study did not collect information on lifestyle factors or patient choices with regard to treatment and care that may have potentially identified other explainable reasons for mortality rates. It is possible that some of these unmeasured confounders could explain or mask the observations.
Northeast Scotland and the Islands do not have the same high levels of deprivation seen in some other areas of Scotland (e.g., the central industrial belt of Scotland). There are, however, significant pockets of deprivation and overall the area is less affluent than, for example, parts of England and Wales. Deprivation scores are collected at the level of the smallest area possible and this method has previously been found sensitive enough to detect mortality differences for common cancers in Scotland (Campbell et al, 2000). Extension of the work to the whole of Scotland will encompass all deprivation groups and this work is in development.
Analysis of travel time for those residing in the mainland region are based on times travelled by private transport, whereas those on island communities are for travel time by air from main island airport to airport in the city where regional cancer centre is located and time to travel by taxi to and from airports. We were unable to control for actual travel time based on accessibility to car ownership or reliability of public transport or hospital transport. Public transport or ambulance transfers could potentially double journey times. The GIS accounted for variation in road classification but we were unable to reliably account for traffic volume, road works and weather conditions that may affect travel times disproportionately.
Our assessment of comorbidity was based on hospital admission data that have previously been shown to compare well with case note review (Soo et al, 2014). We must also acknowledge however that patients with increasing burden of travel will have concurrent comorbidities managed in primary care rather than be admitted to hospital (Wallace et al, 2015). It is also possible that for diseases such as diabetes there is wide variation in the completeness of recording in hospital admission data (Anwar et al, 2011). The combination of these factors could contribute to an underestimation of comorbidities in our patient population.
Access to health services depends on a wide range of factors and we were unable to account for interactions between supply and demand of services; for example, capacity of services and qualified personnel available at the time of referral, diagnosis and treatment.
Comparison with other studies
Previous research in the early 2000s found that increasing distance from a cancer centre was associated with less chance of diagnosis before death for rural patients with stomach, breast and colorectal cancers, but there were also contradictory findings showing evidence of shorter time to treatment for women with breast cancer (Campbell et al, 2000; Robertson et al, 2004). Our adjusted analyses found that between 2007 and 2013, people with cancer with an increased burden of travel were treated more quickly irrespective of cancer site. It is worth noting that previous research pre-dated the introduction of the Scottish Referral Guidelines for Suspected Cancer, and hence it is possible that these have had a particular impact in rural areas of the region (NHS Scotland, 2016).
In a study from a broadly analogous geographical region, increased travel time to a GP was associated with more advanced stage at diagnosis for breast and colorectal cancers and increased risk of death for prostate cancer (Jones et al, 2008a). More recently, evidence suggests that cancer services tend to be located farther from areas with more cancer cases, and that longer travel times are associated with worse survival (Murage et al, 2016). A study of all country-specific cancer diagnoses for the period 1991–1995 found that increasing distance from a cancer centre was associated with less chance of diagnosis before death, a marker of more advanced disease, for stomach, breast and colorectal cancers and poorer survival after diagnosis for prostate and lung cancers (Campbell et al, 2000). It is important to note, therefore, that the paradoxical findings in our study have remained following our adjustments for more advanced disease.
Previous studies have also explored the possibility that increased distance from health services affects the type of treatment received. Those living further away from cancer centres are less likely to receive surgery and/or receive chemotherapy or radiotherapy (Campbell et al, 2002; Jones et al, 2008b; Lin et al, 2015). Patients who were >1 h from a hospital had lower admission rates to a specialist cancer centre (Baird et al, 2008), suggesting that patients may not want to spend much time travelling to hospital.
‘Distance bias’ however has also been attributed to more favourable outcomes. Patients who travel ⩾50 miles to their treating hospital are reported to be more likely to have surgery at high-volume hospitals (Gunderson et al, 2013), concluding that patients who visit hospital from a long distance may wish for more aggressive treatment. In other studies, greater travel distance was positively associated with receipt of therapy (Massarweh et al, 2014), and greater travel times to hospital were associated with better chances of survival for breast and lung cancers (Jones et al, 2008a). It is important to note that we adjusted for treatment type in our analyses and suggest that differential treatment cannot be the sole explanation of poorer cancer outcomes for those with an increased burden of travel.
Studies have also shown no evidence for an association between distance or travel time from health facilities in relation to health outcomes. Later-stage breast cancer was not associated with travel time to the nearest mammography facility (Celaya et al, 2010) and travel time had no effect on stage of colorectal cancer at diagnosis or receipt of treatment (Crawford et al, 2012).
Possible explanations, implications and further research
The contradictory findings on time to diagnosis and mortality are perplexing. The fact that we have controlled for the presence of metastatic disease at diagnosis limits the possibility that our results simply reflect later-stage presentation by people who live further from key healthcare facilities. The findings suggest that what happens to patients subsequent to their diagnosis may be much more important. Residing in areas with increasing burden of travel might limit treatment choices once a diagnosis has been made. Faced with two treatment options, patients might weigh the costs in terms of time, expense and inconvenience of travel against the perceived benefits, for example, choosing surgery over chemotherapy to limit time in hospital (Greenberg et al, 1998). Lengthy or difficult travel to a cancer centre or hospital could also limit engagement with post-primary treatment follow-up, with consequent implications for the effective management of treatment effects and the identification of other follow-up needs. Further research is essential to understand the true interplay of these and other factors.
For the first time we have sought differences between burden of travel and the cancer care pathway for both island and mainland communities. We investigated whether patients from an island community were more likely to have their diagnosis and begin treatment on the same or next day and found evidence that this was so. In addition, many island patients in the region benefit from subsidised air travel and accommodation that potentially could encourage greater engagement with follow-up than their mainland counterparts with increasing burden of travels. Our findings suggest, however, that even these additive advantages during the early stages of the cancer pathway do not translate to equitable outcomes with people living closer to their cancer treatment centre.
Finally, it is important to consider that our results could also reflect some successes. Rural and remote populations have been the focus of improved quality of care for several years. The quicker time to treatment observed here could wholly or partly result from targeted interventions, for example, mobile screening units and public awareness campaigns. It is possible that our findings actually reflect greater geographical equity than would have been the case at earlier periods in recent history.
Future researchers should confirm these findings in the wider United Kingdom and beyond, but the key challenge now is to elucidate the mechanisms underpinning the observed paradoxical relationship between burden of travel, process of care and cancer outcomes. We recommend further mixed methods studies using our approach to data linkage and supplemented by qualitative research to fully understand how cancer services can be best configured to suit the needs of diverse populations.
Conclusions
Island dwellers and mainland residents with >60 min of journey to their cancer treatment centre are diagnosed more quickly and are more likely to receive treatment within the recommended time even after important confounders have been controlled for. This does not however translate into reduced mortality at 1 year following GP referral. Our results are somewhat unexpected and paradoxical. The effects remain even when we control for the most obvious explanation, more advanced disease in patients with increased burden of travels.
Acknowledgments
This study was funded by Cancer Research UK (Grant number C10673/A17593). The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication. All authors are independent of Cancer Research UK.
Author contributions
PM conceived the study and designed the study with inputs from SF, DHB, AL, CB and CD. CD and ZF created the GIS variables that were linked to the main database by colleagues at Grampian Data Safe Haven. MT devised and conducted the analysis in conjunction with SF. All authors contributed to the refinement and interpretation of the analysis. MT wrote the manuscript with comments from all the other authors. All authors approved the final version.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Ahamad A (2011) Geographic access to cancer care: a disparity and a solution. Postgrad Med J 87: 585–589. [DOI] [PubMed] [Google Scholar]
- Anwar H, Fischbacher CM, Leese GP, Lindsay RS, McKnight JA, Wild SH Scottish Diabetes Research Epidemiology Group (2011) Assessment of the under-reporting of diabetes in hospital admission data: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabet Med 28: 1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G, Flynn R, Baxter G, Donnelly M, Lawrence J (2008) Travel time and cancer care: an example of the inverse care law? Rural Remote Health 8: 1003. [PubMed] [Google Scholar]
- Baldwin LM, Cai Y, Larson EH, Dobie SA, Wright GE, Goodman DC, Matthews B, Hat LG (2008) Access to cancer services for rural colorectal cancer patients. J Rural Health 24: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster D, Crichton J, Muir C (1994) How accurate are Scottish cancer registration data? Br J Cancer 70: 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster DH, Stockton D, Harvey JC, Mackay M (2002) Reliability of cancer registration data in Scotland, 1997. Eur J Cancer 38: 414–417. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, Little J (2000) Rural factors and survival from cancer: analysis of Scottish cancer registrations. Br J Cancer 82: 1863–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, Little J (2002) Impact of deprivation and rural residence on treatment of colorectal and lung cancer. Br J Cancer 87: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celaya MO, Berke EM, Onega TL, Gui J, Riddle BL, Cherala SS, Rees JR (2010) Breast cancer stage at diagnosis and geographical access to mammography screening (New Hampshire, 1998-2004). Rural Remote Health 10: 1361. [PMC free article] [PubMed] [Google Scholar]
- Chan L, Hart LG, Goodman DC (2006) Geographic access to health care for rural Medicare beneficiaries. J Rural Health 22: 140–146. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- Chou S, Deily ME, Li S (2014) Travel distance and health outcomes for scheduled surgery. Med Care 52: 250–257. [DOI] [PubMed] [Google Scholar]
- Coory M, Smithers M, Aitken J, Baade P, Ring I (2006) Urban-rural differences in survival from cutaneous melanoma in Queensland. Aust NZ J Public Health 30: 71–74. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Leadbetter S, Richards T, Sabatino SA (2008) Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women. Soc Sci Med 66: 260–273. [DOI] [PubMed] [Google Scholar]
- Crawford SM, Sauerzapf V, Haynes R, Forman D, Jones AP (2012) Social and geographic factors affecting access to treatment of colorectal cancer: a cancer registry study. BMJ Open2: e000410. [DOI] [PMC free article] [PubMed]
- Department of Health England (2007) Cancer Reform Strategy. Department of Health England: London, UK. Available at: http://www.nhs.uk/NHSEngland/NSF/Documents/Cancer%20Reform%20Strategy.pdf (accessed 8 September 2016).
- Greenberg ER, Chute CG, Stukel T, Baron JA, Freeman DH, Yates J, Korson R (1998) Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural areas. N Engl J Med 318: 612–617. [DOI] [PubMed] [Google Scholar]
- Gunderson CC, Tergas AL, Fleury AC, Diaz-Montes TP, Giuntoli RL II (2013) Primary uterine cancer in Maryland: impact of distance on access to surgical care at high-volume hospitals. Int J Gynecol Cancer 23: 1244–1251. [DOI] [PubMed] [Google Scholar]
- Hyndman JC, Holman CD (2000) Differential effects on socioeconomic groups of modelling the location of mammography screening clinics using geographic information systems. Aust NZ J Public Health 24: 281–286. [DOI] [PubMed] [Google Scholar]
- Higgs G (2009) The role of GIS for health utilization studies: literature review. Health Serv Outcomes Res Method 9: 84–99. [Google Scholar]
- Information Services Division (2012) Assessment of SMR01 Data 2010-2011. Scotland Report, May 2012. ISD Scotland, NHS NSS: Edinburgh, UK.
- Information Services Division (2017a) Scottish Cancer Registry. ISD Scotland, NHS NSS: Edinburgh, UK. Available at: http://www.isdscotland.org/Health-Topics/Cancer/Scottish-Cancer-Registry.asp (accessed 26 April 2017).
- Information Services Division (2017b) Quality of Cancer Registration Data in Scotland. ISD Scotland, NHS NSS: Edinburgh, UK. Available at: http://www.isdscotland.org/Health-Topics/Cancer/Cancer%20Registration%20 Data%20Quality%20in%20Scotland.pdf (accessed 26 April 2017).
- Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D (2008. a) Travel times to health care and survival from cancers in Northern England. Eur J Cancer 44: 269–274. [DOI] [PubMed] [Google Scholar]
- Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D (2008. b) Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 44: 992–999. [DOI] [PubMed] [Google Scholar]
- Lin CC, Bruinooge SS, Kirkwood MK, Olsen C, Jemal A, Bajorin D, Giordano SH, Goldstein M, Guadagnolo BA, Kosty M, Hopkins S, Yo JB, Arnone A, Hanley A, Stevens S, Hershman DL (2015) Association between geographic access to cancer care, insurance, and receipt of chemotherapy: geographic distribution of oncologists and travel distance. J Clin Oncol 33: 3177–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarweh NN, Chiang Y-J, Xing Y, Chang GJ, Haynes AB, You N, Feig BW, Cormier JN (2014) Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol 32: 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murage P, Crawford SM, Bachmann M, Jones A (2016) Geographical disparities in access to cancer management and treatment services in England. Health Place 42: 11–18. [DOI] [PubMed] [Google Scholar]
- Murchie P, Campbell N, Smith S, Macdonald G, Samuel L, Leeds J, Royle J, Nicolson M (2013) Is current GP referral practice for cancer in Scotland optimal? A detailed exploration of cancer referral pathways using primary care records and a cancer care pathway database. Commissioned research protocol from the Detect Cancer Early Programme of the Scottish Government, . Eur J of Can Care24. [Google Scholar]
- Murchie P, Raja EA, Lee AJ, Brewster DH, Campbell NC, Gray NM, Ritchie LD, Robertson R, Samuel L (2015) Effect of longer health service provider delays on stage at diagnosis and mortality in symptomatic breast cancer. Breast 24: 248–255. [DOI] [PubMed] [Google Scholar]
- Najafabadi AT, Pourhassan M (2001) Integrating the geographic information system into cancer research. Indian J Cancer 48: 105. [DOI] [PubMed] [Google Scholar]
- National Records of Scotland (2017) Quality of National Records of Scotland (NRS) Data on Deaths. NRS: Edinburgh, UK. Available at: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/deaths/deaths-background-information/quality-of-nrs-data-on-deaths (accessed 26 April 2017).
- Nattinger AB, Kneusel RT, Hoffman RG, Gilligan MA (2001) Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst 93: 1344. [DOI] [PubMed] [Google Scholar]
- NHS Scotland (2016) Scottish Cancer Referral Guidelines. NHS Scotland: Edinburgh, UK. Available at: http://www.cancerreferral.scot.nhs.uk (accessed 6 September 2016).
- National Institute for Health and Care Excellence (NICE) (2016) Suspected cancer: recognition and referral. NICE: London, UK. Available at: https://www.nice.org.uk/guidance/ng12 (accessed 6 September 2016).
- O’Brien ED, Bailie RS, Jelfs PL (2000) Cervical cancer mortality in Australia contrasting risk by Aborginality, age and rurality. Int J Epidemiol 29: 813–816. [DOI] [PubMed] [Google Scholar]
- Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D (2008) Geographic access to cancer care in the U.S. Cancer 112: 909–918. [DOI] [PubMed] [Google Scholar]
- Parks RW, Bettschart V, Frame S, Stockton DL, Brewster DH, Garden OJ (2004) Benefits of specialisation in the management of pancreatic cancer: results of a Scottish population-based study. Br J Cancer 91: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins LA, Graham S, Young R, Lewis B, Flanagan B (2013) Racial disparities in travel time to radiotherapy facilities in the Atlanta metropolitan area. Soc Sci Med 89: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozet A, Westeel V, Berion P, Danzon A, Debieuvre D, Breton JL, Monnier A, Lahourcade J, Dalphin JC, Mercier M (2008) Rurality and survival differences in lung cancer: a large, population-based multivariate analysis. Lung Cancer 59: 291–300. [DOI] [PubMed] [Google Scholar]
- Probst JC, Laditka SB, Wang JY, Johnson AO (2007) Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res 7: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R, Campbell NC, Smith S, Donnan PT, Sullivan F, Duffy R, Ritchie LD, Millar D, Cassidy J, Munro A (2004) Factors influence time from presentation to treatment of colorectal and breast cancer in urban and rural area. Br J Cancer 90: 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScottisH Informatics Project (SHIP) (2003) Available at: http://www.scot-ship.ac.uk/overview (accessed 6 September 2016).
- Soo M, Robertson LM, Ali T, Clark LE, Fluck N, Johnstone M, Marks A, Prescott GJ, Smith WCS, Black C (2014) Approaches to ascertaining comorbidity information: validation of routine hospital episode data with clinician-based case note review. BMC Res Notes 7: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Scottish Government (2008) Better Cancer Care, An Action Plan. The Scottish Government: Edinburgh, UK. Available at: http://www.gov.scot/Resource/Doc/242498/0067458.pdf (accessed 8 September 2016).
- The Scottish Government (2014) Scottish Government Urban Rural Classification. The Scottish Government: Edinburgh, UK. Available at: http://www.gov.scot/Topics/Statistics/About/Methodology/UrbanRuralClassification (accessed 8 June 2016).
- The Scottish Government (2016) The Scottish Index of Multiple Deprivation. The Scottish Government: Edinburgh, UK. Available at: http://www.gov.scot/Topics/Statistics/SIMD (accessed 8 June 2016).
- Underhill CR, Goldstein D, Grogan PB (2006) Inequality in rural cancer survival in Australia is not an insurmountable problem. Med J Aust 185: 479–480. [DOI] [PubMed] [Google Scholar]
- University of Aberdeen (2016) Grampian Data Safe Haven. University of Aberdeen: Aberdeen, UK. Available at: http://www.abdn.ac.uk/iahs/facilities/grampian-data-safe-haven.php (accessed 7 October 2016).
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM (2015) Managing patients with multimorbidity in primary care. BMJ 350: h176. [DOI] [PubMed] [Google Scholar]
- Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, Campbell C, Andersen RS, Hamilton W, Olesen F, Rose P, Nafees S, van Rijswijk E, Hiom S, Muth C, Beyer M, Neal RD (2012) The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 106: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westeel V, Pitard A, Martin M, Thaon I, Depierre A, Dalphin JC, Arveux P (2007) Negative impact of rurality on lung cancer survival in a population-based study. J Thorac Oncol 2: 613–618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.