Abstract
The aim of this study is to investigate the role of tumor necrosis factor-α (TNF-α) in apoptosis and autophagy of mouse osteoblast MC3T3-E1 cells, as well as the crosstalk between autophagy and apoptosis. Mouse osteoblast MC3T3-E1 cells were cultured in vitro and treated with 5-fluorouracil (5-FU), rapamycin, 3-methyl adenine (3-MA) and TNF-α either alone or in combination, respectively. MTT assays were used to monitor the cell viability upon different treatments. Annexin-V-FITC/propidium iodide (PI) staining was used to detect the apoptotic rate of osteoblasts. Autophagic structure and apoptotic bodies were visualized by transmission electron microscopy (TEM). Western blot analysis was performed to detect the autophagic marker LC3-II/I, p62 and apoptotic marker cleaved caspase-3. TNF-α inhibits MC3T3-E1 cell viability in a dose-dependent and time-dependent manner. Annexin-V-FITC/PI staining, coupled with TEM, showed that TNF-α induced cell apoptosis and autophagy in MC3T3-E1 cells. The autophagy inducer rapamycin ameliorated TNF-α-induced apoptosis. In contrast, 3-MA, which is an autophagy inhibitor, caused an exaggerated induction of TNF-α-induced apoptosis. TNF-α upregulated autophagy marker LC3-II/I, but downregulated p62 in osteoblasts. Combined treatment of rapamycin and TNF-α further exaggerated this effect, whereas co-treatment of 3-MA and TNF-α decreased LC3-II/I, but increased p62 compared with TNF-α alone. In addition, TNF-α caused an induction of apoptotic marker cleaved caspase-3. TNF-α-mediated induction of cleaved caspase-3 was downregulated by rapamycin, but upregulated by 3-MA, respectively. TNF-α induced both autophagy and apoptosis in osteoblasts, and upregulated autophagy protects the cell by reducing TNF-α-induced apoptosis.
Keywords: Tumor Necrosis Factor-alpha, Osteoblasts, Antibiotics, Apoptosis
Significance of this study.
What is already known about this subject?
The imbalance between bone formation and resorption results in a variety of bone diseases.
TNF-α is an inhibitor of osteoblast differentiation and anactivator of osteoclastogenesis.
TNF-α induces cell apoptosis in osteoblasts.
Several autophagy-related proteins have impact on osteoblastbiology.
What are the new findings?
Autophagy is involved in TNF-α-induced MC3T3-E1 cell apoptosis.
Autophagy and apoptosis are in balance in response to TNF-α in MC3T3-E1 cells.
TNF-α regulates LC3, p62 and cleaved caspase-3 expression in MC3T3-E1 cells.
How might these results change the focus of research or clinical practice?
TNF-α play essential roles in regulating bone homeostasis inseveral chronic immune and inflammatory joint diseases.
TNF-α inhibitors are effective for achieving disease control in these diseases.
Studying TNF-α induced autophagy of osteoblasts and the crosstalk between autophagy and apoptosis may reveal the pathogenesis of bone disease and provide new ideas and therapeutic targets for the treatment and prevention of bone and joint diseases.
INTRODUCTION
The bone strength and integrity depend on maintaining a delicate balance between bone formation and bone resorption. The imbalance between bone formation and resorption results in a variety of bone diseases, such as femoral head necrosis, osteoporosis, traumatic bone disease, and metabolic bone disease.1–3 Osteoblast cells are bone-forming cells that are involved in synthesis and secretion of bone matrix, bone mineralization, and bone remodeling. Bone remodeling involves the osteoclasts-mediated removal of old and damaged bone (bone resorption) and osteoblasts-mediated subsequent replacement of new bone (bone formation). A number of cytokines are involved in the maintenance of the balance between bone resorption and formation rates, including tumor necrosis factor-α (TNF-α). Accumulating evidence suggests that TNF-α is an inhibitor of osteoblast differentiation and an activator of osteoclastogenesis.4–6 In addition, numerous studies also suggest that TNF-α induces cell apoptosis in osteoblasts.7–9 Based on these observations, TNF-α is found to associate with the pathogenesis of inflammatory joint disease including ankylosing spondylitis, which is characterized by simultaneous bone destruction and excessive formation, as well as rheumatoid arthritis that is characterized by massive juxta-articular bone destruction.6 10
Autophagy is a very common biological phenomenon mediated by lysosomes in eukaryotic cells.11 It is an emergency response of cells to cell stress including nutrient deprivation, hypoxia, or infection, and is beneficial for cell survival. However, excessive autophagy or autophagic dysfunction can induce serious illnesses. Recently, it has been discovered that osteocyte autophagy plays an important role in steroid-induced avascular necrosis of the femoral head, suggesting that the level of autophagy affects osteocyte apoptosis.1 Furthermore, it has been reported that several autophagy-related proteins have impact on osteoblast biology. A knock-in mouse model with deletion of both LC3-interacting region (LIR) and ubiquitin-like modifier activating enzyme (UBA) domains in the NBR1 (autophagy receptor) locus leads to increased osteoblast differentiation and activity.12 However, the function of autophagy in osteoblast is still largely unexplored. Based on these findings, we hypothesized that autophagy might play an important role in osteoblast apoptosis.
In this study, it was reported for the first time that osteoblasts underwent autophagy and apoptosis on TNF-α treatment. When the autophagy level was changed, the level of TNF-α-induced apoptosis changed in a reverse fashion, suggesting that TNF-α was likely to promote apoptosis of osteoblasts by inhibiting autophagy, and was so found to be involved in the pathogenesis of various bone diseases. TNF-α-mediated osteoblast differentiation, apoptosis, and autophagy are the triggers for a varieties of bone diseases, such as femoral head necrosis, osteoporosis, traumatic bone disease, metabolic bone disease, and many inflammatory joint diseases, such as rheumatoid arthritis and ankylosing spondylitis pathogenesis.13 14 Studying TNF-α-induced autophagy of osteoblasts and the crosstalk between autophagy and apoptosis may reveal the pathogenesis of bone disease and provide new ideas and therapeutic targets for the treatment and prevention of bone and joint diseases.
MATERIALS AND METHODS
Cell culture and treatments
Mouse osteoblast MC3T3-E1 cells was purchased from American Type Culture Collection (CRL2596, Manassas, Virginia, USA). Cells were grown in α-MEM (Invitrogen, Carlsbad, California, USA) containing 10 per cent fetal bovine serum (FBS). Cultures were maintained at 37°C in humidified atmosphere with 5 per cent CO2 in air. MC3T3-E1 cells (2.0×104 cells/well) were seeded in six-well plates for 48 hours as control. For other experiments, cells were treated with 20 µg/mL 5-fluorouracil (5-FU) for 36 hours, 500 ng/mL rapamycin (Alexis, Lausen, Switzerland) for 6 hours, 10 nM 3-methyl adenine (3-MA, Sigma-Aldrich, St Louis, Missouri, USA) for 12 hours or 20 ng/mL TNF-α for 48 hours, respectively. For the combined treatment, cells were treated with 20 ng/mL TNF-α alone for 42 hours and then combined with 500 ng/mL rapamycin for additional 6 hours, or cells were treated with 20 ng/mL TNF-α alone for 36 hours, followed by the combined treatment of 20 ng/mL TNF-α and 10 nM 3-MA for additional 12 hours. All cells with different treatment were cultured for 24 hours.
MTT assay
MC3T3-E1 cells (2.0×103 cells/well) were seeded in 96-well plates and incubated for 2 hours prior to TNF-α treatment. Cells were treated with 1, 5, 20 and 100 ng/mL TNF-α (Sigma-Aldrich) for 24, 48 and 72 hours. Also, 10 µL MTT was added into each well and incubated for 4 hours at 37°C. Culture medium was then removed and MTT formazan crystals were resolved in 100 µL dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Absorbance was measured at a wavelength of 490 nm by the use of microplate reader (Model 680, Bio-Rad Laboratories, Hercules, California, USA). Similarly, MC3T3-E1 cells were incubated with 0.5, 5, 50 and 500 ng/mL rapamycin, or treated with 1, 10, 100 and 1000 nM 3-MA. Cell were harvested after 6, 12, 24 and 48 hours, and MTT assays were performed to monitor the cell viability. All procedures were repeated at least three times.
Flow cytometry
Annexin-V-FITC (fluorescein isothiocyanate)/propidium iodide (PI) staining was performed using Annexin-V-FITC/PI apoptosis detection kit (KGI Biotech, Nanjing, China) according to the manufacturer's instruction. MC3T3-E1 cells with different treatment were harvested at specified times, and then resuspended in binding buffer. 1.0×105 cells in 100 µL binding buffer were added into a 5 mL tube. Also, 5 µL Annexin-V-APC reagent and 5 µL PI were added into each tube. The mixture was incubated in the dark at room temperature for 15 min. Then 400 µL phosphate buffered saline (PBS) was added into each tube. The samples were analyzed by flow cytometry (FACS; FACSCalibur, BD Biosciences, San Jose, California, USA) with excitation wavelength at 488 nm and emission wavelength at 530 nm. Mod FitL T software was used for quantification of cell apoptosis. All experiments were performed in triplicate and repeated at least three times.
Western blot
Protein lysates from MC3T3-E1 cells were prepared in radioimmunoprecipitation assay (RIPA) lysis buffer containing 1 per cent protease inhibitor. Protein concentration was determined by BCA assay. Equal amounts of protein lysate were resolved by sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) gel. Proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories). Membranes were blocked with 5 per cent non-fat milk in PBS-Tris buffer for 1 hour, followed by incubation with primary antibody at 4°C overnight. Also, 5 per cent non-fat milk was used to wash away the excess antibody. Membranes were then incubated with secondary antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) at room temperature for 2 hours. ECL reagents (GE Healthcare, Piscataway, New Jersey, USA) were used for protein detection. The X-ray films were analyzed and plotted using GraphPad software (San Diego, California, USA). All experiments were repeated at least four times.
Transmission electron microscopy
4×106 cells were collected (n=3 for each sample) and fixed with 2.5 per cent glutaraldehyde for 12 hours, followed by 1 per cent osmium tetroxide for 1 hour. Samples were dehydrated with 50 per cent, 70 per cent, 90 per cent and 100 per cent acetone, and embedded in paraffin for 12 hours, followed by overnight incubation at 60°C. Samples were cut at 50–100 nm thickness and stained with 3 per cent uranyl acetate and lead nitrate. All specimens were examined with the HT-7700 transmission electron microscope (TEM; Hitachi, Tokyo, Japan). Autophagy and apoptosis were analyzed based on cell organelle structures, quantity and ratio of apoptotic cell and autophagic vacuoles.
Statistical analysis
All experimental data were analyzed using SPSS V.19.0 statistical software. Mann-Whitney U test was performed between two groups in pairwise fashion. Data are expressed as means±SD. p<0.05 was considered statistically significant.
RESULTS
TNF-α inhibits MC3T3-E1 cell viability in a dose-dependent and time-dependent manner
In order to investigate the effect of TNF-α on MC3T3-E1 cell viability, MTT assays were performed. As shown in table 1, TNF-α inhibits MC3T3-E1 cell viability dose-dependently and time-dependently. It was shown that the addition of 20 ng/mL TNF-α to MC3T3-E1 cells for 48 hours exhibited no significant inhibitory effects on the cell viability. In subsequent experiments, a dose of TNF-α at 20 ng/mL and an incubation time of 48 hours were selected for studies. Similarly, MTT assays were performed to test the effects of autophagy inducer rapamycin and autophagy inhibitor 3-MA on MC3T3-E1 cell viability. According to the results of MTT assays, 500 ng/mL rapamycin treatment for 6 hours and 10 nM 3-MA treatment for 12 hours were selected for subsequent experiments, respectively. The selected dose and incubation time for treatment showed no significant inhibitory effect on cell viability (data not shown).
Table 1.
Effect of tumor necrosis factor-α on cell viability in MC3T3-E1 cells
| Culture time/ optical density value (hours) |
Control | 1 ng/mL | 5 ng/mL | 20 ng/mL | 100 ng/mL |
| 24 | 0.860±0.025 | 0.843±0.056 | 0.805±0.033 | 0.790±0.142 | 0.613±0.137* |
| 48 | 0.812±0.053 | 0.805±0.123 | 0.773±0.152 | 0.734±0.045 | 0.547±0.173** |
| 72 | 0.787±0.104 | 0.810±0.089 | 0.750±0.063 | 0.587±0.245** | 0.398±0.067** |
*p<0.05, **p<0.01.
Autophagy is involved in TNF-α-induced MC3T3-E1 cell apoptosis
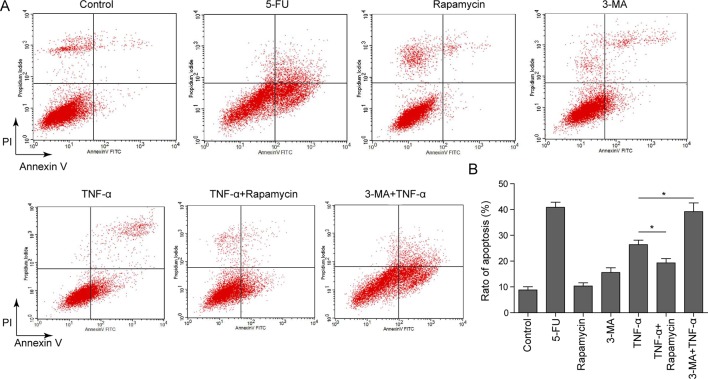
It has been generally accepted that TNF-α induces osteoblast apoptosis.7–9 We next examined whether autophagy was involved in TNF-α-induced apoptosis in MC3T3-E1 cells. MC3T3-E1 cells were treated with the 5-FU, which served as a positive control, rapamycin, 3-MA, or TNF-α either alone or in combination. Annexin-V-FITC/PI staining was performed to monitor the apoptotic rate of each treatment. As expected, 5-FU and TNF-α induced MC3T3-E1 cell apoptosis, whereas rapamycin and 3-MA alone had no significant effect on cell apoptosis (figure 1A, B). More important, the autophagy inducer rapamycin ameliorated TNF-α-induced apoptosis. In contrast, 3-MA, which is an autophagy inhibitor, caused an exaggerated induction of TNF-α-induced apoptosis (figure 1A, B). These findings indicate that autophagy is involved in TNF-α-induced cell apoptosis in MC3T3-E1 cells.
Figure 1.
Effect of different chemicals on cell apoptosis in MC3T3-E1 cells. (A) MC3T3-E1 cells were treated vehicle control, 5-fluorouracil (5-FU) (20 µg/mL, 36 hours), rapamycin (500 ng/mL, 6 hours), 3-methyl adenine (3-MA) (10 nM, 12 hours), tumor necrosis factor-α (TNF-α) (20 ng/mL, 48 hours), rapamycin+TNF-α (rapamycin, 500 ng/mL, 6 hours; TNF-α, 20 ng/mL, 48 hours) or 3-MA+TNF-α (3-MA, 10 nM, 12 hours; TNF-α, 20 ng/mL, 48 hours). Cells were stained with Annexin-V-FITC and propidium iodide (PI). The apoptotic rate was determined by flow cytometry. (B) Quantitative data of Annexin-V-FITC/PI staining. Data were presented as mean±SD in triplicate. *p<0.05 versus corresponding control.
Autophagy and apoptosis are in balance in response to TNF-α in MC3T3-E1 cells
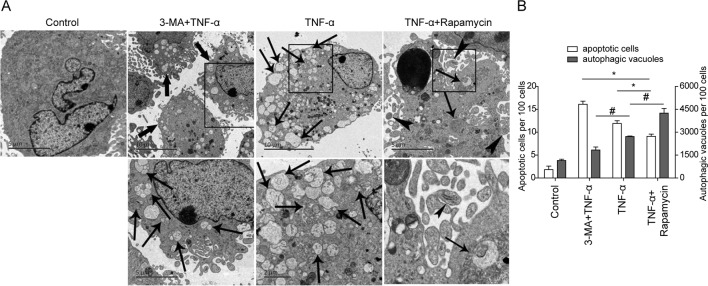
To confirm the results of FACS analysis, TEM was performed to visualize the autophagic structure and apoptotic bodies in MC3T3-E1 cells. As shown in figure 2, the untreated cells showed normal nucleus, mitochondria and other organelles, and no autophagic vacuoles or apoptotic bodies were detected by TEM (figure 2A). In contrast, both autophagic vacuoles and apoptotic bodies were observed in TNF-α-treated cells, suggesting that TNF-α induces cell apoptosis and autophagy in osteoblasts (figure 2A, third panel). Consistent with the results of FACS, 3-MA+TNF-α treatment significantly increased the number of apoptotic cells compared with TNF-α-treated cells. And less autophagic vacuoles were detected in these cells (figure 2A, second panel). As shown in figure 2A, fourth panel, increased autophagic vacuoles were visualized in rapamycin+TNF-α-treated cells, but decreased apoptotic cells were found in this group. These findings suggested that TNF-α-induced autophagy and apoptosis are in balance in osteoblast.
Figure 2.
Detection of autophagy and apoptosis in MC3T3-E1 cells by transmission electron microscopy (TEM). (A) MC3T3-E1 cells were treated vehicle control, tumor necrosis factor-α (TNF-α) (20 ng/mL, 48 hours), rapamycin+TNF-α (rapamycin, 500 ng/mL, 6 hours; TNF-α, 20 ng/mL, 48 hours) or 3-methyl adenine (3-MA)+TNF-α (3-MA, 10 nM, 12 hours; TNF-α, 20 ng/mL, 48 hours). In TNF-α-treated cells, both autophagic bodies (thin black arrow) and apoptotic cells (thick black arrow) could be detected. Co-treatment of rapamycin and TNF-α increased autophagic vacuoles and bodies (thin black arrow) significantly. In 3-MA+TNF-α-treated cells, increased apoptotic cells (arrow head), but decreased autophagic vacuoles (thin black arrow) were found. Boxed areas in black were magnified and shown in the lower panel. (B) Quantitative data of TEM. The number of autophagic bodies per 100 cells and number of apoptotic cells per 100 cells were presented. *p<0.05 versus corresponding control (apoptotic cells); # p<0.05 versus corresponding control (autophagic vacuoles).
TNF-α regulates LC3, p62 and cleaved caspase-3 expression in MC3T3-E1 cells
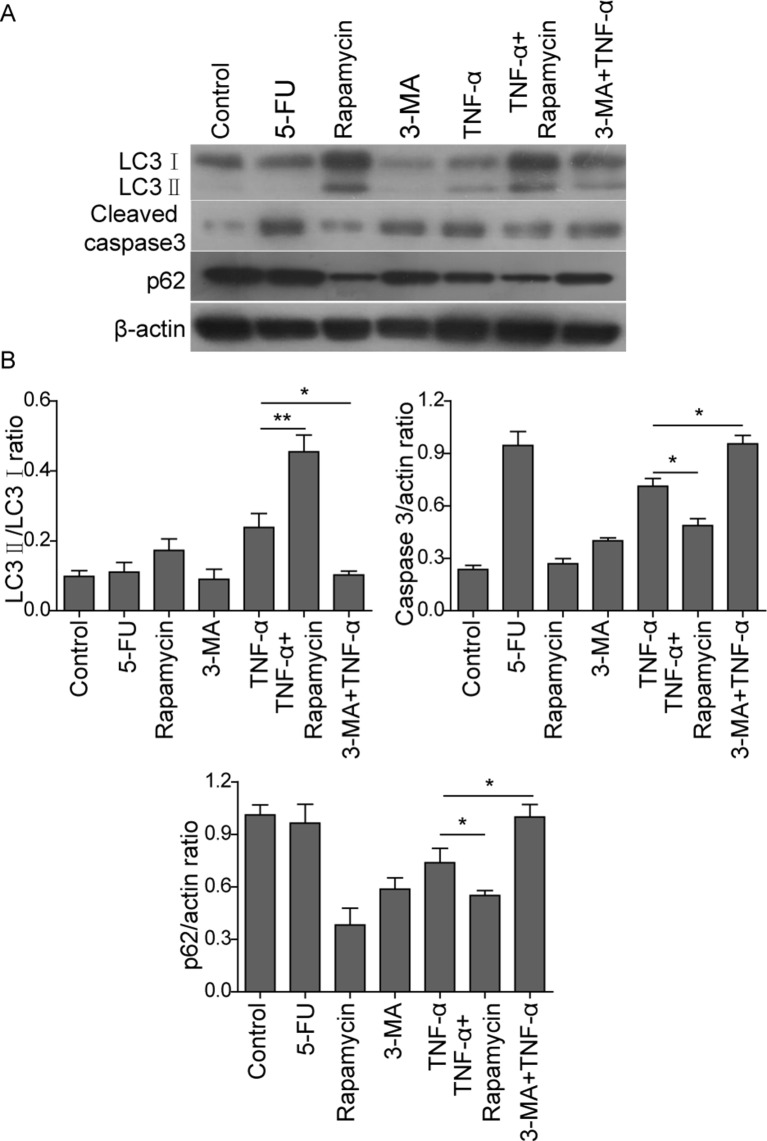
In order to unravel the mechanisms of TNF-α-induced autophagy and apoptosis in MC3T3-E1 cells, the autophagic marker proteins LC3, p62, as well as cleaved caspase-3, which is the hallmark of apoptosis, were analyzed by western blot. In mammalian cells, cytosolic LC3 (LC3-I) is conjugated to phosphatidylethanolamine to form LC3-II on the autophagosomes. LC3-II could be used as an indicator for monitoring autophagy.15 In addition to LC3, p62, which is an LC-3 binding protein, is used as a marker of autophagic defect.15 As shown in figure 3A, B, the 5-FU group showed no significant change in LC3-II/I and p62 levels compared with the control group, suggesting that 5-FU did not induce autophagy in osteoblasts. TNF-α induced increase in LC3-II/I level, but decrease in p62 level compared with control, indicating that TNF-α induced autophagy in MC3T3-E1 cells. Combined treatment of rapamycin and TNF-α further exaggerated this effect, whereas co-treatment of 3-MA and TNF-α decreased LC3-II/I, but increased p62 compared with TNF-α alone (figure 3A, B). On the other hand, rapamycin and 3-MA alone had no significant effect on cell apoptosis, whereas 5-FU caused a remarkable increase in cleaved caspase-3 level (figure 3A, B). Besides autophagy, TNF-α resulted in an induction of cleaved caspase-3 expression, suggesting that TNF-α also induced cell apoptosis in osteoblasts (figure 3A, B). TNF-α-mediated induction of cleaved caspase-3 was inhibited by autophagy inducer rapamycin, but upregulated by autophagy inhibitor 3-MA (figure 3A, B). Taken together, these findings confirmed that TNF-α induced both autophagy and apoptosis in osteoblasts. Upregulated autophagy protected the cells by reducing TNF-α induced apoptosis.
Figure 3.
Western blot analysis of autophagic and apoptotic markers in in MC3T3-E1 cells. (A) MC3T3-E1 cells were treated with vehicle control, 5-fluorouracil (5-FU) (20 µg/mL, 36 hours), rapamycin (500 ng/mL, 6 hours), 3-methyl adenine (3-MA) (10 nM, 12 hours), tumor necrosis factor-α (TNF-α) (20 ng/mL, 48 hours), rapamycin+TNF-α (rapamycin, 500 ng/mL, 6 hours; TNF-α, 20 ng/mL, 48 hours) or 3-MA+TNF-α (3-MA, 10 nM, 12 hours; TNF-α, 20 ng/mL, 48 hours). Expressions of LC3, p62 and cleaved caspase-3 were analyzed by western blot. β-Actin served as a loading control. (B) Quantitative data of western blot. Data were presented as mean±SD in triplicate. *p<0.05,** p<0.01 versus corresponding control.
DISCUSSION
Bone tissue results from the many cell lineages, including osteoblasts, osteocytes and osteoclasts. Imbalance between bone resorption by osteoclasts and bone formation by osteoblasts leads to a variety of bone diseases, such as Paget's disease of bone, fibrous dysplasia and osteoporosis.1–3 The treatment of bone loss is based on the stimulation of bone formation by osteoblasts or the inhibition of bone resorption by osteoclasts.
Proper balance between bone resorption and bone formation is tightly regulated and maintained by multiple cytokines, growth factors, and hormones.13 16 TNF-α derived from activated macrophages, monocytes, or bone marrow cells is involved in the inflammatory response of tissues and cells, bone reconstruction and resorption. It plays an important role in the pathogenesis of bone by promoting osteoclastogenesis and inhibiting osteoblast differentiation and apoptosis.4 17In addition, nuclear factor-κB and Fas/FasL signaling pathways are involved in TNF-α-induced osteoblast differentiation and apoptosis, as well as osteoclastogenesis.17 18 Numerous studies have demonstrated that TNF-α regulates autophagy and apoptosis in many diseases, such as cardiovascular diseases, breast cancer, and autoimmune diseases.4 14 19 The crosstalk between apoptosis and autophagy indicates that regulating autophagy might be an effective approach to controlling cell apoptosis and delaying the development of disease. It has been reported that autophagy is activated in osteoclasts of human rheumatoid arthritis in aTNF-α-dependent manner and regulates osteoclast differentiation and bone resorption.11 However, whether TNF-α-induced osteoblast apoptosis is associated with the autophagy machinery remains uninvestigated.
In this study, we have demonstrated that mouse osteoblast MC3T3-E1 cells underwent apoptosis and autophagy on TNF-α treatment by FACS, TEM, and western blot. Moreover, rapamycin, which is an autophagy inducer, decreased TNF-α-induced apoptosis (figures 1–3). Conversely, 3-MA, an autophagy inhibitor, caused an induction of TNF-α-induced apoptosis (figures 1–3). The results of Annexin-V-FITC/PI staining showed that TNF-α caused apoptosis in osteoblasts (figure 1), which is consistent with previous studies.7–9 Furthermore, TNF-α-mediated apoptosis was inhibited when autophagy was induced by rapamycin. In contrast, 3-MA exerted opposite effect on TNF-α-mediated apoptosis (figure 1). In order to visualize the cell death more accurately, TEM was performed to monitor the cell autophagy and apoptosis. TNF-α-treated cells displayed abundant vacuolization, a widely known morphological indicator for autophagic cell death, as well as apparition of membrane blebs termed apoptotic bodies (figure 2). It is obvious that co-treatment of rapamycin increased the number of autophagic vacuoles, but decreased the number of apoptotic cells. The addition of 3-MA caused a significant increase of apoptotic cells (figure 2). These findings indicate that apoptosis of osteoblasts could be regulated by autophagy.
During autophagy, LC3-II is recruited to autophagosomal membranes, and it is degraded by lysosomal hydrolases after the fusion of autophagosomes with lysosomes.15 Therefore, LC3-II itself is used as a reliable marker of autophagy. p62 (also known as SQSTM1), which is an ubiquitin-binding and LC3-binding protein, is increased when autophagy is impaired.15 The increase in p62 level in tissues and cells indicates the possibility of an insufficiency in autophagy. In the current study, TNF-α caused an induction of LC3-II/I, but a reduction of p62 (figure 3), suggesting that TNF-α induces autophagy in osteoblasts. In addition, rapamycin exaggerated TNF-α-caused induction of LC3-II/I and reduction of p62. As expected, 3-MA had the opposite effect on the expression of LC3-II/I and p62. For the cell apoptosis, cleaved caspase-3, an apoptotic marker, was detected. The results of western blot were consistent with the FACS and TEM results. These data suggest that autophagy exerts a protection effect on TNF-α-induced apoptosis in osteoblasts. The underlying regulatory mechanisms still need to be investigated in the future studies.
The current study demonstrated for the first time that TNF-α induces autophagy in osteoblasts, and the level of autophagy affects the activity of osteoblast apoptosis. These preliminary findings provide considerable scientific value and merit further investigation. In summary, the role of TNF-α in osteoblast apoptosis and autophagy, and the crosstalk between autophagy and apoptosis, may shed new light on the pathogenesis of bone and joint disease. This may provide new ideas and therapeutic targets for the treatment and prevention of bone and joint disease.
CONCLUSION
TNF-α induces autophagy in osteoblasts, and upregulated autophagy protects the cells by reducing TNF-α-induced apoptosis.
Footnotes
Contributors: LZ: conception and design; JW: acquisition of data; DS: analysis of data; ZH: molecular biology experiment; TL: writing paper; WW, JN and XM: paper amendment.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was published Online First. The author's name Zhou Huaying has been amended to read Huaying Zhou and the affiliation details updated.
References
- 1. Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J Bone Miner Res 2012;27:1439–47. 10.1002/jbmr.1668 [DOI] [PubMed] [Google Scholar]
- 2. Zheng L, Wang W, Ni J, et al. The association of eNOS gene polymorphism with avascular necrosis of femoral head. PLoS One 2014;9:65 6 00 00. 10.1371/journal.pone.0087583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng L, Wang W, Ni J, et al. Plasma interleukin 33 level in patients with osteonecrosis of femoral head: an alarmin for osteonecrosis of the femoral head? J Investig Med 2014;62:635–7. 10.2310/JIM.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 4. Glass GE, Chan JK, Freidin A, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A 2011;108:1585–90. 10.1073/pnas.1018501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mucci JM, Scian R, De Francesco PN, et al. Induction of osteoclastogenesis in an in vitro model of gaucher disease is mediated by T cells via TNF-α. Gene 2012;509:51–9. 10.1016/j.gene.2012.07.071 [DOI] [PubMed] [Google Scholar]
- 6. Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol 2014;5:48. 10.3389/fimmu.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology 1997;138:3849–58. 10.1210/endo.138.9.5370 [DOI] [PubMed] [Google Scholar]
- 8. Jilka RL, Weinstein RS, Bellido T, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998;13:793–802. 10.1359/jbmr.1998.13.5.793 [DOI] [PubMed] [Google Scholar]
- 9. Pavalko FM, Gerard RL, Ponik SM, et al. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol 2003;194:194–205. 10.1002/jcp.10221 [DOI] [PubMed] [Google Scholar]
- 10. Danks L, Takayanagi H. Immunology and bone. J Biochem 2013;154:29–39. 10.1093/jb/mvt049 [DOI] [PubMed] [Google Scholar]
- 11. Lin NY, Beyer C, Giessl A, et al. Autophagy regulates TNFα-mediated joint destruction in experimental arthritis. Ann Rheum Dis 2013;72:761–8. 10.1136/annrheumdis-2012-201671 [DOI] [PubMed] [Google Scholar]
- 12. Whitehouse CA, Waters S, Marchbank K, et al. Neighbor of Brca1 gene (Nbr1) functions as a negative regulator of postnatal osteoblastic bone formation and p38 MAPK activity. Proc Natl Acad Sci U S A 2010;107:12913–8. 10.1073/pnas.0913058107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira E, Porter RM, Wehling N, et al. Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow. J Biol Chem 2013;288:29494–505. 10.1074/jbc.M113.471268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang ZQ, Ma YX, Li MH, et al. 5-Hydroxymethylfurfural protects against ER stress-induced apoptosis in GalN/TNF-α-injured L02 hepatocytes through regulating the PERK-eIF2α signaling pathway. Chin J Nat Med 2015;13:896–905. 10.1016/S1875-5364(15)30095-9 [DOI] [PubMed] [Google Scholar]
- 15. Tanida I, Waguri S. Measurement of autophagy in cells and tissues. Methods Mol Biol 2010;648:193–214. 10.1007/978-1-60761-756-3_13 [DOI] [PubMed] [Google Scholar]
- 16. Thomas MV, Puleo DA. Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res 2011;90:1052–61. 10.1177/0022034510393967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon SJ, Ahn IE, Jung H, et al. Temporal differential effects of proinflammatory cytokines on osteoclastogenesis. Int J Mol Med 2013;31:769–77. 10.3892/ijmm.2013.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Briolay A, Lencel P, Bessueille L, et al. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem Biophys Res Commun 2013;430:1072–7. 10.1016/j.bbrc.2012.12.036 [DOI] [PubMed] [Google Scholar]
- 19. Bin G, Cuifang W, Bo Z, et al. Fluid shear stress inhibits TNF-α-induced osteoblast apoptosis via ERK5 signaling pathway. Biochem Biophys Res Commun 2015;466:117–23. 10.1016/j.bbrc.2015.08.117 [DOI] [PubMed] [Google Scholar]