Abstract
Background
Long term mortality may be increased following traumatic brain injury (TBI); however the degree to which survival could be reduced is unknown. We aimed to model life expectancy following post-acute TBI to provide predictions of longevity and quantify differences in survivorship with the general population.
Methods
A population based retrospective cohort study using data from the Rochester Epidemiology Project (REP) was performed. A random sample of patients from Olmsted County, Minnesota with a confirmed TBI between 1987 and 2000 was identified and vital status determined in 2013. Parametric survival modelling was then used to develop a model to predict life expectancy following TBI conditional on age at injury. Survivorship following TBI was also compared with the general population and age and gender matched non-head injured REP controls.
Results
769 patients were included in complete case analyses. Median follow up time was 16.1 years (IQR 9.0–20.4) with 120 deaths occurring in the cohort during the study period. Survival after acute TBI was well represented by a Gompertz distribution. Victims of TBI surviving for at least 6 months post-injury demonstrated a much higher ongoing mortality rate compared to the US general population and non-TBI controls (hazard ratio 1·47, 95% CI 1·15–1·87). US general population cohort life table data was used to update the Gompertz model’s shape and scale parameters to account for cohort effects and allow prediction of life expectancy in contemporary TBI.
Conclusions
Survivors of TBI have decreased life expectancy compared to the general population. This may be secondary to the head injury itself or result from patient characteristics associated with both the propensity for TBI and increased early mortality. Post-TBI life expectancy estimates may be useful to guide prognosis, in public health planning, for actuarial applications and in the extrapolation of outcomes for TBI economic models.
Keywords: Craniocerebral trauma, Survival analysis, Mortality
INTRODUCTION
Injury is a major public health problem, predicted by the World Health Organization to become the 4th largest global burden of disease by 2020.[1] Traumatic brain injury (TBI) comprises an important subset of injury cases, but despite its importance in terms of mortality, morbidity and socio-economic costs important epidemiological aspects, such as life expectancy, remain largely undefined.[2]
There is a growing body of evidence suggesting that long-term mortality is increased following TBI compared to the non-head injured population. For example Ventura and colleagues (2010) reported a standardised mortality ratio of 2·5 (95%CI 2·3–2·7) compared to the general population for adult patients discharged from hospital with significant TBI.[3] However, no previous study has quantified the absolute extent to which length of survival might be reduced in a representative sample of patients following TBI, or extrapolated observed survival to predict future life expectancy. Such information could be beneficial in a number of different areas.
Firstly, longevity estimates would be of interest to patients, carers and clinicians as a guide to long term prognosis following in injury. Secondly, an indication of the long term burden of patients with chronic complications would be helpful in planning public health services for TBI. Thirdly, accurate life expectancy estimates are necessary for actuarial applications and medico-legal settlements. Finally, cost-effectiveness evaluations for TBI interventions need to extrapolate outcomes over a lifetime horizon to fully reflect health effects and prevent bias.[4] It is therefore imperative that valid estimates of long term survival specific to victims of TBI are available.
The aim of this study was to increase understanding of survival following acute TBI and provide extrapolative estimates of life expectancy. Specific objectives were to develop parametric statistical models to accurately reflect survival after TBI and allow extrapolation for prediction of life expectancy, and estimate the relative difference in hazard of death between patients with TBI and the general population.
METHODS
Study design
A population based cohort study was performed, retrospectively analysing data from the Rochester Epidemiology Project (REP); a comprehensive demographic and medical records-linkage system for all residents of the Olmsted County, Minnesota, USA.[5] Survival following acute TBI was investigated using parametric survival analysis methodology.
Setting
Olmsted County (2010 census population, 144,248) is situated in the upper mid-west region of the US. The county is primarily served by the Mayo Clinic, one of the largest private medical practices in the world, and the Olmsted Medical Center, a community hospital with long-term collaborative research ties to the REP. Its population is 90% white with an age and sex distribution comparable with the US total population. Olmsted county residents are more highly educated and have higher median income than the general US population.[6] Mortality rates are consistent with the wider Minnesota population, but slightly lower than those from the total national population.[6]
Since 1907 every Mayo Clinic patient has been assigned a unique identifier, used to record and link medical information from all health care providers in any setting within Olmsted County, including nursing home, primary, secondary or tertiary healthcare.[5] From 1966 the REP has maintained an electronic database recording diagnostic codes, surgical procedure codes and demographic information abstracted from medical records and assigned at every medical contact. The coding system is based on the International Classification of Diseases system (ICD-8 and ICD-9), uses an 8 digit number, and was developed specifically for clinical and research purposes. Additionally, all medical records are archived in a single, unified, continuously updated file; historically in paper form but more recently in searchable electronic format. The REP therefore represents an essentially complete record of the entire health experience within the geographically defined population of Olmsted County, regardless of age, socioeconomic status, or insurance coverage.[7] Furthermore, the REP database additionally provides an ongoing census of individuals as they move in and out of the community, recording address changes at each medical attendance.[8]
Study population
The source population comprised Olmsted County residents with any code suggestive of TBI in the REP diagnostic index from 1/1/1985 to 31/12/1999. A 15% random sample, determined was selected for review of their complete medical records to identify a study population of incident TBI cases. The review was performed by trained nurse abstractors under the supervision of a board-certified physiatrist and neuropsychologist. TBI was defined as any traumatically induced injury that contributed to physiological disruption of brain function. The cohort thus included the entire spectrum of TBI severities from those assessed in primary care to critical care admissions. The criteria used for the identification of TBI within clinical records are detailed in the web appendix.
Significant TBI typically results in high early mortality; as long term survival of patients beyond the acute period was of interest, cases dying within 6 months of injury were excluded. This 6 month time period is the most established endpoint to evaluate acute outcomes in clinical TBI studies.[9] Full information on ED and hospital admissions was first available electronically from 1987 and cases were consequently restricted to those presenting after this date. The final sample therefore consisted of adult patients sustaining a confirmed TBI between 1/1/1987 to 31/12/1999 aged >16 years, who survived beyond 6 months and consented to data use.
To provide a comparison population for judging the external validity of TBI parametric models, non-TBI controls were also examined. In these analyses each TBI case was matched to an Olmsted County resident of the same gender and similar birth year (within one year), but who did not have a REP diagnostic code potentially suggestive of TBI. This matched sample was judged likely to have similar characteristics to those sustaining TBI, thus providing a survival experience against which the predicted survival following TBI could be evaluated.
Data collection
Demographic and injury information was abstracted from each patient’s clinical records by trained research nurses and entered into an electronic database. Vital status was determined on the 31st September 2013 by review of REP medical records, Olmsted County obituary notices, local death certificates, and State of Minnesota death tapes. Persons for whom death was not recorded were considered censored as of the date they were last known to be Olmsted County residents.
Statistical analyses
The cohort’s characteristics were initially examined using descriptive statistics. Observed survival experience was then examined using the Kaplan-Meier product limit method to compare empirical cumulative incidence functions categorised by age-group at TBI and other patient variables. Log-rank tests were used to test the null hypothesis that survival curves did not differ across categories of dichotomous variables; and log-rank test for trend used to test ordinal variables.
Parametric survival modelling then proceeded in four stages.[10,11] Firstly the plausibility of alternative parametric functions from the generalised F distribution for describing survival time after TBI was evaluated. Graphical analyses were performed to assess survival time distribution, proportional hazards or accelerated failure time assumptions. To account for the fact that standard parametric models might not accurately reflect the true hazard function associated with TBI, flexible modelling of the baseline hazard with cubic splines was additionally assessed.
Secondly, potentially suitable parametric survival models were applied to the data. Age at head injury alone was examined in the primary model, with age at head injury, gender and other patient characteristics (including TBI severity, comorbidity and hospitalisation) considered as explanatory variables in separate secondary models. Goodness of fit metrics were assessed, using Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), mean squared differences in observed and predicted survival, and a comparison of predicted survival functions against observed Kaplan-Meier curves, to select the model(s) that best fitted REP data. AIC and BIC provide a numerical measure of both how well a model fits the data and how complex the model is, and can therefore be used to identify the most favourable model from a range of possibilities. Mean squared differences in observed and predicted survival (lower is better numerical value is better), and a comparison of predicted against observed survival functions (closer visual fit is better), offer a direct evaluation of well a model mirrors the actual data.
Thirdly the external validity of the chosen models was assessed by evaluating the concordance of predicted survival against published estimates, and regional and national general population life tables. Extrapolated survival curves were also compared with those of age-matched non-TBI controls sampled from the REP. Final model selection was determined by the best statistical fit to REP data whilst providing credible long term survival predictions.
The final model provides survival and hazard curves based on the survivorship experience of patients who had a head injury between 1988 and 1999. Since that time there has been a secular trend of improving life expectancy in the general US population and the model may therefore slightly underestimate survival of contemporary TBI cases. The shape and scale of the model’s baseline hazard function were therefore updated in the final analysis stage, based on temporal changes in survival curves observed in the US general population.
The indicated parametric distribution was fitted to the adult age-range (>16 years) of published US cohort life tables between 1930 and 2010 (the last available publication) using least squares non-linear regression. The proportional change in shape and scale parameters over time from 2000 were then determined by non-linear regression and the parameters of the baseline hazard function of the final derived REP model updated accordingly.[12]
Statistical analyses were carried out in Stata version 12·1 (StataCorp, College Station, USA). A two-sided p-value of <0.05 was considered to be statistically significant. Thorough model diagnostics were performed to ensure correct specification and validity at each step of the modelling procedure. Full details on statistical analyses, including scenario sensitivity analyses to investigate the potential influence of non-informative censoring, are presented in the web appendix. All analyses were pre-specified in a study protocol submitted to the Mayo Clinic institutional review board. Ethical approval was provided by the Mayo Clinic and Olmsted Medical Centre institutional review boards. Patient consent for use of personal data was obtained in accordance with Minnesota and Federal law.
Role of the funding source
The study was sponsored and funded by the Mayo Clinic. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Sample characteristics
46,114 unique Olmsted County residents were assigned a diagnostic code potentially indicative of TBI between 1985 and 1999. 323 patients were excluded who refused authorisation to disclose personal information for research. The medical records of a random sample of 7,175 cases (15%) were examined in detail, confirming 1,429 incident cases of TBI with 1,257 injured after 01/01/1987. Of these 769 patients were aged >16 years at injury, survived at least 6 months post injury and were included in complete case analyses. There was missing data on patient ethnicity in 21·7% of cases (161 unknown, 6 refused disclosure) and TBI mechanism in 0·4% of patients. All other patient characteristics had complete information. Figure 1 presents the derivation of the final study sample.
Figure 1.
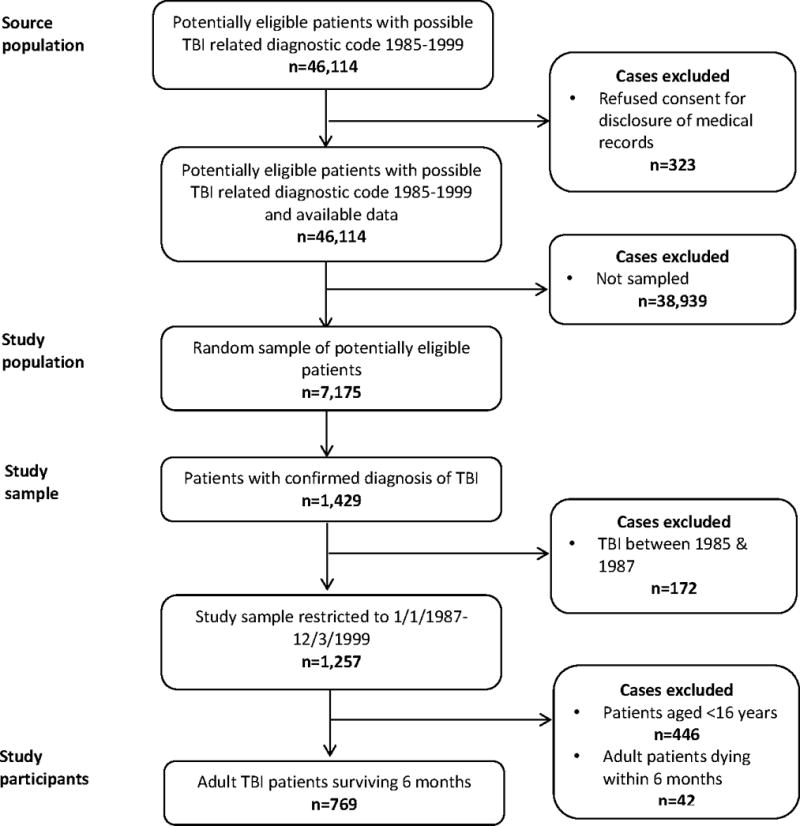
Flow chart delineating the derivation of the study sample.
Median follow up time was 16.1 years (IQR 9·0–20·4) with 120 deaths occurring during the study period. Four hundred and forty six patients (58.0%) were administratively right censored at the end of follow up in September 2013 (median follow up=19.3 years IQR 16·4–22·2). Two hundred and three (26·4%) cases left Olmsted County prior to the end of the study and were non-administratively censored (median follow time 4.9 years IQR 1·4–10·2). The reverse Kaplan-Meier estimate of overall median follow up was 17.3 years (95%CI 16·6–18·0). The completeness index (ratio of total observed to potential person-time of follow up) was 77·7%.
The median age of the study sample was 30·3 years (Inter-quartile range 21·5–43·7) with males accounting for 50·2% of cases (95% confidence interval (CI) 46·7–53·7%). White ethnicity dominated, representing 92·9% (95%CI 90·8–94·9) of enrolled patients. The commonest modes of injury were road accidents (43·7%), falls (24·7%), sports-related trauma (9·2%) and assaults (7·8%). A significant minority of patients presented with a concomitant extra-cranial injury (23·3%, 95% CI 20·3–26·3%). Mild head injury preponderated and was responsible for 93·2% (95% CI 91·5–95·0%) of included cases. Patient characteristics are summarised in table 1.
Table 1.
Patient characteristics
| Patient characteristic (n=769) |
Summary statistics (%, 95%CI) |
|---|---|
| Age (Years, median, IQR) | 30·3 years (21·5–43·7) |
| Male Gender | 50·2% (46·7–53·7) |
| White ethnicity* | 92·9% (90·8–94·9) |
| Extra-cranial injury | 23·3% (20·3–26·3) |
| Mild TBI | 93·2% (91·5–95·0) |
| Mode of injury** | |
| • Road accident | 43·7% (40·2–47·2) |
| • Fall | 24·7% (21·7–27·8) |
| • Sports-related | 9·2% (7·2–11·3) |
| • Bunt assault | 7·8% (5·9–9·7) |
| • Other | 14·6% (12·1–17·1) |
| Mortality | 15·6% (13·0–18·2) |
available case analysis: n=602;
available case analysis n=766
Empirical cumulative incidence curves for the REP TBI population calculated using the Kaplan-Meier method and categorised by age at first TBI are shown in Figure 2. A log rank test for trend indicated significantly decreased survival as age group at first TBI increased: (young adult (16–39 years); middle aged (40–64 years); elderly (>65 years) subgroups, p<0·001). No significant difference in empirical survivor function was evident in crude analyses examining the presence of extra-cranial injury, gender and ethnicity (log rank tests, p=0·34–0·43). However, there was some evidence of increased mortality rates associated with male gender after adjustment for age at TBI (stratified log rank test, p=0·08). Of note, a significant difference in survival was apparent between patients with mild and moderate/severe TBI in univariate analysis (log rank test p<0.01). However the effect of TBI severity was lost after adjustment for age (with or without gender, stratified log rank test, p=0.53–0.55). Survival curves categorised by gender, TBI severity and other patient characteristics, are presented in the web appendix.
Figure 2. Cumulative incidence of death categorised by age-group.
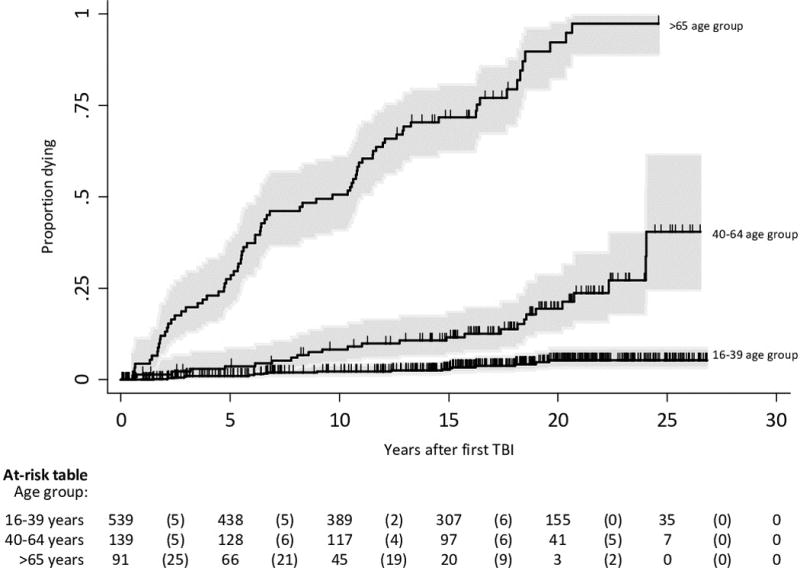
Shading indicates 95% confidence intervals. Vertical tick marks represent censored observations. The numbers at risk of death for each age group are shown in the table for every 5 years since TBI and the number of deaths within each interval is shown in parentheses
Parametric survival analysis
Primary model development
The primary analysis modelled the effect of age at first TBI on long-term survival. The Weibull model and Gompertz distributions were identified as potential candidate models based on linear log cumulative hazard plots and approximately linear smoothed log-hazard plots respectively. Proportionality of hazards with increasing age at TBI was confirmed graphically using log cumulative hazard, log hazard and kernel smoothed hazard plots. For both Gompertz and Weibull models age at TBI demonstrated an approximately quadratic relationship with log-hazard and was modelled using a first degree fractional polynomial of power 2. There was no evidence of change in distribution shape with increasing age, unexplained heterogeneity, or time-varying age coefficients.
A Gompertz model demonstrated the best fit to the REP data with lower information criterion statistics (AIC 529·7 v 531·9, BIC 543·6 v 545·8) and lower sum of squared errors (1,074 v 32,335) compared to the Weibull model. Survival curves from this model demonstrated satisfactory fit to the observed Kaplan-Meier curves for each age group, with almost all predicted survival probabilities (calculated for the median age of each age category) remaining within the 95% confidence interval bounds as demonstrated in Figure 3. All model diagnostics were unremarkable, with no evidence for any model misspecification, as detailed in the web appendix.
Figure 3. Gompertz model predicted survival probabilities compared to the observed Kaplan-Meier curves over the study period.
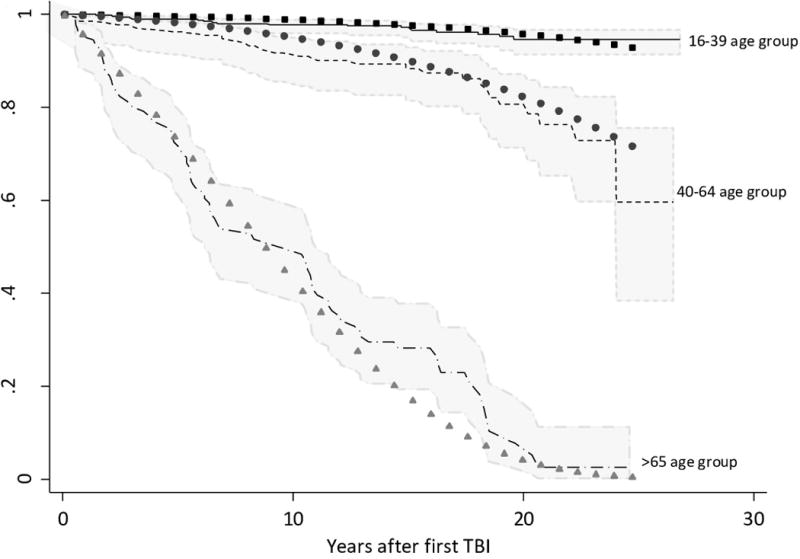
Lines represent the Kaplan-Meier derived survival function for young, middle aged and elderly age groups. 95% confidence intervals for this empirical survivor function are shaded in grey. Symbols present the predicted survivor function from the Gompertz model for the median age of patients within each age group (25, 52 and 79 years respectively).
Extrapolated TBI survival curves from the Gompertz model demonstrated lower relative survival compared with those derived from US general population cohort life tables from the year 2000. TBI patients also demonstrated decreased survival when compared to age and gender matched non-TBI REP controls in a Gompertz proportional hazards model (hazard ratio 1·47, 95% CI 1·15–1·87). Predicted median survival following a TBI at the ages of 25, 52 and 79 years were 45·8 years (95%CI 38·0–53·6), 30.6 years (95%CI 26·8–34·5) and 9·0 years (95%CI 7·8–10·3) respectively; compared to 53.5 (95%CI 40·7–66·4), 36·7 (95%CI 29·7–43·7) and 11.5 (10·0–13·0) for non-TBI controls.
The Gompertz TBI survival curves demonstrated plausible extrapolation of long term survival, based on theoretical considerations, comparisons with general population survival patterns, and correlation with published survival estimates following TBI. Gompertz distributions are well known to accurately represent long term survival in general adult populations and therefore has face validity based on a priori reasoning. Illustrative extrapolated survival functions for TBI patients at 25, 52 and 79 years of age were indistinguishable in pattern (showing a lower relative survival for patients following TBI) compared to age and gender matched non-TBI REP controls, Minnesota general population period life tables from the same period, and corresponding year 2000 US general population cohort survival curves (Figure 4). Median survival times reported in the current study were also not discordant with previously estimates reported by Ventura 2010, Felix-Harrison 2009 and 2004, and Strauss 1998. Full details of an evaluation of the model’s external validity are presented in the web appendix.
Figure 4. Comparison of predicted extrapolative estimates of survival following TBI sustained at certain ages using a Gompertz distribution compared to the year 2000 US general population cohort life table.
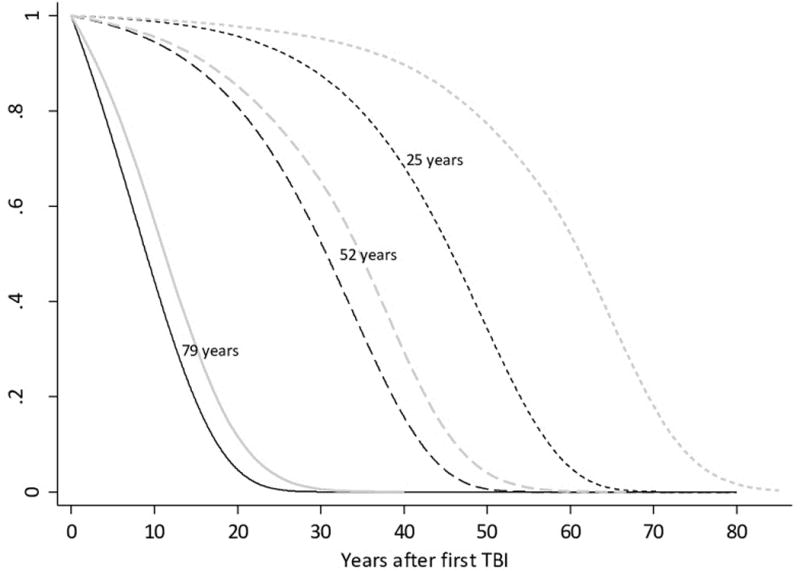
Black lines present the survival curves estimated from the Gompertz model following TBI sustained at illustrative ages. Grey lines indicate the corresponding US general population survival curves for patients of the same age based on cohort life table data.
Model updating
Survival curves derived from US general population cohort life tables demonstrated increasing ‘rectangularisation’ and rightward shift between 1930 and 2010. Gompertz distributions showed an excellent fit to the corresponding hazard functions with high adjusted R2 (0.99) and good visual fit of observed and fitted values. The shape and log scale parameters of these Gompertz distributions displayed a shallow concave relationship with time, best fitted by a quadratic function when using non-linear regression. Model fit was satisfactory with excellent visual concordance between fitted and observed values, high adjusted R2 (>0·999) and low sum of mean squared errors (0·0004 and 0·0003 for shape and log-scale parameters respectively). Full details on the modelling of secular trends in US general population survivorship, and the final updated Gompertz model for predicting long term survival after TBI, are described in the web appendix.
Additional analyses
Details of a secondary model including gender as an explanatory variable to predict survival, and the results of sensitivity analyses investigating non-informative censoring, are shown in the web appendix.
DISCUSSION
Summary of results
Survival after acute TBI followed a Gompertz distribution, with increasing age at head injury having an approximately quadratic relationship with the log-hazard of dying. Victims of TBI, surviving for at least 6 months post-injury, demonstrated a much higher ongoing mortality rate compared to non-TBI controls and the US general population. Gompertz distributions fitted to US general population cohort life tables manifested progressively increasing shape, and more negative log-scale parameters, between 1930 and 2010 allowing adjustments to the survival model to enable the prediction of survival in contemporary TBI patients.
Strengths and limitations
This study has a number of strengths. We studied a fully population based sample including the full spectrum of TBI severity and report one of the longest follow up periods of any published TBI cohort; providing a unique opportunity to describe survival after TBI and allow extrapolative estimates of life expectancy. Moreover, REP coding and data collection has demonstrated very high accuracy and reliability which should ensure that no incident cases of TBI have been missed.[5] Furthermore, the REP census enumeration has been validated, demonstrating excellent concordance with decennial US censuses and random-digit dialling surveys.[8] Finally, the parametric survival analysis methodology fully conformed to state of the art modelling recommendations.[10,11]
Conversely, there are several potential sources of systematic error, which could challenge the internal validity of results. Firstly, the analyses are predicated on non-informative censoring. However, it may be possible that patients leaving Olmsted County, with consequently short non-administrative censoring times, have a different prognosis to those who remained until to the end of the study. Reassuringly, such patients had very similar observed characteristics to remaining patients and sensitivity analyses simulating alternative assumptions for the distribution of survival times in patients lost to follow up demonstrate that the results were not susceptible to major change in plausible scenarios for censoring mechanisms (see web appendix).
Secondly, selection bias could also have arisen during the retrospective record review, with inclusion of non-TBI patients and consequent overestimation of survival. Cases who presented only with post concussive symptoms were eligible for enrolment and it is possible that non-specific symptoms were incorrectly attributed to trivial recent head trauma. The number of patients excluded for non-participation in the REP was negligible (<0.7%) and is unlikely to have influenced results.
Thirdly, study participants were included over a staggered 13 year accrual period and the reported results assume conditional survival probabilities are the same for subjects enrolled early and late in the study. Small cohort effects with increased longevity, arising from treatment differences or demographic trends, are likely in later birth cohorts within the recruited sample and could result in slightly underestimated survival in the final updated extrapolation model.
Lastly, although the REP sample size is not dissimilar to previously reported TBI cohorts, there were limited numbers in some subgroups, particularly non-mild TBI patients. This may have prevented detection of small, but clinically significant, differences in survival across TBI severities, decreased the power to observe important interactions between explanatory variables, or reduced the precision of model predictions.
Comparison to previous studies
A number of previous studies have investigated the long term survival of patients following TBI compared with the general population or non-head injury controls.[3,13–21] The finding of higher long term mortality following TBI has been consistently observed in a range of different settings (Europe, North America, Australasia), varying TBI populations (hospitalised, outpatient, and rehabilitation samples), contrasting study designs (population and non-population based studies, matched or un-matched samples), and alternative effect estimates (standardised mortality ratios, risk ratios, and odds ratios). Our results are concordant with these studies, but the novel use of parametric survival analysis, computing a hazard ratio between TBI and non-TBI patients, comparing TBI survival projections with general population life tables, and predicting post-TBI life expectancy are additional unique contributions to knowledge in this field.
Previous investigations have primarily had an epidemiological focus on whether TBI is an independent risk factor for premature death per se, often reporting effect estimates adjusted for multiple confounders. This approach has limited relevance to the prediction of post-acute mortality where crude estimates of absolute longevity are required conditional on certain demographic characteristics. A small number of studies have attempted to predict post-TBI life expectancy, projecting survival by adjusting abridged period life tables with age-specific mortality risks. This approach gives very coarse survival functions and is critically limited by the improbable assumption that contemporary cross-sectional mortality rates will continue for the lifespan of a subject, thus substantially underestimating longevity.
Interpretation of findings
To be relevant for the prediction of post-TBI life expectancy, the temporal and geographical generalisability of the final survival model requires close scrutiny. The external validity of the REP population has been studied in detail and the current findings are likely to be applicable to the upper mid-west and US white population.[6] Furthermore, Minnesota life expectancies are congruent with many Northern and Western European countries further increasing the applicability of results. Conversely, the proportion of female patients sustaining TBI was relatively high in the REP sample and the primary model, unadjusted for gender, may therefore be unrepresentative of other mixed head injury populations.
Model predictions are based on a historical population, possibly reflecting bygone survival experiences. The use of external data in clinical prediction models is well established and updating shape and scale parameters to reflect modern mortality rates should increase external validity.[22] However, the adjusted estimates are based on the unverifiable assumptions that future general population survivorship will continue to follow a Gompertz distribution with increasing shape and more negative log-scale parameters; and that secular trends in patients with TBI will mirror those in the general population. Additionally, recent cohort life tables, used to investigate secular trends in longevity, will be largely reliant on projected, rather than observed, survival at older ages.
The underlying reasons for the observed increase in long term mortality rates following TBI compared to the general population have not been fully delineated. Cognitive and emotional changes are common after TBI and could lead to behavioural and social problems, such as substance misuse or other risk-taking actions, associated with premature death.[23,24] However, epidemiological evidence to support this hypothesis is limited. It is also possible that head injury may directly lead to life-shortening medical and psychiatric disorders. Recent systematic reviews and population based cohort studies have concluded that TBI of any severity may predispose to Parkinson’s disease and epilepsy;[25,26] and severe TBI is associated with an increased risk of dementia.[27] Moreover, there is also a high incidence of psychiatric conditions, including schizophrenia, depression and bipolar disorders compared to the general population,[28] although whether this represents causation or association remains contentious.
Alternatively, the observed increase in mortality could be confounded by patient characteristics associated with both the propensity for TBI and increased early mortality. Patients with TBI are often young, low socio-economic status, male adults who may indulge in hazardous and unhealthy behaviours leading to lower life expectancy.[29] Likewise, co-morbidities, including degenerative and non-neurological disorders, are risk factors for sustaining TBI in the elderly.[2] The observed increase in premature mortality could therefore be independent of the TBI itself, but instead be dependent on the latent health behaviours and medical problems typical in victims of TBI. The finding in this REP cohort by Brown and colleagues (2013) that there was no significant difference in the risk of death between patients with TBI who survived 6 months, and controls who sustained non-head injuries, and likely to share similar pre-morbid characteristics, supports this position.[30] Our finding of a lack of association between TBI severity and life expectancy in patients surviving the acute insult, consistent with previous literature,[30,31] further corroborates this position.
Conclusions
Regardless of the aetiology, the clear finding of decreased life expectancy in patients surviving TBI has implications for clinical practice, public health, and health economic evaluations. Patients and families will be interested in the long term prognosis following their injuries. Knowledge of premature mortality, in conjunction with other reported findings of increased risk of substance misuse and suicide, may also highlight areas for preventative community interventions. Furthermore, these results do not support the common practice of using general population survivorship to extrapolate outcomes in TBI economic models.
Supplementary Material
Acknowledgments
Role of the funding source
The study was sponsored and funded by the Mayo Clinic. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Authors’ contributions
GF conceived and designed the study, performed the analyses, interpreted the findings and wrote the report. JR collected the data from the REP database. JM provided statistical assistance. AB supervised the project. JR, JM and AB also provided substantial contributions to the interpretation of results and writing of the manuscript. All authors gave final approval to the final version of the manuscript.
Contributor Information
Dr Gordon Ward Fuller, Emergency Medicine Research in Sheffield, Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA, UK.
Ms Jeanine Ransom, Email: ransom@mayo.edu, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota, USA, Telephone: +1 507 284 5537, Fax: +1 507 284 9542.
Dr Jay Mandrekar, Email: mandrekar.jay@mayo.edu, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota, USA, Telephone: +1 507 255 3116, Fax: +1 507 255 7696.
Professor Allen W Brown, Email: brown.allen@mayo.edu, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA, Telephone: +1 507 255 1625, Fax: +1 507 255 7696.
References
- 1.Soreide K. Epidemiology of major trauma. The British journal of surgery. 2009;96:697–698. doi: 10.1002/bjs.6643. [DOI] [PubMed] [Google Scholar]
- 2.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nature reviews Neurology. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Ventura T, Harrison-Felix C, Carlson N, Diguiseppi C, Gabella B, Brown A, Devivo M, Whiteneck G. Mortality after discharge from acute care hospitalization with traumatic brain injury: A population-based study. Archives of physical medicine and rehabilitation. 2010;91:20–29. doi: 10.1016/j.apmr.2009.08.151. [DOI] [PubMed] [Google Scholar]
- 4.Drummond MF. Methods for the economic evaluation of health care programmes. 3rd. Oxford: Oxford University Press; 2005. [Google Scholar]
- 5.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the rochester epidemiology project: Half a century of medical records linkage in a us population. Mayo Clinic proceedings. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: An illustration from the rochester epidemiology project. Mayo Clinic proceedings. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: The rochester epidemiology project (rep) medical records-linkage system. International journal of epidemiology. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The rochester epidemiology project. American journal of epidemiology. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishak KJ, Kreif N, Benedict A, Muszbek N. Overview of parametric survival analysis for health-economic applications. PharmacoEconomics. 2013;31:663–675. doi: 10.1007/s40273-013-0064-3. [DOI] [PubMed] [Google Scholar]
- 11.Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: Inconsistencies, limitations, and a practical guide. Medical decision making: an international journal of the Society for Medical Decision Making. 2013;33:743–754. doi: 10.1177/0272989X12472398. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham VJ. Non-linear regression techniques in data analysis. Medical informatics = Medecine et informatique. 1985;10:137–142. doi: 10.3109/14639238509010848. [DOI] [PubMed] [Google Scholar]
- 13.Baguley IJ, Nott MT, Howle AA, Simpson GK, Browne S, King AC, Cotter RE, Hodgkinson A. Late mortality after severe traumatic brain injury in new south wales: A multicentre study. The Medical journal of Australia. 2012;196:40–45. doi: 10.5694/mja11.10090. [DOI] [PubMed] [Google Scholar]
- 14.Himanen L, Portin R, Hamalainen P, Hurme S, Hiekkanen H, Tenovuo O. Risk factors for reduced survival after traumatic brain injury: A 30-year follow-up study. Brain injury: [BI] 2011;25:443–452. doi: 10.3109/02699052.2011.556580. [DOI] [PubMed] [Google Scholar]
- 15.McMillan TM, Teasdale GM, Weir CJ, Stewart E. Death after head injury: The 13 year outcome of a case control study. Journal of neurology, neurosurgery, and psychiatry. 2011;82:931–935. doi: 10.1136/jnnp.2010.222232. [DOI] [PubMed] [Google Scholar]
- 16.Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: A retrospective cohort study. Archives of physical medicine and rehabilitation. 2009;90:1506–1513. doi: 10.1016/j.apmr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Harrison-Felix C, Whiteneck G, DeVivo M, Hammond FM, Jha A. Mortality following rehabilitation in the traumatic brain injury model systems of care. NeuroRehabilitation. 2004;19:45–54. [PubMed] [Google Scholar]
- 18.Colantonio A, Escobar MD, Chipman M, McLellan B, Austin PC, Mirabella G, Ratcliff G. Predictors of postacute mortality following traumatic brain injury in a seriously injured population. The Journal of trauma. 2008;64:876–882. doi: 10.1097/TA.0b013e31804d493e. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliff G, Colantonio A, Escobar M, Chase S, Vernich L. Long-term survival following traumatic brain injury. Disability and rehabilitation. 2005;27:305–314. doi: 10.1080/09638280400018338. [DOI] [PubMed] [Google Scholar]
- 20.Shavelle RM, Strauss D, Whyte J, Day SM, Yu YL. Long-term causes of death after traumatic brain injury. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2001;80:510–516. doi: 10.1097/00002060-200107000-00009. quiz 517–519. [DOI] [PubMed] [Google Scholar]
- 21.Fazel S, Wolf A, Pillas D, Lichtenstein P, Langstrom N. Suicide, fatal injuries, and other causes of premature mortality in patients with traumatic brain injury: A 41-year swedish population study. JAMA psychiatry. 2014;71:326–333. doi: 10.1001/jamapsychiatry.2013.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW. Clinical prediction models: A practical approach to development, validation, and updating. New York; London: Springer; 2009. [Google Scholar]
- 23.Neyens DM, Boyle LN. Crash risk factors related to individuals sustaining and drivers following traumatic brain injuries. Accident; analysis and prevention. 2012;49:266–273. doi: 10.1016/j.aap.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Bjork JM, Grant SJ. Does traumatic brain injury increase risk for substance abuse? J Neurotrauma. 2009;26:1077–1082. doi: 10.1089/neu.2008.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marras C, Hincapie CA, Kristman VL, Cancelliere C, Soklaridis S, Li A, Borg J, af Geijerstam JL, Cassidy JD. Systematic review of the risk of parkinson’s disease after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Archives of physical medicine and rehabilitation. 2014;95:S238–244. doi: 10.1016/j.apmr.2013.08.298. [DOI] [PubMed] [Google Scholar]
- 26.Yeh CC, Chen TL, Hu CJ, Chiu WT, Liao CC. Risk of epilepsy after traumatic brain injury: A retrospective population-based cohort study. Journal of neurology, neurosurgery, and psychiatry. 2013;84:441–445. doi: 10.1136/jnnp-2012-302547. [DOI] [PubMed] [Google Scholar]
- 27.Sivanandam TM, Thakur MK. Traumatic brain injury: A risk factor for alzheimer’s disease. Neuroscience and biobehavioral reviews. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Orlovska S, Pedersen MS, Benros ME, Mortensen PB, Agerbo E, Nordentoft M. Head injury as risk factor for psychiatric disorders: A nationwide register-based follow-up study of 113,906 persons with head injury. The American journal of psychiatry. 2014;171:463–469. doi: 10.1176/appi.ajp.2013.13020190. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. The Journal of head trauma rehabilitation. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 30.Brown AW, Leibson CL, Mandrekar J, Ransom JE, Malec JF. Long-term survival after traumatic brain injury: A population-based analysis controlled for nonhead trauma. The Journal of head trauma rehabilitation. 2014;29:E1–8. doi: 10.1097/HTR.0b013e318280d3e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan TM, Teasdale GM, Stewart E. Disability in young people and adults after head injury: 12–14 year follow-up of a prospective cohort. Journal of neurology, neurosurgery, and psychiatry. 2012;83:1086–1091. doi: 10.1136/jnnp-2012-302746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


