Abstract
Background
This randomized, controlled trial assessed the impact of a tailored navigation intervention versus a standard mailed intervention on colorectal cancer (CRC) screening adherence and screening decision stage (SDS).
Methods
Primary care patients (n=945) were surveyed and randomized to a Tailored Navigation Intervention (TNI) Group (n=312), Standard Intervention (SI) Group (n=316), or usual care Control Group (n=317). TNI Group participants were sent colonoscopy instructions and/or stool blood tests according to reported test preference, and received a navigation call. The SI Group was sent both colonoscopy instructions and stool blood tests. Multivariable analyses assessed intervention impact on adherence and change in SDS at 6 months.
Results
The primary outcome, screening adherence (TNI Group: 38%, SI Group: 33%, Control Group: 12%), was higher for intervention recipients than controls (p=0.001 and p=0.001, respectively), but the two intervention groups did not differ significantly (p=0.201). Positive SDS change (TNI Group: +45%, SI Group: +37%, and Control Group: +23%) was significantly greater among intervention recipients than controls (p=0.001 and p=0.001, respectively), and the intervention group difference approached significance (p=0.053). Secondary analyses indicate that tailored navigation boosted preferred test use, and suggest that intervention impact on adherence and SDS was attenuated by limited access to screening options.
Conclusions
Both interventions had significant, positive effects on outcomes compared to usual care. TNI versus SI impact had a modest positive impact on adherence and a pronounced effect on SDS.
Impact
Mailed screening tests can boost adherence. Research is needed to determine how preference, access, and navigation affect screening outcomes.
Keywords: colorectal neoplasms, mass screening, health behavior, primary health care, decision making
Introduction
There will be an estimated 143,640 new cases of and 51,690 deaths from colorectal Cancer (CRC) in 2012.[1] Late diagnosis will account for many of CRC-related deaths. CRC screening can detect colorectal adenomas before they progress to cancer; and can find early cancer, when the disease can be more readily cured. The American Cancer Society (ACS) and the United States Preventive Services Task Force (USPSTF) encourage CRC screening by adults who are 50 or more years of age, asymptomatic, and are at average risk for CRC.[2] Colonoscopy every 10 years and annual stool blood test (SBT) use are the most frequently performed CRC screening tests in primary care, the setting in which most screening takes place.[3]
While CRC screening is increasing in the United States, rates lag behind those for breast and cervical cancer screening.[4] Healthy People 2020 has called for CRC screening rates of at least 70 percent.[5] Effective behavioral interventions must be identified and applied in primary care to achieve this goal. The Guide to Community Preventive Services has reported that patient-oriented mailed contacts and reminders, along with methods for increasing access or reducing structural barriers (e.g., patient navigation contacts) can increase CRC screening adherence.[6]
It has been suggested that providing patients access to their preferred CRC screening test is a new strategy that could further boost screening adherence. [7–9] To date, little research has been reported concerning the impact of CRC screening test preference on screening adherence. Recently, Inadomi et al. [9] found that primary care patients were more likely to screen when they were given access to a screening test that was in accord with their personal preference than when they were provided with tests they did not prefer. In a randomized, controlled trial of interventions designed to increase CRC screening, Siddiqui et al. [10] reported that screening decision stage (i.e., decided against, never heard of, not considering, undecided, decided to do), which is based on the Precaution Adoption Process Model, was a strong predictor of screening adherence. Decision staging, originally defined by the Precaution Adoption Process Model, has been incorporated into the Preventive Health Model (PHM). The PHM is an explanatory framework developed to identify predictors of health behavior and to guide interventions that aim to influence health behavior. In the PHM, personal representations related to given behaviors (e.g., salience and coherence, perceived susceptibility, worries and concerns, response efficacy, and social support) and decision stage are conceived of as factors that condition one’s preference for available behavioral alternatives, and influence the likelihood of action.
This paper presents findings from an NCI-funded randomized controlled trial designed to determine the impact of a preference-based tailored navigation intervention versus a standard intervention and usual care on CRC screening outcomes. The tailored navigation arm of the study provides adult primary care patients access to their preferred CRC screening test (colonoscopy and/or SBT), while the standard intervention provides access to both tests. To our knowledge, this study is the first time a preference-based navigation intervention has been used to promote CRC screening adherence.
Methods
Study Design and Participants
The study, which was conducted between 2007 and 2011, included 10 primary care practices affiliated with the Christiana Care Health System (CCHS) in Delaware that used a common medical record system (Centricity). The CCHS provides health care services across the spectrum of care and provides over two-thirds of all health care in Delaware. The system includes primary care, specialty referral care, tertiary care, home health care, and long-term care. The system includes two acute care hospitals with over 42,000 inpatient admissions and more than 125,000 visits to the emergency department annually, and a network of 15 primary care practices in family medicine, internal medicine, pediatrics, and obstetrics/gynecology.
Following procedures approved by the institutional review boards of Thomas Jefferson University and CCHS, the research team identified potential study participants in 10 sequential patient recruitment “waves,” each of which lasted for three to four months. For each wave, members of the research team searched computerized and manual searches of the electronic medical records to identify patients who were 50 to 79 years of age, had no prior diagnosis of colorectal neoplasia or inflammatory bowel disease, had visited one of the participating practices within the previous two years, had complete contact information, and were not compliant with American Cancer Society CRC screening guidelines.
Potentially eligible patients were mailed an introductory letter and were called to verify eligibility and obtain verbal consent. Patients who were not initially reached by telephone were considered for inclusion in later study waves. Patients who consented and enrolled were mailed a $20 gift card.
Baseline Telephone Survey
Potentially eligible patients were mailed an introductory letter and were called to verify eligibility, obtain verbal consent, and administer a baseline survey questionnaire. Patients who were not initially reached by telephone were considered for inclusion in later study waves. Patients who consented and enrolled were mailed a $20 gift card. The baseline (pre-randomization) survey collected data on participant sociodemographic characteristics, as well as a number of Preventive Health Model (PHM) items used in earlier research.[11,12]
PHM items were measured on a 5-point Likert-type scale (1 = strongly disagree to 5 = strongly agree) and items were averaged to construct 5 separate PHM scales (1 = low to 5 = high): perceived susceptibility to CRC (3 items, α = 0.84), screening salience and coherence, (3 items, α = 0.73), screening response efficacy (2 items, α = 0.48), screening worries and concerns (2 items, α = 0.77), and screening social support and influence (4 items α = 0.64). To complement these measures which have been previously reported to be valid across population subgroups,[13] we added a global PHM measure (14 items, Cronbach’s α = 0.74).
In accordance with prior research,[14,15] we determined each respondent’s test-specific SDS for both colonoscopy and SBT screening (1 = decided against, 2 = never heard of, 3 = not considering, 4 = undecided, or 5 = decided to do). We defined each participant’s preferred screening test as the one with the highest reported stage in a head-to-head comparison of test-specific SDS. For example, if a participant reported that s/he had decided to do colonoscopy screening, and was undecided about doing SBT, we identified colonoscopy as the preferred screening test. Equal preference was assigned when test-specific SDS was the same for both tests. We also defined each participant’s overall SDS as the highest stage reported for both tests. Overall SDS is considered to indicate an individual’s proximity to actual adherence. As participating practices did not routinely use other tests (e.g., flexible sigmoidoscopy, barium enema x-ray) to screen for CRC, we did not assess test-specific SDS with respect to those tests.
Study Groups and Interventions
Following completion of the baseline survey, participants were randomly assigned to one of three study groups: a Tailored Navigation Intervention (TNI) Group, a Standard Intervention (SI) Group, or a usual care Control Group. We included a usual care group to assess screening in the absence of intervention. A mailed intervention group was added, because prior research showed that this approach increased CRC screening compared to usual care[15]. We hypothesized that a preference-based navigation intervention would increase screening adherence significantly compared to a both a mailed intervention and usual care. In the study, we used random assignment to study groups, with blocking stratified by study wave. This strategy was implemented through electronic allocation files.
As mentioned above, the Control Group received usual care following randomization. The SI Group received a mailed informational booklet on CRC screening, a personalized letter that included a nurse contact telephone number that could be used to obtain information about scheduling a colonoscopy appointment, and an SBT kit. Participating primary care practices did not authorize the study nurse to schedule a colonoscopy appointment, but did allow the nurse to provide participants with the name and telephone number of a gastroenterology or surgical provider approved by the practice. In this manner, all SI Group participants were given access to both colonoscopy and SBT screening tests. A reminder letter was mailed at 30 days post-randomization.
TNI Group participants were also mailed the CRC screening booklet. In addition, participants were sent CRC screening test materials keyed to each individual’s preferred CRC screening test. Initially, test preference was determined using responses reported on the baseline survey. TNI Group participants who preferred colonoscopy received a letter and message page that included a nurse navigator contact telephone number. Participants with an equal preference for colonoscopy and SBT also were mailed a letter and message page with the nurse navigator contact telephone number, plus an SBT kit. Finally, participants who preferred SBT received a letter and message page with only an SBT kit. After this initial mailing, a trained navigator called each participant to verify and update (if necessary) the individual’s test preference, discuss any concerns or barriers that the participant may have, and encourage performance of the preferred test. For participants who reported a change in the preferred test from the one identified at baseline, the navigator arranged to have needed preferred test materials sent to the participant. Finally, reminders were sent to participants: those who preferred SBT or had equal colonoscopy/SBT preference received a reminder at 30 days after randomization, while those who preferred colonoscopy received a reminder at 90 days.
Endpoint Survey and Medical Records Review
Six months after randomization, each study participant was contacted by telephone for administration of an endpoint survey. Survey interviewers were blinded to the participants’ study group assignment. The endpoint survey collected data on PHM items (PHM scales) and PAPM variables (including test-specific SDS and overall SDS), as well as self-reported screening adherence (any type of guidelines-recommended CRC screening test performed and corresponding date). Twelve months after randomization, an endpoint medical records review was also conducted to assess screening adherence. Study research assistants who performed these reviews were masked to the participants’ study group assignment.
Study Endpoints and Analyses
The study’s primary endpoint was CRC screening adherence within 6 months after randomization. We also considered a 12-month window after randomization to assess delayed screening performance as an additional endpoint of interest. Screening adherence was defined as the performance of any CRC screening test recommended in guidelines that applied in 2007, the time the study was initiated (i.e., colonoscopy, SBT, flexible sigmoidoscopy, or barium enema x-ray). Any such screening that was reported at the endpoint survey or was found in the laboratory and medical records reviews was counted, as long as it was accompanied by a date within the target window.[12,14,15] A secondary study endpoint was defined as change in overall SDS between the baseline and the endpoint surveys. Change in overall SDS was measured at 6 months only, given the timing of the endpoint survey. Baseline-to-endpoint changes in participant perceptions about CRC screening (PHM scales) were additional secondary endpoints (see supplementary online table).
All analyses followed the intent-to-treat principle, with participants analyzed in the group that they were randomized to, irrespective of the contacts they may have received. Participants who did not complete an endpoint survey were excluded from the analyses of secondary outcomes. Analyses of screening were based on logistic regression. A Generalized Estimating Equations approach that accounted for potential within-practice clustering yielded results that were almost identical results to those obtained using ordinary logistic regression. Therefore, results of the latter are presented. According to a pre-specified data analysis plan, the main analyses controlled for study wave and practice, as well as participant age, sex, and race. According to a pre-specified data analysis plan, the main analyses controlled for study wave and practice, as well as participant age, sex, and race. Additional analyses that controlled for all baseline participant characteristics yielded very similar results (not presented). The analysis of baseline-to-endpoint change in overall SDS (any forward change versus no change or backward change) was based on logistic regression, and the analyses of the baseline-to-endpoint change in PHM variables were based on linear regression. The comparison of the TNI Group versus the SI Group was performed with alpha 0.05, while testing of each intervention group versus the Control Group was performed with alpha 0.025 for each.
The study was powered to detect differences of screening rates across the three study groups of the order of 10% (i.e., projected screening rates: 30% in the TNI Group, 20% in the SI Group, and 10% in the Control Group). Thus, the study’s sample size had 80% power for the comparison of TNI versus SI (alpha = 0.05), 88% power for the comparison of the SI versus Control (alpha = 0.025), and greater than 95% power for the comparison of the TNI versus Control (alpha = 0.025).
Results
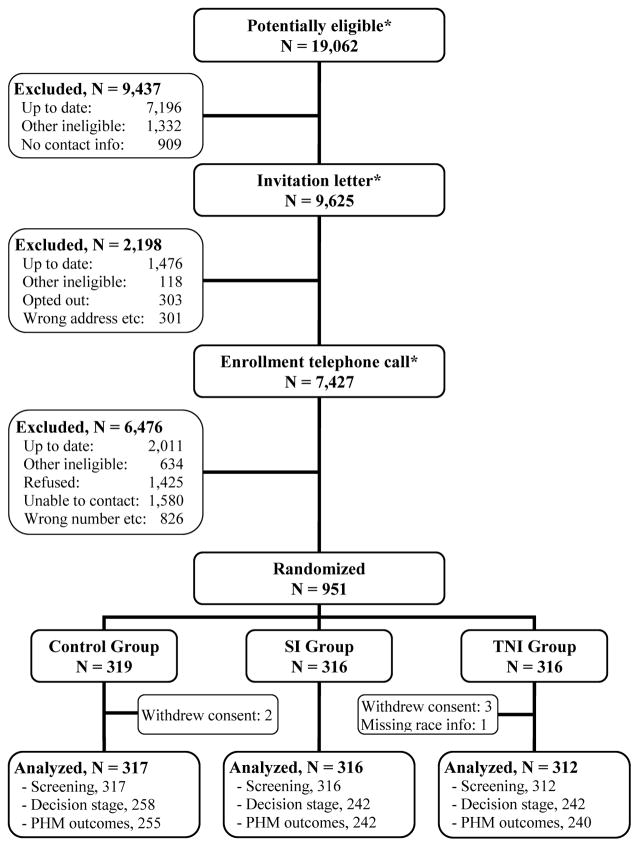
A total of 19,062 patients were screened for eligibility, and 951 enrolled in the study and were randomized (Figure 1). Six participants were excluded from the analyses, including five participants who withdrew consent and one participant for whom race was not reported. Endpoint medical records data were reviewed for 945 (99%) participants, and endpoint survey data were obtained for 742 (79%) participants.
Figure 1. Intervention trial schema.
SI: Standard Intervention. TNI: Tailored Navigation Intervention.
(*) Numbers prior to randomization include some patients more than once (patients who were not contacted or not enrolled initially, but who were recontacted in subsequent study waves).
Most participants were women, white, non-Hispanic, and married; had education beyond high school; and tended to have highly favorable perceptions about CRC screening (Table 1). The three study groups were comparable on participants’ baseline characteristics, with the exception of screening test preference at baseline.
Table 1.
Participants’ baseline characteristics (N = 945).
| Control Group (N = 317) | SI Group (N = 316) | TNI Group (N = 312) | |
|---|---|---|---|
| Age (years), n (%) | |||
| 50–59 | 223 (70) | 222 (70) | 213 (68) |
| 60–69 | 69 (22) | 70 (21) | 67 (21) |
| 70–79 | 25 (8) | 24 (10) | 32 (10) |
| Sex, n (%) | |||
| Female | 181 (57) | 201 (64) | 207 (66) |
| Male | 136 (43) | 115 (36) | 105 (34) |
| Race, n (%) | |||
| White | 248 (78) | 249 (79) | 243 (78) |
| Non-white | 69 (22) | 67 (21) | 69 (22) |
| Ethnicity, n (%) | |||
| Non-Hispanic | 310 (98) | 308 (98) | 301 (99) |
| Hispanic | 5 (2) | 7 (2) | 4 (1) |
| Education | |||
| High school or less | 131 (42) | 136 (43) | 134 (43) |
| Greater than high school | 183 (58) | 177 (57) | 175 (57) |
| Marital status, n (%) | |||
| Married (or living as married) | 189 (60) | 200 (63) | 197 (64) |
| Single/divorced/widowed | 127 (40) | 116 (37) | 113 (36) |
| Global PHM scale, n (%) | |||
| Low (1.0–3.0) | 54 (17) | 38 (12) | 43 (14) |
| High (3.1–5.0) | 261 (83) | 278 (88) | 267 (86) |
| Perceived susceptibility, n (%) | |||
| Low (1.0–3.0) | 267 (85) | 269 (85) | 269 (87) |
| High (3.1–5.0) | 48 (15) | 47 (15) | 40 (13) |
| Screening salience and coherence, n (%) | |||
| Low (1.0–3.0) | 11 (3) | 9 (3) | 11 (4) |
| High (3.1–5.0) | 304 (97) | 307 (97) | 299 (96) |
| Screening response efficacy, n (%) | |||
| Low (1.0–3.0) | 47 (15) | 43 (14) | 52 (17) |
| High (3.1–5.0) | 267 (85) | 272 (86) | 253 (83) |
| Worries and concerns, n (%) | |||
| Low (1.0–3.0) | 229 (73) | 227 (72) | 223 (72) |
| High (3.1–5.0) | 84 (27) | 89 (28) | 87 (28) |
| Social support and influence, n (%) | |||
| Low (1.0–3.0) | 48 (15) | 48 (15) | 47 (15) |
| High (3.1–5.0) | 264 (85) | 268 (85) | 262 (85) |
| Screening decision stage, n (%) | |||
| Decided against / Never heard of | 16 (5) | 14 (4) | 12 (4) |
| Not considering / Undecided about | 70 (22) | 56 (18) | 65 (21) |
| Decided to do | 231 (73) | 246 (78) | 235 (75) |
| Preferred screening test, n (%) | |||
| Stool blood test | 58 (18) | 75 (24) | 52 (17) |
| Equal preference (stool blood test / colonoscopy) | 151 (48) | 119 (38) | 125 (40) |
| Colonoscopy | 108 (34) | 122 (39) | 135 (43) |
Counts may not sum to each group’s total because of occasional missing data.
Non-whites were primarily African Americans, but also included some Asians and members of other racial groups (15 in Control Group, 6 in SI Group, 7 in TNI Group).
The study’s preference-based intervention strategy guided the mailed distribution of colonoscopy instructions and/or SBTs to the TNI Group at baseline as follows: SBTs only (17%), both colonoscopy instructions and SBTs (40%), and colonoscopy instructions only (43%). In addition, a navigation telephone call was delivered to 252 (81%) TNI Group participants. The mailed intervention strategy prescribed that all SI Group participants should be mailed both colonoscopy instructions and SBTs. Six-month endpoint survey completion rates were similar across the three groups (TNI Group: 78%, SI Group: 77%, Control Group: 81%, p = 0.298), and medical records were found and reviewed for all study participants.
Overall CRC screening adherence at 6 months (TNI Group: 38%, SI Group: 33%, Control Group: 12%, Table 2) was significantly higher in both intervention groups than in the Control Group (adjusted p = 0.001 for both comparisons). However, adherence was not significantly different in the TNI Group and the SI Group (adjusted p = 0.201). We also assessed study intervention impact on adherence at 12 months, in order to account for possible under-reporting of CRC screening data in electronic medical records due to factors such as colonoscopy scheduling difficulties, and delays in entering screening test results in the medical record. We found that screening rates at 12 months were higher across all three study groups than rates at 6 months (TNI Group: 43%, SI Group: 36%, Control Group: 18%, Table 2), but the pattern of intervention effects across the study groups was comparable. Adherence at 12 months was not significantly different between the TNI and SI Groups (p = 0.118).
Table 2.
CRC screening adherence at 6 and 12 months after randomization, by study group (N = 945).
| Control Group (N = 317) | SI Group (N = 316) | TNI Group (N = 312) | |
|---|---|---|---|
| Any screening within 6 months | |||
| n (%) | 38 (12%) | 103 (33%) | 117 (38%) |
| Adjusted OR (95% CI) | 1.00 | 3.69 (2.42, 5.64) | 4.60 (3.02, 7.02) |
| P-value | Ref | 0.001 | 0.001 |
|
| |||
| Any screening within 12 months | |||
| n (%) | 57 (18%) | 115 (36%) | 133 (43%) |
| Adjusted OR (95% CI) | 1.00 | 2.68 (1.83, 3.90) | 3.48 (2.39, 5.07) |
| P-value | Ref | 0.001 | 0.001 |
OR: odds ratio (adjusted for study wave and practice, and participant age, sex, and race).
CI: confidence interval.
In terms of forward change in SDS, most participants indicated that they had decided to do screening at baseline (TNI Group: 75%, SI Group: 78%, Control Group: 73%). At endpoint, we observed a marked shift from the lower decision stages to the decided-to-do or screened stages (TNI Group: 91%, SI Group: 87%, Control Group: 81%). Forward change in overall SDS (TNI Group: 64%, SI Group: 52%, and Control Group: 39%, Table 3) was significantly greater in the TNI and SI Groups than in the Control Group (adjusted p = 0.001 for both comparisons), and was more pronounced in the TNI Group than in the SI Group (adjusted p = 0.053).
Table 3.
Change in CRC screening decision stage, by study group (N = 733).
| Control Group (N = 255) | SI Group (N = 239) | TNI Group (N = 239) | |
|---|---|---|---|
| Change in screening decision stage | |||
| Any forward change, n (%) | 57 (22%) | 87 (36%) | 107 (45%) |
| Adjusted OR (95% CI) | 1.00 | 2.37 (1.50, 3.76) | 3.58 (2.28, 5.63) |
| P-value | Ref | 0.001 | 0.001 |
|
| |||
| Types of forward changes, n (%) | |||
| Undecided to Decided to do | 26 (46%) | 26 (30%) | 25 (23%) |
| Decided to do to Screened | 22 (39%) | 45 (52%) | 69 (64%) |
| Other forward changes | 9 (16%) | 16 (18%) | 13 (12%) |
OR: odds ratio (adjusted for study wave and practice, and participant age, sex, and race).
CI: confidence interval.
We performed secondary analyses to explore the impact of baseline screening test preference and study group on overall adherence at 6 months. When controlling for screening test preference, we found a statistically significant difference in overall adherence across the three study groups (p = 0.001). The results also suggested that intervention versus Control Group effects were stronger among participants who preferred SBT screening and were weaker among participants who preferred colonoscopy screening (p = 0.099 for the interaction between group and screening test preference). To better understand the influence of screening test preference, we assessed adherence within preference categories (Table 4).
Table 4.
CRC screening adherence at 6 months after randomization, by screening test preference and study group (N = 945).
| Test Preference | Control Group (N = 317) | SI Group (N = 316) | TNI Group (N = 312) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any test | SBT | CX | Any Test | SBT | CX | Any test | SBT | CX | ||||
|
|
|
|
||||||||||
| N | (%) | (%) | (%) | N | (%) | (%) | (%) | N | (%) | (%) | (%) | |
| Prefer SBTƗ | 58 | (7) | (3) | (3) | 75 | (39) | (35) | (4) | 52 | (42) | (42) | (0) |
| Equal preference± | 151 | (12) | (3) | (9) | 119 | (33) | (29) | (4) | 125 | (46) | (34) | (12) |
| Prefer CX≠ | 108 | (15) | (0) | (15) | 122 | (29) | (17) | (11) | 135 | (28) | (6) | (22) |
|
| ||||||||||||
| All preferences | 317 | (12) | (2) | (10) | 316 | (33) | (26) | (7) | 312 | (38) | (23) | (14) |
SBT: stool blood test. CX: colonoscopy.
Comparison of study groups for participants who preferred SBT: SI vs Control, OR = 9.96, 95% CI: 3.19 to 31.17, p = 0.001; TNI vs Control, OR = 11.27, 95% CI: 3.46 to 36.74, p = 0.001; TNI vs SI, OR = 1.13, 95% CI: 0.53 to 2.41, p = 0.750.
Comparison of study groups for participants who had an equal preference for colonoscopy and SBT: SI vs Control, OR = 3.69, 95% CI: 1.94 to 7.02, p = 0.001; TNI vs Control, OR = 6.08, 95% CI: 3.26 to 11.35, p = 0.001; TNI vs SI, OR = 1.65, 95% CI: 0.96 to 2.83, p = 0.069.
Comparison of study groups for participants who preferred colonoscopy: SI vs Control: OR = 2.31, 95% CI: 1.18 to 4.55, p = 0.015; TNI vs Control, OR = 2.43, 95% CI: 1.25 to 4.74, p = 0.009; TNI vs SI, OR = 1.05, 95% CI: 0.60 to 1.84, p = 0.882.
Among participants who preferred SBT screening at baseline, overall adherence was higher in both intervention groups than in the Control Group (p = 0.001 for both comparisons), but was not different in the TNI Group and SI Group (p = 0.750). Intervention versus control group effects were largely accounted for by increased SBT use in the intervention groups.
For participants who had an equal preference for colonoscopy and SBT screening, we also found that overall adherence was higher in the two intervention groups than in the Control Group (p = 0.001 for both comparisons), but adherence also tended to be higher in the TNI Group than the SI Group (p = 0.069). Intervention group versus Control Group differences were again due largely to increased SBT use. The TNI Group versus SI Group difference, however, was due to markedly higher colonoscopy performance and slightly higher SBT use in the TNI Group.
Finally, among participants who preferred colonoscopy screening, overall adherence was significantly higher in the intervention groups than the Control Group (p = 0.015 for SI and 0.009 for TNI), and adherence was comparable in the two intervention groups (p = 0.862). It should be noted that adherence among Control Group participants who preferred colonoscopy screening reflected only colonoscopy performance. The TNI Group versus the Control Group difference in overall adherence reflected higher colonoscopy performance and SBT use; while the SI Group difference from the Control Group reflected only higher SBT use.
Changes in participants’ perceptions about CRC screening (PHM scales) were generally very modest. With the exception of response efficacy, effects were comparable across the three study groups (results shown in supplementary online table).
Discussion
This study was designed to test the impact of a novel preference-based navigation intervention relative to a standard mailed intervention and usual care on overall screening adherence and change in SDS. In terms of the first outcome, we found that both intervention strategies were superior to usual care. Although screening adherence was higher in the TNI Group than in the SI Group, the difference was not statistically significant. In terms of findings reported elsewhere concerning the effect of patient navigation on CRC screening, the level of overall adherence observed in the TNI Group falls within the range of rates reported in studies that involved community primary care practice patient populations.[16–18] In addition, the overall adherence rate observed in the SI Group is comparable to rates reported elsewhere in response to mailed stool blood tests.[14,17,19] Our analyses suggest that incorporating test preference with navigation did not significantly increase screening adherence over the mailed intervention. Results of secondary analyses provide important insights into why the impact of the preference-based strategy used here was muted.
In accordance with study protocol, the standard and tailored intervention strategies tested in this study provided differential access to screening tests for the SI and TNI Group. That is, all SI Group participants were mailed both a SBT kit and colonoscopy instructions, while TNI Group participants were mailed screening materials that were keyed to their screening test preference. It appears that these differences in intervention group access may have attenuated the potential impact of preference-based navigation. The most compelling evidence supporting this view is found among study participants in the TNI Group and SI Group who had an equal preference for colonoscopy and SBT screening. That is, overall adherence in the TNI Group was 13 percentage points higher than in the SI Group; and this difference reflected higher colonoscopy and SBT screening use in the TNI Group than the SI Group. Further analyses of the impact of test preference, access to screening tests, and navigation could help inform the development of more effective personalized intervention methods.
This report also addressed study intervention impact on change in SDS. To our knowledge, there are no other reports in the literature that have assessed behavioral intervention impact on change in CRC screening SDS. [13] We found that all study groups exhibited forward change in SDS from baseline to endpoint; but both TNI Group and SI Group participants were significantly more likely to exhibit forward change in SDS than Control Group participants. Importantly, most of the positive change in SDS in the two intervention groups reflected movement from the decided-do-do screening stage to actual screening (TNI Group: 64% and SI Group: 52%), while only 39% of the controls moved from the decided-to-do stage to screening.
Other studies have assessed the impact of stage-based interventions on change in stage of readiness to engage in CRC screening. Vernon, et al, conducted an RCT, where constructs of the Transtheoretical Model (TTM) were used as the basis for a tailored website intervention.[20] The other two study groups included a public website intervention and a control group. No statistically significant differences were seen in screening adherence among the study groups. However, all three groups showed statistically significant positive stage movement from baseline to 6 months. Pignone et al. used the TTM in delivering a stage-based intervention in a two arm RCT intervention study.[21] Intervention participants viewed a video about CRC screening and then were exposed to an educational brochure based on the participant’s stage of change. Intention to screen, measured at baseline and endpoint, increased significantly between the intervention and control groups. Findings presented here concerning intervention impact on overall SDS contribute to the small but growing literature on the distribution CRC screening decision stage related to different theoretical models among study participants, and on the use of educational messages to move patients towards taking action.[14,15,17–20] A large proportion of intervention group patients were found to be in the decided-to-do stage and did not go on to screen. Research is needed to identify obstacles to this transition, and to determine how to help individuals move towards action.
Generalizability of findings from the current study may be limited by the inclusion of a relatively small proportion of non-white participants. Elsewhere, we have reported that exposure to a mailed intervention was associated with race-related disparity in CRC screening adherence that favored whites. [10] Findings reported here may be more applicable to a predominantly white patient population than to more diverse patient populations. Study results may also have been influenced by factors exogenous to the investigation. That is, the state of Delaware initiated an aggressive CRC screening promotion program during the time period the study was in effect. In addition, participating primary care practices engaged in varied initiatives during the study period that included efforts to boost CRC screening. As a result, study participants may have received messages that encouraged screening over and above those provided by the study. Given randomization, we assume that these effects had little impact on study group differences, but may have had a modest effect on overall screening rates.
In summary, findings from the current study indicate that mailing CRC screening materials was an effective strategy for increasing overall adherence and SDS among primary care patients, as compared to usual care. Tailored navigation also increased adherence and SDS relative to usual care. In terms of the intervention groups, we found that preference-based navigation did not significantly boost overall adherence to a level that was significantly higher than that achieved by mail, but increased participant performance of their preferred screening test in comparison to the mailed intervention, especially colonoscopy use. Furthermore, tailored navigation was more effective than the mailed intervention in moving patients forward in SDS. Navigators were not empowered to schedule colonoscopy appointments and primary care providers of participants were not engaged in the intervention process. These factors may have limited the effects of the navigation intervention compared to the mailed-only strategy. Research is needed to determine if an intervention strategy that maximizes screening test access, incorporates patient preference, and engages providers can achieve produce substantially higher screening rates than those that can be realized by a mailed strategy.
Supplementary Material
Acknowledgments
Sources of support: This research was supported by an NIH/NCI grant number CA116576 and a small grant from Olympus America. Stool blood tests were donated by Quest Diagnostics.
Footnotes
None of the co-authors has a conflict of interest to report for this manuscript.
Clinical Trial Registration #: NCT00617071
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. Atlanta, GA: American Cancer Society; 2012. [cited 2012 August 23]. Available from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. [Google Scholar]
- 2.U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Oct, 2008. AHRQ Publication 08-05124-EF-3. [Google Scholar]
- 3.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: Recommendations and practices, 2006–2007. American Journal of Preventative Medicine. 2009;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention. Colorectal Cancer Screening Rates. Atlanta, GA: CDC; 2011. [cited 2012 August 23]. Available from http://www.cdc.gov/cancer/colorectal/statistics/screening_rates.htm. [Google Scholar]
- 5.U.S. Department of Health and Human Services. Healthy People 2020 – Topics and Objectives: Cancer. Washington, DC: 2010. [cited 2012 August 23]. Available from: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=5. [Google Scholar]
- 6.Centers for Disease Control and prevention. The Guide to Community Preventive Services. Atlanta, GA: CDC; 2012. Cancer Prevention & Control: Client-oriented interventions to increase breast, cervical and colorectal cancer screening. [cited 2012 August 23]. Available from: http://www.thecommunityguide.org/cancer/screening/client-oriented/index.html. [Google Scholar]
- 7.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 Suppl 1):S10–6. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 8.Shokar NK, Carlson CA, Weller SC. Informed decision making changes test preferences for colorectal cancer screening in a diverse population. Ann Fam Med. 2010;8(2):141–150. doi: 10.1370/afm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Yunghui VL, et al. Adherence to Colorectal Cancer Screening: A Randomized Clinical Trial of Competing Strategies. Archives of Internal Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui AA, Sifri R, Hyslop T, Andrel J, Rosenthal M, Vernon SW, et al. Race and response to colon cancer screening interventions. Prev Med. 2011;52(3–4):262–264. doi: 10.1016/j.ypmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Myers RE, Jepson C, Ross E, Wolf T, Balshem A, Millner L, et al. Modeling Adherence to Colorectal Cancer Screening. Prev Med. 1994;23(2):142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- 12.Vernon SW, Myers RE, Tilley BC. Development and Validation of an Instrument to Measure Factors Related to Colorectal Cancer Screening Adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–832. [PubMed] [Google Scholar]
- 13.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2855–2861. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- 14.Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Cocroft J, et al. Tailored navigation in colorectal cancer screening. Med Care. 2008;46(9):S123–131. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- 15.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 16.Myers R, Vernon SW, Tilley B, Li S, Lu M. Predictors of colorectal cancer screening compliance in high-risk workers. In abstract presented at the American Society of Preventive Oncology; March 14–16; Houston, TX. 1999. [Google Scholar]
- 17.Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: A randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costanza ME, Luckmann R, Stoddard AM, Avrunin JS, White MJ, Stark JR, et al. Applying a stage model of behavior to change to colon cancer screening. Prev Med. 2005;42:707–719. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Lasser KE, Murillo J, Medlin E, Lisboa S, Valley-Shah L, Fletcher RH, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: Results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: Sometimes more is just the same. Ann Behav Med. 2011;41(3):284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening: A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.