Abstract
During exposure to chronic stress, some individuals engage in active coping behaviors that promote resiliency to stress. Other individuals engage in passive coping that is associated with vulnerability to stress and with anxiety and depression. In an effort to identify novel molecular mechanisms that underlie vulnerability or resilience to stress, we used nonbiased analyses of microRNAs in the ventral hippocampus (vHPC) to identify those miRNAs differentially expressed in active (long-latency (LL)/resilient) or passive (short-latency (SL)/vulnerable) rats following chronic social defeat. In the vHPC of active coping rats, miR-455-3p level was increased, while miR-30e-3p level was increased in the vHPC of passive coping rats. Pathway analyses identified inflammatory and vascular remodeling pathways as enriched by genes targeted by these microRNAs. Utilizing several independent markers for blood vessels, inflammatory processes and neural activity in the vHPC, we found that SL/vulnerable rats exhibit increased neural activity, vascular remodeling and inflammatory processes that include both increased blood–brain barrier permeability and increased number of microglia in the vHPC relative to control and resilient rats. To test the relevance of these changes for the development of the vulnerable phenotype, we used pharmacological approaches to determine the contribution of inflammatory processes in mediating vulnerability and resiliency. Administration of the pro-inflammatory cytokine vascular endothelial growth factor-164 increased vulnerability to stress, while the non-steroidal anti-inflammatory drug meloxicam attenuated vulnerability. Collectively, these results show that vulnerability to stress is determined by a re-designed neurovascular unit characterized by increased neural activity, vascular remodeling and pro-inflammatory mechanisms in the vHPC. These results suggest that dampening inflammatory processes by administering anti-inflammatory agents reduces vulnerability to stress. These results have translational relevance as they suggest that administration of anti-inflammatory agents may reduce the impact of stress or trauma in vulnerable individuals.
Introduction
Chronic stress can have a profound influence on mood, and can promote an increased risk for depression and anxiety.1, 2, 3 Engaging in passive coping strategies following exposure to stress may increase susceptibility to the adverse effects of stress,4, 5, 6, 7 whereas active coping and perceived controllability of stressful situations can mitigate the adverse effects of stress.4, 5, 6, 7 Chronic social defeat stress in rodents is an effective model to assess the impact of different coping strategies during stress on the subsequent effects of stress on behavior and endocrine function.8, 9 In our previous work, we showed that following several days of social defeat, male Sprague Dawley rats show a bimodal distribution in their latencies to be defeated. Short-latency (SL/vulnerable) rats are characterized by passive coping and increased anxiety-like and depressive-like behaviors, while long-latency (LL/resilient) rats appear to be protected against the adverse effects of defeat stress by engaging in ‘active coping’ including increased upright posturing and resistance to defeat.8, 10
The neural substrates underlying resilience or vulnerability to stress are the subject of much interest. Recently, we demonstrated that epigenetic modifications occur in the ventral hippocampus (vHPC) of SL/vulnerable rats.11 Lesions to the vHPC can decrease anxiety-like behavior in rats,12, 13, 14 and the vHPC has been suggested as a key mediator of anxiety.12, 13, 14, 15 Increased vHPC activity may underlie anxiety-like states such as those observed in rats vulnerable to social defeat.10 However, there have been no detailed investigations on the specific role of the vHPC in mediating resilience or vulnerability to stress or the processes in the vHPC that might mediate these individual differences. The goal of the studies described here was to determine the mechanisms in the vHPC that underlie the anxiety-like profile of rats vulnerable to social defeat.
In a previous study from our lab, we identified several miRNAs in the brain and blood that were differentially expressed in the brains of resilient and vulnerable rats.10 Here, we sought to examine miRNAs in the vHPC and subsequent pathways analyses to identify novel pathways involved in stress vulnerability and resiliency.
In the brain, evidence of inflammation in the brain has been observed in stress-related disorders, such as depression and anxiety. Brain inflammation can be detected via inflammatory processes such as increased microglia proliferation and recruitment to site of inflammation,16 increased expression of pro-inflammatory cytokines (such as Il16, vascular endothelial growth factor (VEGF) and HMBG1), increased blood–brain barrier (BBB) permeability, and vascular remodeling. Inflammation is thought to be the only established cause of vascular remodeling in the adult brain.17, 18 Vascular remodeling is characterized by an increase in BBB permeability, endothelial sprouting and angiogenesis,17, 18 and VEGF appears to be a primary mediator of these events following inflammation in the adult brain.17, 19, 20 Intriguingly, vascular remodeling occurs following sustained neural activity,21, 22, 23, 24 and while the mechanism of neuronal activity-induced vascular remodeling is unclear it is likely to involve release of cytokines, such as VEGF, which has been shown to induce vascular remodeling following neural activity.17, 19, 20, 24, 25, 26 Thus, if the vHPC shows sustained increases in neural activity as we hypothesized in stress vulnerable rats, increased vascular remodeling is also likely to occur, as well as an increase in inflammation, as assessed by expression of microglia, pro-inflammatory cytokines, angiogenic molecules and increased BBB permeability. Here, we hypothesized that vulnerability to stress would be characterized by vascular remodeling accompanying increased neural activation in the vHPC. We further tested this hypothesis by determining whether pharmacologically inducing inflammation in the vHPC produced vulnerability to stress and whether blocking inflammation promoted resiliency.
Materials and methods
Subjects
Male Sprague Dawley rats (275–300 g on experimental day 1) were used as control or intruder rats. Male Long-Evans retired breeders (600–800 g) were used as residents. The rats were purchased from Charles River (Wilmington, MA, USA) and were singly housed immediately upon entering the facility, and remained singly housed throughout all the experiments. The rats were placed on 12:12 h light:dark schedule (lights on at 0700 h) with food and water available ad libitum. The rats were randomly assigned to experimental conditions before starting the experiments. All the experiments were conducted between 0900 h and 1300 h. The rats were given at least 1 week to acclimate before any testing, during which time they were handled extensively. All the procedures were approved by the Children’s Hospital of Philadelphia’s Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Use of Laboratory Animals. The samples sizes were selected based on our previously published work.8, 10, 27
Social defeat paradigm and identification of SL/vulnerable and LL/resilient rats
The social defeat paradigm used was adapted from the model designed by Klaus Mizcek,28 and the specific paradigm used in these studies that allows the identification of SL/vulnerable and LL/resilient rats has been previously published.8, 9, 29, 30 Briefly, the male Sprague Dawley rats are used as intruders, and are placed into the home cage of a resident rat that has been pre-screened for aggression. On the display of a defeat posture, assessed by the intruder rat being in a submissive supine position for >2 s, the rats were separated by a wire mesh divider for the remainder of the time (30 min total). If attacks were made, and no defeat occurred, the rats were separated at 15 min, and remained in the resident’s cage from the time of separation until 30 min. Control (nonstressed) rats were placed in novel cages for 30 min during the time social defeat was occurring. To identify SL/vulnerable and LL/resilient rats (represented as only SL or LL in figures/figure legends, but SL/vulnerable and LL/resilient in text), the average latency of each rat over the course of 7 days was entered into an R script used to perform cluster analyses on averages of defeat latencies (code available at www.github.com/cookpa/socialdefeat). The analysis provides probabilities for resiliency, with 1 indicating resilience and 0 indicating vulnerability. During the screening, rats whose value was between 0.10 and 0.9 were removed from the experiments.
Social interaction
Social interaction testing was conducted using our previously published protocol.10 Briefly, the experimental rats were placed in a novel chamber and allowed 10 min to explore and interact with a well-socialized stimulus rat (male Sprague Dawley of similar age). The interactions were measured by the amount of time the experimental rat spent interacting with the stimulus rat. Percent time for social interactions was coded by an experimenter blind to group conditions.
Experiment 1: miRNA array to determine novel substrates of stress vulnerability and resiliency
The rats were exposed to social defeat for 7 days to reveal SL/LL phenotypes. The rats were killed 24 h after the last defeat by rapid decapitation, the vHPC was immediately dissected and flash frozen in 2-methyl-butane (Supplementary Table 1). The FlashTag Biotin HSR RNA labeling kit (Affymetrix, Santa Clara, CA, USA) was used to biotin label total RNA from blood and tissue samples. 250 ng of tissue-derived RNA was analyzed on Affymetrix GeneChip microRNA 3.0 arrays. Further analyses of miRNA data were described in detail in a previous publication from our group.10 RNA quality was assessed using standard 260/280 and 260/230 ranges for purity. We analyzed a total of 347 microRNAs after removal of probe sets whose hybridization intensities were too low to be reliable, based on deviation from linearity (mean log2 intensity value of less than 2). A Benjamini–Hochberg correction was applied to the results to control for the false discovery rate, and we considered any resulting microRNA with an adjusted P-value <0.1 to be significant. Ingenuity Pathway Analysis (IPA) software was used to assess biologically significant pathways predicted to be affected by the identified miRNAs.
Experiment 2: Expression of genes mediating vascular remodeling in the vHPC
To simultaneously profile a large number of genes involved in vascular remodeling, we used Qiagen’s RT2 Profiler PCR Array for Angiogenesis per the manufacturer’s instructions. This array targets 84 established inflammatory and angiogenic genes (a full list of results is included in Supplementary Table 2). Qiagen’s PCR Array Data Analysis Web Portal was used to quantify the PCR results using the ΔΔCT method and to perform statistical analyses. Data were quality controlled and normalized to the internal controls and housekeeping genes included in the arrays.
Experiment 3: Assessment of blood vessel density markers in the vHPC of rats following social defeat
To assess blood vessels in the brain, we examined three blood vessel markers including von Willebrand Factor (VWF), glucose transporter-1 (GluT1) and fluorescein isothiocyanate (FITC)-labeled blood vessels. For VWF and GluT1 assessment, the rats were exposed to social defeat for 7 days to reveal SL/LL conditions and killed 24 h after the final social defeat. The brains were sliced in 20 μm sections for immunohistochemistry (IHC) or punched and used for western blot analysis. The images from IHC were taken on a Leica DM4500 microscope. Two images were taken for each animal containing a whole slice each containing dHPC or vHPC (see Supplementary Figure 1 showing coordinates examined). An experimenter blind to conditions then counted the number of Iba1-, FosB- or VWF-immunopositive cells in the left and right side of each section. The average of these values for each region was statistically analyzed. GluT1 (Abcam, Cambridge, UK, Cat. No. ab652) expression was assessed by western blotting,27, 31 and each of the bands (45 kDa astrocytic isoform, and 55 kDa endothelial isoform) were normalized to β-actin density (Sigma, St. Louis, MO, USA, Cat. No. A2228). Secondary antibodies were goat anti-rabbit IRDYE 800CW and goat anti-mouse IRDYE 680RD. The membranes were analyzed on a Licor Odyssey Infrared Imager. The band intensity was measured by densitometry in ImageJ, and after normalization to β-actin bands were further normalized by percent of control values on each membrane to allow for intergel comparisons. The blood vessels were assessed by IHC for VWF28, 29 (Sigma, Cat. No. F3520) and the average number of VWF-immunostained blood vessels was counted by experimenters blind to the treatment groups. FITC labeling of blood vessels was conducted in a separate group of rats after the 7th d of social defeat according to a previously published protocol.32, 33, 34 Intracardiac perfusion of FITC reliably stains blood vessels in the brain (via staining endothelial cell nuclei33), and can be used to assess BBB permeability through measurement of extracellular FITC intensity following perfusion.32, 33, 34 After chronic social defeat, the rats were intracardiacally perfused with 50 ml of FITC (1 mg FITC per 10 ml phosphate-buffered saline) for 5 min and then brains were rapidly frozen in 2-methylbutane. Paraformaldehyde perfusion was not used following FITC perfusion as post-FITC perfusion with paraformaldehyde can cause diffusion of FITC and lead to false negative results.34 The brains were sectioned into 50 μm slices and immediately imaged on an Olympus Fluoview FV1000 Confocal Microscope using a FITC filter, and identical settings were used for each image to allow comparison of fluorescent intensity across images. Z-stack images were created in ImageJ.
Experiment 4: Measuring inflammatory markers and BBB permeability markers following social defeat
We examined BBB permeability with two different markers (FITC extravasation in vHPC and plasma levels of S100β) and assessed the quantity of microglia (number of Iba1-immunopositive microglia) as a more typical marker of inflammation.35, 36, 37, 38, 39, 40, 41 FITC extravasation, previously used to assess BBB permeability in several other studies32, 33, 34 was analyzed from images collected above in Experiment 3. To assess FITC extravasation, the Z-stack images were opened in ImageJ and a mask was created for the outline of each blood vessel to remove fluorescence located within blood vessels from the analysis. The amount of FITC outside of each vessel was analyzed using a MATLAB program (code available upon request). The program provided identification and quantification of fluorescent intensity within and outside of blood vessels in 1 μm intervals. Importantly, the fine spatial resolution of this program allowed us to look at FITC diffusion near each vessel, thus removing any potential bias caused by having differences in total blood vessel expression between subjects. Background was subtracted in each condition to place group plateaus at Y=0 for normalization. For a complementary analysis of BBB permeability, we examined plasma S100β by ELISA (Abnova, Taipei, Taiwan, Cat. No. KA0037). S100β is a soluble astrocytic protein that has been reliably shown to reflect increased BBB permeability.24, 36, 42, 43, 44, 45, 46, 47 and 48 Iba1 IHC (Wako, Richmond, VA, USA, Cat. No. 019-19741) was performed on sections from FITC-injected rats in this experiment, and the number of Iba1-expressing cells were counted in ImageJ by an experimenter blind to conditions.
Experiment 5: Assessing neural activity in brain regions involved in anxiety-like behavior and responses to stress
To assess long-term changes in neural activity, we performed IHC staining (as described in Experiment 3) neural activity marker FosB/ΔFosB (Santa Cruz Biotechnology Cat. No. sc-48), which is known to increase following chronic neural activity.49 We examined neural activity in the vHPC (both CA1 and CA3), basolateral amygdala and prefrontal cortex, because these regions are involved in responses to stress and in anxiety-like behaviors.12, 13, 14, 15, 50, 51 CA1 and CA3 subregions were assessed separately because there could be functional differences following different patterns of neural activity in CA1 and CA3 regions.52, 53, 54
Experiment 6: Testing the effects of delivering pro-inflammatory cytokines
VEGF164 was used to test whether increasing vascular remodeling and inflammation was sufficient to induce vulnerability in naive rats undergoing the resident intruder task. VEGF164 stimulates inflammation, increases BBB permeability and increases vascular density in the rat brain.17, 55, 56 Detailed surgical procedures for implantation of microinjection cannula have been published elsewhere.27, 31 Two hundred nanograms of rat recombinant VEGF164 (Cell Signaling, Danvers, MA, USA) or vehicle control (phosphate-buffered saline) was administered into the left lateral ventricle (from bregma: posterior 1.1 mm, lateral 1.5 mm, ventral 3.2 mm) in a volume of 1 μl at 1 h pre-defeat during 5 days of social defeat. On day 6, we tested the rats for social interaction (described above). The rats were killed 24 h after social interaction. IHC was performed for VWF, Iba1 and FosB/ΔFosB as described above.
Experiment 7: Testing the effects of anti-inflammatory drug treatment to promote resiliency
Naive rats were socially defeated for 4 days to identify SL/vulnerable rats. On days 5–7 SL/vulnerable rats and control rats were treated with 1 mg kg−1 meloxicam, a non-steroidal anti-inflammatory drug that may inhibit Cox-2 at some doses, or saline vehicle intraperitoneally 1 h before daily social defeat. On day 8, the rats were tested for social interaction, then immediately killed by rapid decapitation. We examined VWF and Iba1 to validate inhibition of inflammatory processes and vascular remodeling following treatment with meloxicam, The purpose of this experiment was to determine whether animals identified already as stress vulnerable could be treated with a peripherally administered anti-inflammatory drug to reduce central inflammation and behavioral vulnerable, as this finding would have great translational relevance. Preliminary experiments assessed other possible pharmacological approaches such as a VEGF antibody or VEGF receptor. These were difficult to solubilize and did not produce the expected anti-inflammatory effects and so were not considered further. Peripherally administered meloxicam inhibits inflammation in the hippocampus,57 and we hypothesized that meloxicam would decrease stress vulnerability.
Statistical analyses
For statistical comparisons of two groups, we used the Student’s t-test; and for comparisons of more than two groups, we used an analysis of variance followed by Bonferroni post hoc tests. An α level of 0.05 (two-tailed) was set for significance. More detail is provided in the figure legends. All statistical analyses were made in SPSS version 17, R (IBM, Armonk, NY, USA) or Graphpad Prism 5.
Results
Experiment 1: Identification of miRNAs associated with inflammation and vascular remodeling in the vHPC of SL/vulnerable rats
Two microRNAs were significantly different between SL/vulnerable and LL/resilient rats. The miR-455-3p levels were significantly higher in LL compared with SL rats and significantly positively correlated with defeat latency (Figures 1a and b). miR-30e-3p was significantly lower in LL/resilient rats compared with SL/vulnerable rats and significantly negatively correlated with defeat latency (Figures 1c and d). IPA revealed significant associations with inflammatory and immune processes implying that both miR-30e-3p and miR-455-3p may have a role in immunological responses. The SL/vulnerable condition was simulated in IPA by increasing miR-30e-3p within the network, which resulted in a predicted activation of the immune response (Supplementary Figure 2). When we examined the result of increased miR-30e-3p and decreased miR-455-3p, we identified vascular remodeling as being significantly upregulated in the SL-modeled condition (Supplementary Figure 3). The LL condition was simulated in IPA by increasing miR-455-3p and decreasing miR-30e-3p within the network, resulting in predicted inhibition of the immune response and inhibition of vascular remodeling (Supplementary Figure 4). Based on these findings, we predicted that vulnerability would be associated with increased vascular remodeling and inflammatory processes in the vHPC.
Figure 1.
miRNAs and RNAs associated with vascular remodeling increased in vHPC of SL/vulnerable rats. (a) LL/resilient rats exhibited increased levels of miR-455-3p compared with SL/vulnerable rats (n=5–7 per group; F2,15=11.49, P=0.0009). (b) There was a significant positive correlation between levels of miR-455-3p with average latency to be defeated (r=0.79, P<0.001). (c) LL/resilient rats exhibited reduced levels of miR-30e-3p in the vHPC compared with SL/vulnerable rats (n=5–7 per group; F2,15=11.12, P=0.01). (d) There was a significant negative correlation between levels of miR-30e-3p with average latency to be defeated (r=−0.86, P<0.001). For a and c, data represent mean±s.e.m. (e) Angiogenesis array data in SL/vulnerable and LL/resilient rats showing fold change from control indicated by the dashed line (error bars represent s.e.m.). *P<0.05. The letter 'a' denotes statistical significance relative to control and 'b' denotes statistical significance relative to SL. LL, long latency; miRNA, microRNA; SL, short latency; vHPC, ventral hippocampus.
Experiment 2: Changes in expression of vascular remodeling markers in vHPC of SL/vulnerable rats
To broadly assess markers of vascular remodeling, we examined vHPC tissue from SL/vulnerable and LL/resilient rats using an array designed to examine a variety of targets associated with vascular remodeling (Figure 1e). In SL/vulnerable rats, eight unique targets were different from controls, with six of these targets being upregulated (Cxcl1, Mapk14, Nrp2, Pdgfa, Pecam1, Hgf). The two downregulated targets were Ifng and Plau. Only three of the targets in LL rats were significantly different from controls. Figf and the repressors of angiogenesis Timp2 and Timp3 were increased in the LL rats relative to controls.58, 59, 60 Two targets were significantly different between SL/vulnerable and LL/resilient rats including Figf, which was increased in the LL/resilient rats, and the VEGF co-receptor Nrp2, which was increased in the SL/vulnerable rats.61 Taken together with results from our miRNA analysis and subsequent IPA modeling, these data strongly suggest that vulnerability is characterized by increased vascular remodeling in the vHPC.
Experiment 3: SL/vulnerable rats exhibit increased blood vessel density in the vHPC
To directly assess vascular remodeling, we examined three independent measures of blood vessel density and two independent measures of vascular permeability.
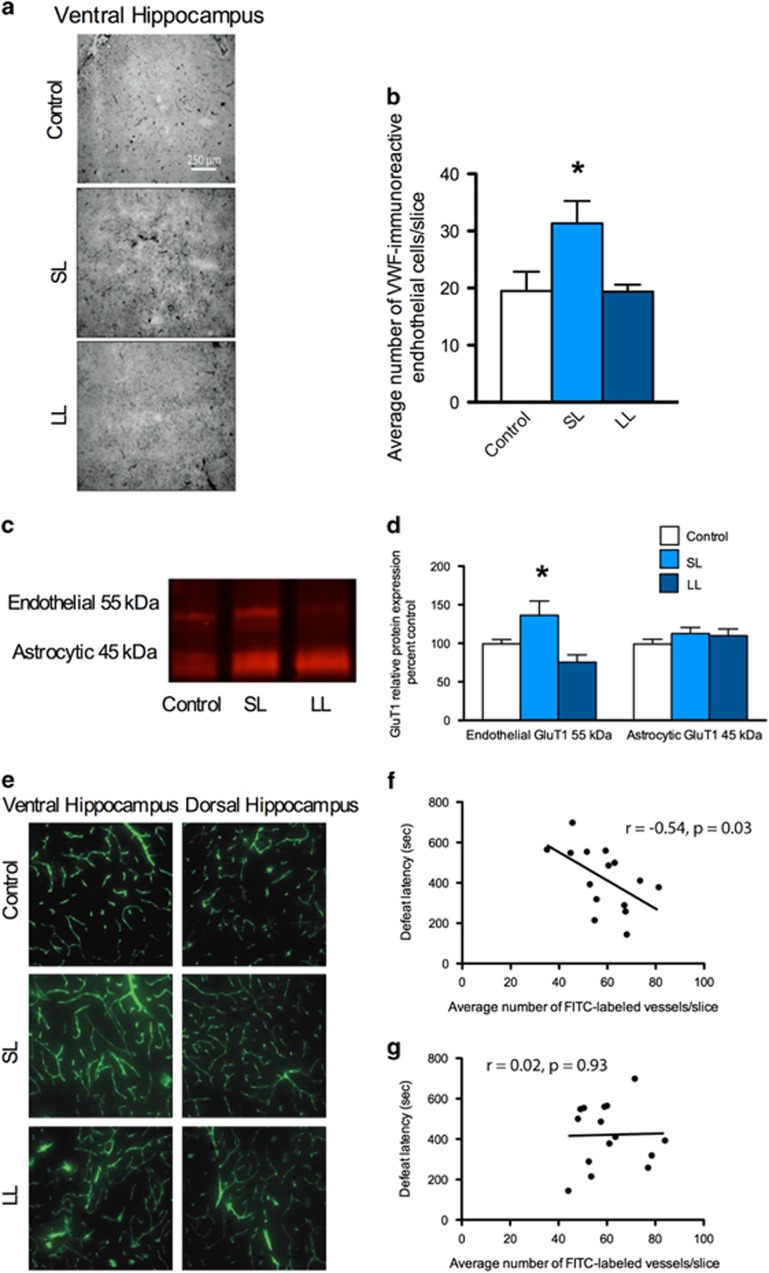
First, we assessed the density of VWF, which is a well-validated marker for blood vessels and vascular remodeling.62, 63, 64 The number of VWF-labeled vessels in the vHPC of SL rats was increased relative to the LL/resilient rats (Figures 2a and b) and VWF staining was similar between control and LL rats. There were no significant differences in VWF staining in the cingulate or prelimbic or infralimbic prefrontal cortex (Supplementary Figure 5). Second, we examined protein expression of glucose transporter-1 (GluT1; Figures 2c and d). There was an increase in expression of endothelial but not astrocytic GluT1 in the vHPC of SL/vulnerable rats, further suggesting a specific increase in brain blood vessel expression in SL/vulnerable rats. Third, in a separate cohort of rats perfused with FITC, there was a strong negative correlation (r=−0.54) between the number of FITC-labeled vessels in the vHPC and defeat latencies (Figures 2e and f). There was not a significant correlation between FITC-labeled vessels and defeat latencies in the dHPC (Figure 2g). Collectively, these data show that SL/vulnerable rats have increased blood vessel density in the vHPC.
Figure 2.
Increases in blood vessel density in rats vulnerable to stress. (a) Representative images of VWF immunohistochemistry. (b) VWF labeling was increased in the vHPC of SL/vulnerable rats relative to LL/resilient rats (n=6–7 per group; F2,3=6.02, P=0.006). There was no difference in the SL/vulnerable nor LL/resilient rats relative to the controls. (c) Representative western blot images of endothelial GluT1, which was (d) increased in the vHPC of SL/vulnerable rats relative to LL/resilient rats (n=6–7 per group; F2,17=5.65, P=0.013); there was no difference in GluT1 expression between SL/vulnerable nor LL/resilient rats relative to control rats. The astrocytic GluT1 was not altered. (e) Fifty micrometers confocal z-stacks of FITC-labeled blood vessels. (f) There was a negative correlation between latency to social defeat and average number of FITC-labeled vessels in the vHPC (n=15, r=−0.54, P=0.03). (g) There was not a significant correlation between latency to social defeat and the average number of FITC-labeled vessels in the dHPC (n=15). Data represent mean+s.e.m. *P<0.05. FITC, fluorescein isothiocyanate; LL, long latency; SL, short latency; vHPC, ventral hippocampus; VWF, von Willebrand Factor.
Experiment 4: Markers for inflammation increased in the vHPC of vulnerable rats
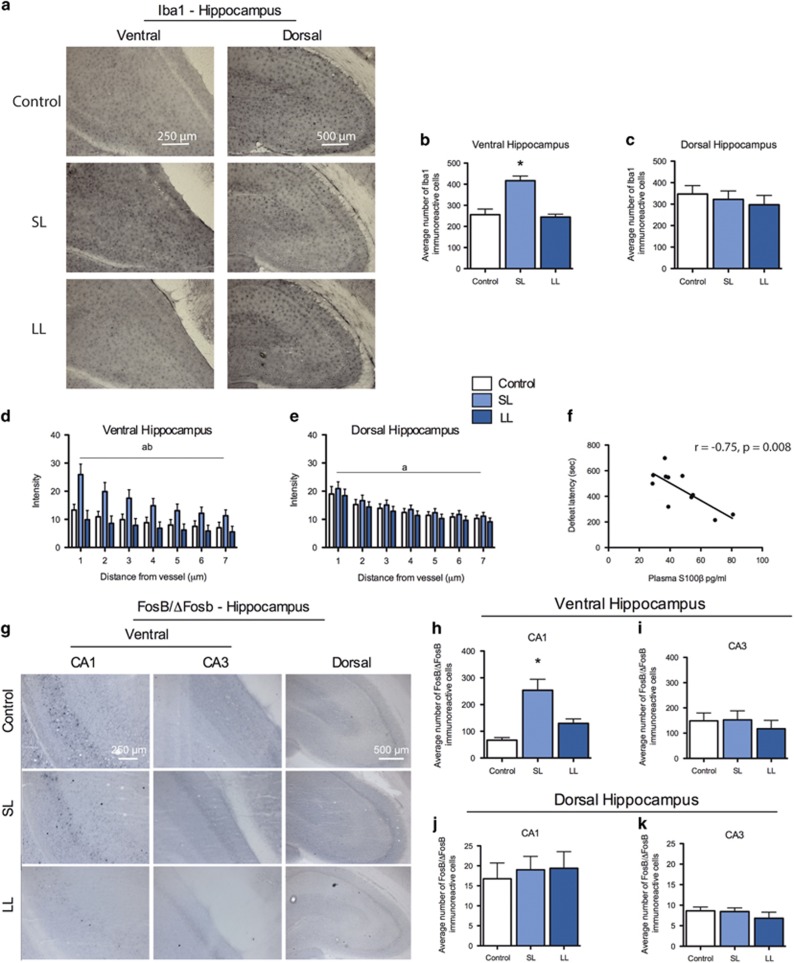
It is likely that the increased blood vessel density in the vHPC of vulnerable rats is a result of inflammation as inflammation is critical for inducing vascular remodeling in the adult brain.65 We assessed microglial activity in the vHPC and dHPC, BBB permeability, which are processes upregulated during inflammation.36, 46, 47 We found increased number of Iba1-immunopositive microglia in the vHPC of SL/vulnerable rats (Figures 3a and b), but not in the dHPC (Figure 3c). There was no difference in Iba1 expression between control and LL rats in either region of the hippocampus.
Figure 3.
Increases in inflammatory and neural activity in the vHPC of vulnerable rats. (a) Representative immunohistochemistry images for Iba1 showing regions analyzed in bar graphs. (b) Iba1 expression was increased in the vHPC of SL/vulnerable rats relative to control and LL/resilient rats (n=7; F2,19=20.78, P<0.0001). (c) There was no difference in expression of Iba1 in the dorsal hippocampus between any of the groups (n=8 per group). (d) Measurement of FITC extravasation showed FITC was significantly increased up to 7 μm away from blood vessels in SL/vulnerable rats relative to control and LL/resilient rats (main effect of group, F2,112=25.83, P=0.0001; and main effect of distance, F6,112=4.35, P=0.001). (e) There were no significant main effect of group between any groups in the dHPC but there was a significant main effect of distance (F6,119=10.76, P=0.0001). (f) Plasma S100β was negatively correlated with defeat latencies (n=11, r=−0.75, P=0.0009). (g and h) The long-term neuronal activity marker FosB/ΔFosB was increased in the vHPC CA1 region of SL/vulnerable rats (n=5) relative to control (n=8) and resilient LL rats (n=5; F2,15=18.16, P<0.0001). (i) There was no difference in FosB/ΔFosB expression in the vHPC CA3 region. (j and k) There was no difference in FosB/ΔFosB expression between groups in the CA1 (n=8 per group) or CA3 region (control n=7, SL n=8, LL n=8) of the dHPC (P>0.05). The letter 'a' denotes significant effect of distance and 'b' denotes significant group effect with SL/vulnerable higher than other groups. Data represent mean+s.e.m. *P<0.05. dHPC, dorsal hippocampus; FITC, fluorescein isothiocyanate; LL, long latency; SL, short latency; vHPC, ventral hippocampus.
To assess BBB permeability specifically in the hippocampus, we measured FITC extravasation in the vHPC and dHPC after intracardiac perfusion with FITC (same cohort of rats as in Experiment 3). The SL/vulnerable rats had increased FITC extravasation 1–7 μm away from vessels in the vHPC relative to control and LL rats, suggesting increased BBB permeability (Figure 3d). There was no difference between groups in dHPC FITC extravasation (Figure 3e). There was a strong negative (r=−0.75) between defeat latencies and plasma S100β concentration (Figure 3f), which further suggests that SL/vulnerable rats have increased BBB permeability. Taken together, the increased number of microglia and increased BBB permeability observed in the SL/vulnerable rats suggests that vulnerability to chronic stress is characterized by increased inflammatory processes in the vHPC.
Experiment 5: SL/vulnerable rats have increased neuronal activation in the vHPC following social defeat
Because blood vessel density increases in response to long-term alterations in neural activity (potentially via inflammatory mediators released by neurons or astrocytes) and inflammation,17, 24, 46, 66, 67, 68, 69 we predicted that SL/vulnerable rats have increased expression of the long-term neural activity marker FosB/ΔFosB in the vHPC.49, 70 The SL/vulnerable rats had significantly increased FosB/ΔFosB expression in the CA1 region (that is, a key output region) of the vHPC relative to control or LL rats (Figures 3g and i). There was no difference in FosB/ΔFosB expression in the CA3 of the vHPC (Figure 3i) or either the CA1 or CA3 region of the dHPC (Figures 3j and k, respectively). To assess whether the effects of social defeat were present in other brain regions associated with stress, we assessed FosB/ΔFosB in the basolateral amygdala and ventromedial prefrontal cortex (Supplementary Figure 6), and found no difference between groups in FosB/ΔFosB expression.
Experiment 6: Delivery of the pro-inflammatory cytokine VEGF164 promotes stress vulnerability
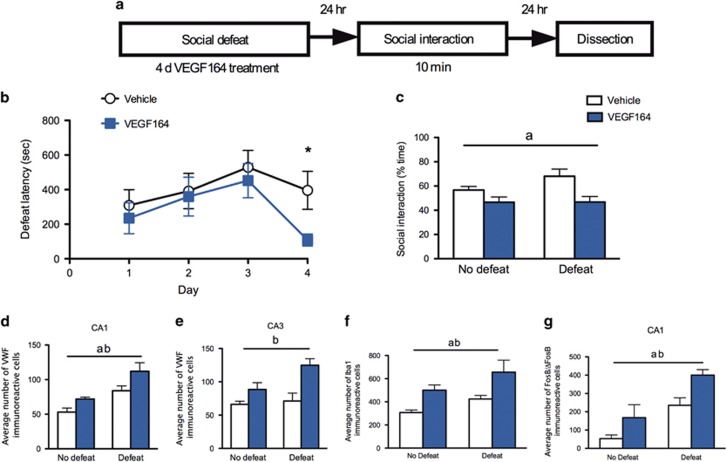
The results above suggest that inflammatory processes, neural activity and vascular remodeling are increased specifically in the vHPC in vulnerable relative to resilient rats. We next asked whether these increases directly produce vulnerability by pharmacologically inducing them using administration of the pro-inflammatory cytokine VEGF164 (experimental design in Figure 4a). Daily pre-defeat VEGF164 treatment decreased defeat latencies on day 4 (Figure 4b) and increased anxiety-like behavior in social interaction test relative to vehicle-treated controls (Figure 4c). Furthermore, VEGF164-treated rats had an increased number of VWF-positive vessels in the vHPC (Figures 4d and e), increased number of microglia (Figure 4f) and increased FosB/ΔFosB expression in the vHPC (Figure 4g), compared with rats treated with vehicle. These effects of VEGF164 were significant drug effects, indicating a significant effect of VEGF164 in both defeated and non-defeated rats; however, the effects were larger in defeated rats. These data suggest that inducing inflammation in the vHPC accompanied by increased vascular remodeling and neuronal activity was sufficient to produce vulnerable SL-like behaviors during and following social defeat. We next asked whether delivering an anti-inflammatory drug through a translationally relevant peripheral route of administration would reduce inflammatory processes in the vHPC and reduce vulnerability to stress.
Figure 4.
Induction of inflammation in the vHPC promotes stress vulnerability. (a) Experimental design layout. (b) Rats treated with VEGF164 had decreased defeat latencies on day 4 (control n=12; VEGF164 n=14; t24=2.66, P=0.01). (c) There was a significant main effect of VEGF on reducing social interaction behavior (n=7–8 per group). Subsets of brains were examined for the following measures. (d) There was a main effect of drug treatment (F1,13=9.4, P=0.009) and social defeat (F1,13=21.49, P=0.005) on increasing blood vessel density in the CA1 region of the vHPC (n=4–5 per group) with VEGF164 significantly increasing density as assessed by VWF and defeated animals having higher VWF expression than non-defeated rats. (e) There was a main effect of drug treatment on VWF expression in the CA3 region of the vHPC (n=4–5 per group; F1,13=17.2, P=0.001) with VEGF164-treated rats exhibiting increased densities. (f) There was a main effect of VEGF164 treatment (F1,10=15.01, P=0.003) and social defeat (F1,10=6.14, P=0.03) on Iba1 expression in the ventral hippocampus with increased Iba1 density in both VEGF-treated rats and in defeated rats. (g) There was a main effect of VEGF164 treatment on neural activity (as assessed by counting FosB/ΔFosB immunopositive cells) in the CA1 region of the vHPC (b: n=3–4 per group, F1,11=8.72, P=0.01). There was also a main effect of defeat on neural activity in the CA1 region with defeated rats exhibiting higher neural activity than non-defeated rats (F1,11=19.52, P=0.001). Data represent mean±s.e.m. and letters denote statistical significance in two-way analysis of variance (ANOVA; P<0.05). The letter 'a' denotes a main effect of defeat, and the letter 'b' denotes a main effect of drug treatment. *P<0.05. vHPC, ventral hippocampus; VWF, von Willebrand Factor.
Experiment 7: Peripheral administration of an anti-inflammatory drug decreases inflammatory processes in the vHPC and reduces stress vulnerability
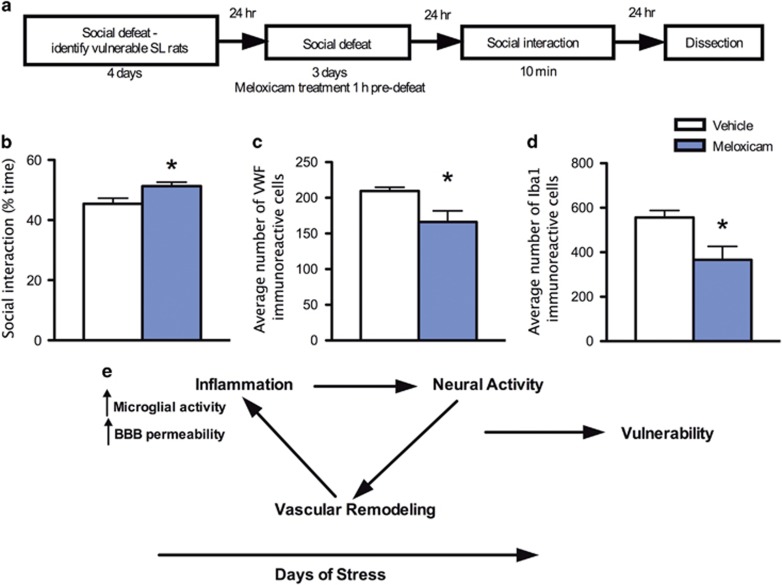
In this experiment, we focused on SL/vulnerable rats to directly test the hypothesis that reducing inflammation in these rats would reduce their vulnerability. Following 4 days of social defeat, the SL/vulnerable rats were identified by a cluster analysis as described in the 'Materials and methods' section. Once identified, the SL/vulnerable rats underwent three more days of social defeat with pretreatment with meloxicam or vehicle on each of these 3 days (Experimental design depicted in Figure 5a). This design was chosen because without social defeat first, there is unlikely to be inflammatory processes in which an anti-inflammatory drug could act to reverse vulnerability. Meloxicam was chosen because it has previously been shown to decrease hippocampal inflammatory markers.57 Meloxicam-treated rats exhibited increased social interaction time (Figure 5b) and these anxiolytic-type effects were accompanied by reductions in blood vessel density (Figure 5c) and number of microglia (Figure 5d) relative to defeated rats treated with vehicle. These data suggest that blocking inflammation during stress promoted a more resilient phenotype associated with reduced anxiety-like behaviors and reduced stress-induced vascular remodeling and microglia density in the vHPC.
Figure 5.
Inhibition of inflammation in the vHPC reduced indices of vulnerability in SL rats. (a) Experimental design layout. (b) Meloxicam decreased time spent in social interaction compared with vehicle (n=5 per group, t8=2.5, P=0.04), (c) reduced VWF staining (control n=4, meloxicam n=5, t7=2.39, P=0.05) (d) and reduced Iba1 staining (n=4 per group, t6=2.79, P=0.03) compared with vehicle treatment of SL rats. (e) Model demonstrating potential mechanism of neural activity and inflammation promoting vascular remodeling in the vHPC during stress and the interaction of these processes leads to vulnerability during stress (passive coping as indicated by shorter latencies to be defeated) and increased anxiety-related behaviors after stress. Data represent mean±s.e.m. *P<0.05. SL, short latency; vHPC, ventral hippocampus; VWF, von Willebrand Factor.
Discussion
Array analyses revealed vascular remodeling as a potential mediator of stress vulnerability
An unbiased approach assessing microRNAs in the vHPC was used to identify novel target genes and functional pathways underlying vulnerability to social defeat. Expression of miR-30e-3p in the vHPC was reduced in resilient animals and miR-455-3p was reduced in vulnerable animals compared with resilient animals. The major goal of using a broad miRNA-based analysis in the current study was to identify novel pathways that could be involved in differential responses to stress. It was these miRNA findings, and subsequent pathway analyses that indicated genes targeted by these microRNAs were associated with vascular remodeling and inflammation in the SL/vulnerable rats. Further analysis indicated that several genes associated with vascular remodeling (that is, Cxcl1, Mapk14, Nrp2, Pdgfa, Pecam1, Hgf) were increased in vHPC of vulnerable rats, and in LL rats two RNAs (Timp2 and Timp3) are known repressors of angiogenesis.58, 59, 60 Several of the targets identified in the angiogenesis array expectedly are also involved in inflammation, which is unsurprising as vascular remodeling in the adult brain depends on inflammatory mediators.17, 18 For instance, Cxcl1, PDGF-A and HGF encode potent pro-inflammatory molecules.71, 72, 73 Mapk14 encodes p38α mitogen-activated protein kinase, which is responsible for induction of pro-inflammatory cytokine release, such as tumor necrosis factor-α.74, 75 Future studies examining the specific role of these targets in SL/vulnerable rats may reveal valuable insights into the role of inflammation and vascular remodeling in stress vulnerability.
Collectively, these data suggested that inflammatory processes and vascular remodeling in the vHPC, which in the adult brain is dependent on inflammation,46, 66 underlie vulnerability to stress. Based on these data, we hypothesized that stress vulnerability would be characterized by vascular remodeling and by the activation of inflammatory processes in the vHPC.
Identification of increased vascular remodeling and inflammatory processes in the vHPC of stress vulnerable rats
We assessed vascular remodeling in the vHPC through three measures, all of which indicated increased density of blood vessels in SL/vulnerable compared with LL/resilient rats. Specifically, the density of blood vessels after intracardiac perfusion of FITC, and the densities of blood vessels stained with the endothelial marker VWF were increased in SL/vulnerable compared with LL/resilient rats. We observed increased expression of endothelial GluT1, another marker of blood vessels that targets glucose uptake sites. The increased expression of glucose transporter was specific to blood vessels as the expression of the astrocyte-specific glucose transporter was not changed by stress. It is important to note that we did not directly assess angiogenesis, the formation of new blood vessels in this study, however, increased vascular density and BBB barrier permeability were assessed, which strongly suggest the vascular remodeling did indeed occur in SL/vulnerable rats, as discussed in depth below.65, 76, 77Together, these results demonstrate that vulnerability to stress is characterized by vascular remodeling in the vHPC such that blood vessel density is increased. One consequence of this is potentially increased glucose availability to the vHPC through increased GluT1 expression in SL/vulnerable rats, which could provide metabolic support following increased neural activity (see below).78
Previous research has demonstrated that morphometric and volumetric changes occur in the brain in response to stress-related disorders, such as increased size of the basolateral amygdala79 and decreased hippocampal volume80, 81 as assessed by changes in neuronal and glial density.82 However, there has been little to no investigation as to whether morphometric changes in blood vessels occur as well.
During vascular remodeling, BBB permeability is increased due to inflammatory molecules that are critical for vascular remodeling.17, 46, 66 In this study, we observed increased FITC extravasation in the vHPC of vulnerable rats, as indicated by the presence of FITC diffusion at greater distances from blood vessels, suggesting increased permeability of the BBB in SL/vulnerable rats. This was confirmed by the finding of increased plasma concentrations of S100β, a soluble astrocytic protein increased in plasma following BBB permeability, and is a well-validated marker of BBB permeability.42, 43, 44, 45 Increased plasma S100β is a clinically relevant finding as it may suggest this measure could be novel biomarker to assess vulnerability in response to traumatic events. Finally, increased number of microglia in the vHPC was observed in vulnerable rats. This suggests increased inflammation in these rats as Iba1 expression is increased in microglia following inflammation and the number of Iba1-immunopositive cells increases following inflammation.35, 36, 37, 38, 39, 40 Upregulation in microglia activity and expression can lead to increased production and release of pro-inflammatory cytokines46, 66, 83 from microglia, which promotes endothelial cell proliferation.84, 85 Iba1 is constitutively expressed in the microglia,86 however increased number of Iba1-positive microglial has been observed in brain regions following trauma, likely as both a response to inflammation, but also as a triggering event in inflammation via the release of cytokines from active Iba1-positive microglia.35, 36, 37, 38, 39, 40 This increase in microglia has been associated with an increase in microglial proliferation and recruitment towards areas of inflammation.37 In some studies, an increase in Iba1-positive expression did not change following inflammation, while a change in microglia morphology from resting to active states has been observed,87 thus there is some controversy as to whether Iba1 expression accurately reflects microglial activation. The LL/resilient rats were protected against the vascular remodeling and inflammatory events observed in vulnerable rats because the vascular markers examined in LL/resilient rats were not different from control rats. This is an important distinction: we propose that resiliency is a robust protection against the effects of stress and hence resilient rats are likely similar to controls. Together, the results suggest that inflammatory processes and vascular remodeling in the vHPC characterize vulnerability to chronic stress.
Vascular remodeling in the vHPC likely has a functional relationship with neural activity and together, neurons, microglia and blood vessels constitute the basic functional unit in the brain known as the 'neurovascular unit'.88 We observed increases in neural activity as assessed by an increase in the number of FosB/ΔFosB cells in the vHPC in vulnerable rats. The vascular, inflammatory and neural components can influence each other’s activity. For example, many inflammatory cytokines, released during stress can affect neural activity, as well as trigger vascular remodeling.1, 3, 26, 30, 46, 89, 90, 91 Conversely, neural activity can promote alterations in the vascular system by releasing pro-inflammatory cytokines, which may effectively remodel the vasculature to support neural activity.24, 26, 92 Thus, greater neural activity may be triggering microglial activity and vascular remodeling with daily stress in SL/vulnerable rats, which could produce increased anxiety-like behavior in SL/vulnerable rats, consistent with the known role of the vHPC in mediating anxiety-like behaviors13, 93 (See Figure 5e).
Increased inflammatory processes and vascular remodeling in the vHPC of rats vulnerable to chronic stress is critical for stress vulnerability
To test the hypothesis that vascular remodeling and inflammatory processes in the vHPC are critical in promoting stress vulnerability, we used pharmacological approaches to reduce or elevate inflammatory processes and examine resultant effects on behavior. The pro-inflammatory cytokine VEGF164 was sufficient to induce many of the inflammatory effects found in the vHPC of SL/vulnerable rats, including increased Iba1 expression, increased blood vessel densities and increased neural activation in the vHPC. Importantly, VEGF was also sufficient to induce decreases in latency to be defeated and increases in anxiety-like behaviors in the social interaction test, characteristic of SL/vulnerable rats.10 These data mirror previous observations that VEGF effects have been linked to mood disorders in several other studies.89, 94, 95
Previous research has shown that VEGF can induce neuroinflammation, and VEGF is considered to be one of the major initiators of inflammation, BBB permeability and vascular remodeling in the adult brain.17, 66, 96, 97, 98 Typically, the pro-inflammatory effects of VEGF precede its effects on vascular remodeling.17 Thus, we propose that increasing VEGF in the vHPC during repeated defeat promoted inflammatory processes and subsequently restructured the cerebral vasculature to allow increased neural activity by increasing delivery of nutrients necessary to sustain neural activity in response to stress. These effects of VEGF also occurred in non-defeated rats suggesting that VEGF treatment was sufficient to produce a vulnerable state even in the absence of stress. Our data might also explain the positive effects of VEGF in the dorsal hippocampus in other studies20, 99 in that increasing vascular remodeling in the dorsal hippocampus, a region with functions distinct from those of the vHPC, could promote neural activity and thus attenuate depression-like behaviors in rodents. The finding that vascular remodeling was necessary for VEGFs effects also fits the timeline in which effects of VEGF were observed, whereby it took 4 days for a significant effect of VEGF treatment to emerge. Thus, VEGFs effects were unlikely to be via an immediate effect such as direct modulation of neural activity or acute induction of inflammation.
To test whether the converse, a decrease in inflammatory processes, could reduce vulnerability, we treated rats already identified as vulnerable with meloxicam. Meloxicam treatment both promoted behavioral resiliency in SL/vulnerable rats by increasing social interactions, and prevented the increase in microglia observed in vehicle-treated rats (suggesting decreased inflammation) and vascular remodeling in SL/vulnerable rats. The effects of meloxicam were modest, likely because meloxicam was peripherally administered, so its anti-inflammatory effects at the vHPC may have been reduced. Future studies aimed at directly reducing inflammatory roles in the vHPC may offer greater insight into the direct role of the vHPC in mediating individual differences in response to stress. Nonetheless, it is intriguing that administrating an anti-inflammatory drug peripherally can have significant effects on reducing stress vulnerability. It is unclear whether the inflammatory effects observed in SL/vulnerable rats are generated in the central nervous system or periphery, and it is possible that many of the inflammatory actions observed in the brain from SL/vulnerable rats originates from peripheral cues, such as peripherally released cytokines or immune cells. In summary, the results with VEGF and meloxicam together suggest that inflammatory processes and blood vessel remodeling in the vHPC are critical substrates underlying the emergence of vulnerability in stressed animals. In particular, the results with meloxicam suggest that anti-inflammatory drugs or treatments that block vascular remodeling during stress may prevent or reverse the adverse impact of stress in vulnerable individuals.
Conclusions and future directions
In this study, we identify the vHPC as a novel brain region important in regulating vulnerability to chronic social defeat. The effects observed here were specific to the vHPC, rather than the dHPC or medial prefrontal cortex. Although the vHPC is known to have a role in mediating anxiety-like behavior,13, 93 this, to the best of our knowledge, is the first demonstration that the vHPC is important for mediating individual differences in response to chronic stress that lead to a vulnerable phenotype. We suggest the anxiety-like behaviors in vulnerable rats derive from increased neural activity in the vHPC and vascular remodeling likely induced by inflammatory mediators that sustain the neural activity. Further studies are required to definitively determine the temporal sequence of these events. These data demonstrate the importance of inflammatory processes and blood vessel remodeling in the vHPC for the development of vulnerability to stress. Further, these results suggest that dampening inflammatory processes by administering anti-inflammatory agents reduces vulnerability to stress. These results have translational relevance as they suggest that administration of anti-inflammatory agents may reduce the impact of stress or trauma in vulnerable individuals.
Acknowledgments
We thank Lucia Peixoto for helpful analyses on the miRNA data. We thank Victoria Siu, Joel Wilson and Hannah Nam for assistance with data collection in the behavioral tests in some experiments. We thank Doug Coulter for the use of his confocal microscopy facilities. This work was supported by the Defense Advanced Research Projects Agency (DARPA) and the U. S. Army Research Office under grant number W911NF1010093 to SB.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Hammen C. Stress and depression. Annu Rev Clin Psychol 2005; 1: 293–319. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 891–907. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Dass SA, Booth J, Suraev A, Vyas A, McGregor IS. Active coping toward predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Horm Behav 2014; 66: 561–566. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Maier SF, Lyons DM, Raskind MA. The neurobiology of the stress-resistant brain. Stress 2011; 14: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun 2008; 22: 662–667. [DOI] [PubMed] [Google Scholar]
- Ono Y, Lin HC, Tzen KY, Chen HH, Yang PF, Lai WS et al. Active coping with stress suppresses glucose metabolism in the rat hypothalamus. Stress 2012; 15: 207–217. [DOI] [PubMed] [Google Scholar]
- Wood SK, Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress 2015; 1: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK. Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Curr Neuropharmacol 2014; 12: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S et al. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience 2015; 305: 36–48. [DOI] [PubMed] [Google Scholar]
- Kenworthy CA, Sengupta A, Luz SM, Ver Hoeve ES, Meda K, Bhatnagar S et al. Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus. Neuroscience 2014; 264: 88–98. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci 2004; 118: 63–78. [DOI] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 2010; 65: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LW, Stegeman RA, Leimgruber NK, Gierse JK, Abdel-Meguid SS. Preliminary crystallographic study of glycosylated recombinant human renin. J Mol Biol 1989; 210: 239–240. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 2004; 28: 273–283. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Ransohoff RM, Cai N, Zhang Q, Martin FJ, Wei T et al. VEGF-mediated inflammation precedes angiogenesis in adult brain. Exp Neurol 2004; 187: 388–402. [DOI] [PubMed] [Google Scholar]
- Muramatsu R, Takahashi C, Miyake S, Fujimura H, Mochizuki H, Yamashita T. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med 2012; 18: 1658–1664. [DOI] [PubMed] [Google Scholar]
- Krum JM, Khaibullina A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: roles for VEGF in brain repair. Exp Neurol 2003; 181: 241–257. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 2007; 104: 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone BJ, Lacoste B, Gu C. Neuronal and vascular interactions. Annu Rev Neurosci 2015; 38: 25–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 1990; 87: 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Gu C. Control of cerebrovascular patterning by neural activity during postnatal development. Mech Dev 2015; 138(Pt 1): 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 2014; 15: 43–53. [DOI] [PubMed] [Google Scholar]
- ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 2014; 15: 6453–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 2004; 36: 827–835. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S et al. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 2011; 152: 4738–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology 1979; 60: 253–259. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 2010; 151: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry 2015; 78: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-beta(1-42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimers Dis 2012; 30: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm KA, Tobet SA. Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: influence of fetal glucocorticoid excess. Brain Struct Funct 2014; 220: 2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Morita S. A new method for visualization of endothelial cells and extravascular leakage in adult mouse brain using fluorescein isothiocyanate. J Neurosci Methods 2011; 202: 9–16. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Bredno J, Wendland M, Derugin N, Ohara P, Wintermark M. High and low molecular weight fluorescein isothiocyanate (FITC)-dextrans to assess blood-brain barrier disruption: technical considerations. Transl Stroke Res 2011; 2: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016; 64: 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HK, Ji K, Min K, Joe EH. Brain inflammation and microglia: facts and misconceptions. Exp Neurobiol 2013; 22: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blon D, Hoornaert C, Daans J, Santermans E, Hens N, Goossens H et al. Distinct spatial distribution of microglia and macrophages following mesenchymal stem cell implantation in mouse brain. Immunol Cell Biol 2014; 92: 650–658. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun 2010; 24: 1058–1068. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318. [DOI] [PubMed] [Google Scholar]
- Klein R, Roggendorf W. Increased microglia proliferation separates pilocytic astrocytomas from diffuse astrocytomas: a double labeling study. Acta Neuropathol 2001; 101: 245–248. [DOI] [PubMed] [Google Scholar]
- Piskunov A, Stepanichev M, Tishkina A, Novikova M, Levshina I, Gulyaeva N. Chronic combined stress induces selective and long-lasting inflammatory response evoked by changes in corticosterone accumulation and signaling in rat hippocampus. Metab Brain Dis 2016; 31: 445–454. [DOI] [PubMed] [Google Scholar]
- Watson P, Shirreffs SM, Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1689–R1694. [DOI] [PubMed] [Google Scholar]
- Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev 2016; 68: 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma 2009; 26: 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargerstock E, Puvenna V, Iffland P, Falcone T, Hossain M, Vetter S et al. Is peripheral immunity regulated by blood-brain barrier permeability changes? PLoS ONE 2014; 9: e101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 2000; 20: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan C, Chrzaszcz M, Choi N, Wainwright MS. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflammation 2010; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, McDole JR, Chen Y, Pirko I, Johnson AJ. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PLoS ONE 2008; 3: e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res 1999; 835: 10–17. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci 2008; 363: 2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res 1996; 732: 145–153. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol 2007; 10: 741–758. [DOI] [PubMed] [Google Scholar]
- Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience 2011; 174: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Miles R. The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front Cell Neurosci 2015; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res 2000; 119: 139–153. [DOI] [PubMed] [Google Scholar]
- Licht T, Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci 2013; 70: 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Glasper ER, Harrell CS, Malviya SA, Otis JS, Neigh GN. Meloxicam blocks neuroinflammation, but not depressive-like behaviors, in HIV-1 transgenic female rats. PLoS ONE 2014; 9: e108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 2003; 9: 407–415. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 2003; 114: 171–180. [DOI] [PubMed] [Google Scholar]
- Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A et al. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer 1998; 75: 246–253. [DOI] [PubMed] [Google Scholar]
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 2006; 108: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Fukumoto M. [Histochemical study using lectin and anti-human von Willebrand factor antibody of oral hemangioma]. Nichidai Koko Kagaku 1989; 15: 431–440. [PubMed] [Google Scholar]
- Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 2006; 54: 385–395. [DOI] [PubMed] [Google Scholar]
- Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K et al. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int J Cancer 2000; 85: 281–288. [DOI] [PubMed] [Google Scholar]
- Risau W. Angiogenesis is coming of age. Circ Res 1998; 82: 926–928. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 2012; 122: 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol 1989; 288: 401–413. [DOI] [PubMed] [Google Scholar]
- Gross PM, Sposito NM, Pettersen SE, Panton DG, Fenstermacher JD. Topography of capillary density, glucose metabolism, and microvascular function within the rat inferior colliculus. J Cereb Blood Flow Metab 1987; 7: 154–160. [DOI] [PubMed] [Google Scholar]
- Suchting S, Bicknell R, Eichmann A. Neuronal clues to vascular guidance. Exp Cell Res 2006; 312: 668–675. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 2004; 132: 146–154. [DOI] [PubMed] [Google Scholar]
- Molnarfi N, Benkhoucha M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor: a regulator of inflammation and autoimmunity. Autoimmun Rev 2015; 14: 293–303. [DOI] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013; 121: 4930–4937. [DOI] [PubMed] [Google Scholar]
- Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Platelet-derived growth factor-BB reflects clinical, inflammatory and angiogenic disease activity and oxidative stress in inflammatory bowel disease. Clin Biochem 2009; 42: 1602–1609. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem 1993; 268: 25009–25014. [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994; 265: 808–811. [DOI] [PubMed] [Google Scholar]
- Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res 1998; 82: 845–851. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E. "Sprouting angiogenesis", a reappraisal. Dev Biol 2012; 372: 157–165. [DOI] [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab 2005; 25: 2–16. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorafshan A, Abdollahifar MA, Karbalay-Doust S. Stress changes the spatial arrangement of neurons and glial cells of medial prefrontal cortex and sertraline and curcumin prevent it. Psychiatry Investig 2015; 12: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Kawahito Y. [The immunologic function and role of allograft inflammatory factor-1]. Nihon Rinsho Meneki Gakkai Kaishi 2014; 37: 139–145. [DOI] [PubMed] [Google Scholar]
- Jia J, Cai Y, Wang R, Fu K, Zhao YF. Overexpression of allograft inflammatory factor-1 promotes the proliferation and migration of human endothelial cells (HUV-EC-C) probably by up-regulation of basic fibroblast growth factor. Pediatr Res 2010; 67: 29–34. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhao YF, Zhao JH. Potential roles of allograft inflammatory factor-1 in the pathogenesis of hemangiomas. Med Hypotheses 2007; 68: 288–290. [DOI] [PubMed] [Google Scholar]
- Kongsui R, Beynon SB, Johnson SJ, Walker FR. Quantitative assessment of microglial morphology and density reveals remarkable consistency in the distribution and morphology of cells within the healthy prefrontal cortex of the rat. J Neuroinflammation 2014; 11: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 2013; 23: 1784–1797. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–185. [DOI] [PubMed] [Google Scholar]
- Clark-Raymond A, Halaris A. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res 2013; 47: 1080–1087. [DOI] [PubMed] [Google Scholar]
- Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 2007; 10: 149–166. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 2006; 163: 1630–1633. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–580. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res 2003; 139: 197–213. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Rocchi G, Escelsior A, Contini P, Ghio M, Colicchio S et al. VEGF plasma level variations in duloxetine-treated patients with major depression. J Affect Disord 2013; 151: 590–595. [DOI] [PubMed] [Google Scholar]
- Galecki P, Galecka E, Maes M, Orzechowska A, Berent D, Talarowska M et al. Vascular endothelial growth factor gene (VEGFA) polymorphisms may serve as prognostic factors for recurrent depressive disorder development. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 117–124. [DOI] [PubMed] [Google Scholar]
- Kirk SL, Karlik SJ. VEGF and vascular changes in chronic neuroinflammation. J Autoimmun 2003; 21: 353–363. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain 2007; 130(Pt 7): 1942–1956. [DOI] [PubMed] [Google Scholar]
- Shimizu F, Kanda T. [Disruption of the blood-brain barrier in inflammatory neurological diseases]. Brain Nerve 2013; 65: 165–176. [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 2009; 34: 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.