Abstract
Telomerase extends the repetitive DNA at the ends of linear chromosomes, and it is normally active in stem cells. When expressed in somatic diploid cells, it can lead to cellular immortalization. Human papillomaviruses (HPVs) are associated with and high-risk for cancer activate telomerase through the catalytic subunit of telomerase, human telomerase reverse transcriptase (hTERT). The expression of hTERT is affected by both high-risk HPVs, E6 and E7. Seminal studies over the last two decades have identified the transcriptional, epigenetic, and post-transcriptional roles high-risk E6 and E7 have in telomerase induction. This review will summarize these findings during infection and highlight the importance of telomerase activation as an oncogenic pathway in HPV-associated cancer development and progression.
Keywords: human papillomavirus, papillomaviruses, oncogenic virus, cancer, HPV E6, HPV E7, hTERT, telomerase
1. Introduction: Human Papillomavirus Infections
Human Papillomavirus Infection and Life Cycle in Stratified Squamous Epithelium
Human papillomaviruses (HPVs) are small, non-envelope, double stranded DNA viruses, and there are more than 200 papillomavirus types identified to date (see curated list at Papillomavirus Episteme (PaVE); https://pave.niaid.nih.gov/#home). All HPVs complete their life cycle in stratified squamous epithelium, either cutaneous or mucosal dependent on their tropism (reviewed in [1,2]). The HPV life cycle begins with infection of basal cells in stratified squamous epithelium. These cells are reached either through microabrasions or at anatomic sites where monolayer, columnar epithelium transition to stratified squamous epithelium. These sites of transition are the found at the cervical transformation zone, the anal verge, and crypts in the oral mucosa, and intriguingly these are also where many HPV-associated cancers occur [3]. There is also evidence the cervix and anal verge contain specific cells that support HPV-associated cancers and have signature gene expression profiles [4,5]. Whether or not these cells represent a preferred host for the virus or more narrowly for the initiation of cancer, we broadly understand that a productive HPV infection begins in the bottom layer of stratified squamous epithelium.
In the basal layer, the HPV genome escapes its viral capsid and is maintained at 50–60 copies per cells (ranging from 10 to 200 copies) [2,6], while the early (E) viral genes are expressed. E1 and E2 support HPV DNA replication and the measured expression of E6 and E7 [2,6]. One function of E6 and E7, as well as E5, is to reduce activation of the innate immune system and avoid viral clearance [7,8,9]. Evading immune sensing and maintaining copies of the viral genome are both critical to establishing an infection.
As cells leave the basal layer, they rise through the suprabasal and spinous layers and progress through differentiation. HPV requires this cellular differentiation program to complete its own viral life cycle. Without it, HPV has an abortive infection. Therefore, as HPV-infected host cells rise through the differentiating layers, HPV progresses through its own life cycle. In these differentiating layers, HPV DNA becomes amplified to several thousand copies per cell [2], and late (L) gene expression is activated. L1 and L2 form the protein shell of HPV, and they incorporate the HPV episomal DNA into new infectious virions. Those released as epithelial cells are sloughed off at the top of stratified squamous epithelium, completing the viral life cycle.
Although HPV requires its host cell to differentiate in order to complete its life cycle, it also requires it host cell to continue to grow when normally it would not. HPV dysregulates the balance of growth and differentiation found in stratified squamous epithelium to do so. In low-risk HPV types, this is manifest as warts. In high-risk HPV types, this leads to dysplasias and carcinoma in situ [10]. This dysregulation of growth and differentiation is driven primarily by the E6 and E7 genes. E6 and E7 drive cells to continue to grow and divide when they otherwise would not, and, to that end, E6 and E7 are expressed in the differentiating layers of stratified squamous epithelium [2,10]. By E6 and E7 disrupting the typical segregation of cell cycle and growth from differentiation, more HPV DNA can be copied and expressed, and more cells infected by HPV can grow.
There are at least 15 HPV types that are defined as high-risk (HR) by their association with cervical cancer [11]. HPV-associated cancers universally express the HR E6 and E7 genes, thus are considered to be HPV’s viral oncogenes. If HR E6 and E7 are introduced into normal diploid cells, they become immortalized [12,13]. If HR E6 and E7 expression is reduced in HPV-positive cervical cancer cell lines, the cells growth arrest [14,15]. This implies that not only are the HR E6 and E7 genes required for oncogenesis, but they are also required for the maintenance of malignant phenotype. There are critical oncogenic pathways that HR E6 and E7 affect. HR E7 targets the retinoblastoma protein (Rb) for degradation in epithelial cells [16,17]. This allows infected epithelial cells to proceed through S phase and the cell cycle. HR E6 targets p53 for degradation to avoid apoptosis [18,19]. It similarly targets PSD-Dlg-ZO-1/2 (PDZ) containing proteins for degradation, disrupting cellular apicobasal orientation and cell-to-cell adhesion in the epithelium, leading to hyperplasia [20,21,22,23,24]. HR E6 also activates gene expression; its most critical gene to activate is human telomerase reverse transcriptase (hTERT), the catalytic subunit of telomerase. It is the degradation of Rb by HR E7 and the activation of hTERT by HR E6 that drives normal keratinocytes to immortalization [12,13].
In this review article, we will describe the roles E6 and E7 have in telomerase induction during HPV infection and in oncogenesis. We will first define telomeric DNA, its role in DNA protection, and the enzymatic function of telomerase. Then, we will highlight the multiple ways HR E6 and E7 derepress hTERT to activate and accelerate telomerase activity. Finally, we will discuss how E6, E7, and hTERT expression changes during oncogenesis.
2. Telomeric DNA and Telomerase
Telomeric DNA caps the ends of linear chromosomes, is repetitive, and is approximately 5000 to 15,000 nucleotides in length in humans [25,26]. No genetic material is found within telomeric DNA itself. Rather, it is bound by the shelterin protein complex to block double strand (dsDNA) repair signaling [27], protecting against non-homologous end joining and erroneous chromosomal break repair [27]. Telomerase, a ribonucleoprotein enzyme complex, extends this repetitive telomeric DNA. The holoenzyme includes the catalytic subunit hTERT that is expressed at rate-determining levels [28,29,30], the telomerase RNA component, TERC or TR, used to extend the six nucleotide repeat 5′ TTAGGG 3′ found in telomeric DNA, and the protein dyskerin [26,31]. Telomerase is typically active during embryonic and fetal development [32] and in stem cells [33]. It is not active in normal somatic cells. However, telomerase activity has been detected in almost all human tumors and immortalized cells in culture [29,30,34].
Without telomerase activity, the linear chromosomes of cellular DNA are serially shortened with every cell cycle and division by 100 to 200 nucleotides [35]. This DNA loss is called the “end replication problem”. As telomeric DNA becomes critically shortened over time, normal somatic diploid cells enter mortality stage one (M1) and undergo either replicative senescence or apoptosis [35,36,37,38]. If these cells continue to cycle beyond stage M1, they lose the protective shelterin protein complexes and enter mortality stage two (M2) or crisis. In crisis, cells signal that there are dsDNA breaks at the ends of chromosomes requiring repair. This genomic instability creates the “end protection problem”. It leads to anaphase bridges and chromosomal breaks that are catastrophic to the cell [39,40,41]. Only clonal cells survive that have had enormous chromosomal rearrangements [41]. Consequently, the extension of telomeric DNA by telomerase allows diploid cells to grow over time, avoiding apoptosis, senescence, and chromosomal rearrangements. It is because of this allowance that telomerase and its rate-determining catalytic subunit hTERT are expressed in nearly all cancers [30,34,42,43].
3. Telomerase and hTERT Activity Driven by HR E6 and E7
Studies in the late 1980s defined the roles HR E6 and E7 played in cellular immortalization, cancer development, and cancer progression [12,13]. In a seminal paper by Kiyono et al. HR E6 and E7 were found to collaborate in the immortalization of both primary fibroblasts and keratinocytes, specifically by dysregulating Rb/p16INK4A and telomerase [44]. HR E7 was important for immortalization, but it did not directly affect telomerase [44]. Rather, HR E6 did. HR E7 was described to increase hTERT driven expression of luciferase and augment telomerase activity driven by HR HPV E6 [45]. In HeLa cells, re-expression of either HR E6 or E7 after their removal led to increased hTERT [46]. Although E7 could synergize the E6 regulation of telomerase and cellular immortalization, HR E6 is the principal trigger and regulator of hTERT expression and telomerase activity (Table 1). Other studies built on these foundational reports.
Table 1.
HR E6 and E7 regulation of hTERT and cellular protein targets for that regulation.
| HPV Gene | Effect on hTERT | Cellular Protein Target |
|---|---|---|
| Chromatin Effects | ||
| E6 and E7 | Promoter methylation changes | |
| E6 | Increase promoter acetylation | HATs and HDACs, mSin3A |
| Transcription Effects | ||
| E6 | Increase transcriptional activators | c-Myc/Max, Sp1 |
| E6 | Decrease transcriptional repressors | c-Myc/Mad, Maz, USF1, NFX1-91 |
| E7 | Increase expression with E6 | |
| RNA Effects | ||
| E6 | Increase transcript stability | NFX1-123, PABPCs |
| E6 | Increase active spliced isoform of hTERT | c-Myc |
| Protein Effects | ||
| E6 | Binds hTERT | hTERT |
HR: high-risk; hTERT: human telomerase reverse transcriptase; HPV: human papillomavirus; HATs: histone acetyltransferases; HDACs: histone deacetylases; mSin3A: SIN3 transcription regulator family member A; c-Myc: MYC proto-oncogene; Max: MYC associated factor X; Maz: MYC associated zinc finger protein; USF1: Upstream transcription factor 1; NFX1-91: Nuclear transcription factor, X-box binding 1, isoform 3; NFX1-123: Nuclear transcription factor, X-box binding 1, isoform 1; PABPCs: cytoplasmic poly(A) binding proteins.
Recent studies have confirmed that low-risk (LR) E6 does not activate telomerase [47] while HR E6 is necessary and sufficient for telomerase activation in keratinocytes [48,49,50,51]. Without HR E6, telomerase is not detected in epithelial cells, and the catalytic subunit of telomerase, hTERT, is not expressed [48,50]. In addition to HR E6 regulating the activity of telomerase, HR E6 was found to bind hTERT itself and repetitive telomeric DNA [52]. Therefore, the role HR E6 has in hTERT, telomerase, and telomeric DNA regulation is multilayered, demonstrated by its redundant actions to drive immortalization.
The E3 Ubiquitin Ligase E6 Associated Protein (E6AP) is important for the activation of hTERT expression and telomerase activity by HR E6 [53,54,55]. E6AP partners with HR E6 to polyubiquitinate and degrade p53 and PDZ-containing proteins [18,19,21,56,57], but this partnership does not lead to the degradation of hTERT or telomerase; instead, it increases hTERT and telomerase. Decreasing HR E6 and E6AP by microRNA (miR375) indirectly reduced hTERT and telomerase activity in cells [58], and the E6 motifs needed to bind E6AP were also required for telomerase activation and immortalization in fibroblasts and keratinocytes [59]. Hence, HR E6 and E6AP (E6/E6AP) function as principal inductors of telomerase, and its catalytic subunit hTERT.
3.1. hTERT: Promoter Regulation
Most research on telomerase regulation has focused on the expression of its catalytic subunit, hTERT. The hTERT gene is constitutively repressed in somatic epithelial cells; this repression occurs at its promoter. The hTERT promoter is approximately 1100 nucleotides in length, with its core promoter being only 200 to 300 nucleotides long [60,61,62]. Normally, transcriptional repressors of hTERT are bound to cis elements in its core promoter, blocking transcription [48,60,61,63,64,65,66,67,68,69]. These cis elements are E boxes, GC-rich sites, and X boxes.
Two E box cis elements flank the transcriptional start site of hTERT [61,62,66], and if these E boxes are mutated or deleted, hTERT expression and telomerase activity are dramatically reduced [50,54]. These E boxes are normally bound by c-Myc as a heterodimer with either Max or Mad. These c-Myc/Max or c-Myc/Mad heterodimers are important for hTERT transcriptional activation or repression, respectively [65,66,70,71,72]. Upstream transcription factor 1 (USF1) also binds to E boxes, competitively and sterically repressing hTERT expression by c-Myc/Max [50,73,74] Although the amount of c-Myc that binds to the hTERT promoter does not correlate to hTERT expression, the presence of c-Myc at the promoter is important [51,72,75]. E6/E6AP are also bound at E boxes in the hTERT promoter [51,54,74,75], and they interact with c-Myc to drive gene expression [51]. The requirement for E6AP to drive hTERT expression at the promoter with HR E6 is controversial [53,54,55,76,77], but the cis E boxes within the hTERT promoter are required for its transcriptional activation with or without HR E6.
There are five GC-rich cis elements in the hTERT promoter 5′ of the transcriptional start site [50,71,74]. Sp1 binds to these elements and transcriptionally activates hTERT expression [50,71]. Maz also is bound at these sites but is a transcriptional repressor [71]. Like deletion of the E boxes, deletion of GC-rich cis elements leads to loss of hTERT promoter-driven transcriptional activation [50].
Finally, there are two X boxes in the hTERT promoter [48]. One is downstream of the hTERT transcriptional start site, lies within the 5′ UTR of hTERT, and overlaps with the downstream E box to which c-Myc/Max binds [48]. The second is upstream of the hTERT core promoter in an inverted position [48]. Nuclear transcription factor X-box binding 1, isoform 3 (NFX1-91) is a repressor of hTERT transcription and is bound constitutively at the hTERT downstream X box [48,78]. NFX1-91 is polyubiquitinated by E6/E6AP and targeted for proteasomal degradation [48]. Its removal from the hTERT promoter leads to transcriptional activation of hTERT [48].
3.2. hTERT: Epigenetic Regulation
Beyond studies of the hTERT promoter cis elements and the transcriptional proteins that bind those elements, epigenetic studies of the hTERT promoter demonstrate important structural chromatin changes that affect transcriptional activation of hTERT [78]. Several studies document the importance of E6/E6AP in opening the hTERT promoter chromatin structure as they change histone acetyltransferase (HAT) and histone deacetylase (HDAC) recruitment to the hTERT promoter [53,78]. The hTERT repressor NFX1-91 not only binds the promoter X box cis element but also binds SIN3 transcription regulator family member A (mSin3A), a transcriptional co-repressor that recruits HDACs to promoters [78]. When NFX1-91 is degraded by E6/E6AP, HDAC activity at the hTERT promoter is lost and HAT activity increases [78], and with this, histone acetylation increases further over time [53].
DNA methylation patterns at the hTERT promoter also shift during an HPV infection and in tissue culture studies of HPV positive cells. Specific regions of the promoter become hypermethylated, while other regions become hypomethylated, during long-term tissue culture of cells with HR E6 and E7 [79,80,81,82]. Although a direct causal association between hTERT promoter methylation and cancer development has not been seen [83], there are changes that parallel increases in hTERT expression, and these changes in methylation patterns correlate with HR and probable HR E6 expression [84].
3.3. hTERT: Post-Transcriptional Regulation
Post-transcriptional regulation of hTERT by alternative mRNA splicing and mRNA stabilization is important for telomerase activity [32,85,86]. In non-HPV studies, c-Myc shifts hTERT mRNA expression from a non-active splice variant to an active form [87]. RNA processing proteins, such as Serine-Arginine Rich Splicing Factors, are also expressed at increased levels in high-grade cervical dysplasias [88], pointing indirectly to HR HPV manipulating RNA processing proteins during oncogenesis.
We found that hTERT and telomerase activity are upregulated post-transcriptionally by HR E6 through the host cellular protein NFX1-123 [86]. NFX1-123 is the longer splice variant of the NFX1 gene (the hTERT transcriptional repressor NFX1-91 is the shorter splice variant) [48,89]. Greater expression of NFX1-123 leads to increased hTERT and telomerase activity with HR E6, and knock down of endogenous NFX1-123 reduces the ability of HR E6 to increase hTERT and telomerase [86,89]. The mechanism by which NFX1-123 augments hTERT expression is through stabilization of the hTERT mRNA, and the 5′ UTR of the hTERT transcript is necessary for this stabilization [86].
NFX1-123 contains two protein motifs important for binding, stabilizing, and augmenting hTERT expression. The R3H domain of NFX1-123 has putative single-stranded nucleic acid binding capabilities [86,89,90], and when this motif is deleted, the stabilization and increased expression of hTERT seen in HR E6 expressing cells is lost [86,89]. Second, the poly(A) binding protein interacting motif (PAM2) of NFX1-123 directs binding of cytoplasmic poly(A) binding proteins (PABPCs) to NFX1-123, and PABPCs increase the stability and translation of genes with poly (A) tailed mRNA [89,91]. Like the R3H domain, when the PAM2 motif of NFX1-123 is mutated or deleted, its ability to augment hTERT expression and telomerase activity by HR E6 is also lost [86,89].
Cytoplasmic poly(A) binding proteins themselves are important in hTERT expression and telomerase activity in HR E6 positive cells. When PABPC types 1 and 4 are knocked down, hTERT and telomerase activity are reduced [92]. Conversely, when PABPC type 4 is overexpressed, hTERT and telomerase are augmented, and cells with either more hTERT or more PABPC type 4 grow better in culture [92].
Collectively, these research findings highlight multiple ways hTERT mRNA is post-transcriptionally regulated. Again, the duplicative mechanisms, from DNA, chromatin, and RNA regulation, that HR E6 uses to increase hTERT and telomerase emphasizes its importance to HPV and oncogenesis.
3.4. hTERT: Beta HPV E6
Most studies examining the regulation of telomerase by HPV have focused on HR HPVs from the α genus. More recent work has examined the role β genus HPVs play in nonmelanomatous squamous cell carcinoma, and how the beta E6 and E7 proteins may also activate oncogenic pathways, whether similar or disparate to α HR E6 and E7 proteins. Work by Galloway et al. has determined the oncogenic potential of β HPV types through direct analysis of their E6 and E7 protein functionality, and specifically how different E6 types activate hTERT expression, telomerase activity, and immortalization [40,93]. β E6 proteins with greater effect on hTERT activation and telomerase activity have improved cellular growth and longevity in culture [93]. This improvement is not only proportional to telomerase activity but also depends on the presence of E6AP [93]. Therefore, like α HR HPV types, several β genus E6 genes drive hTERT expression and telomerase activity.
4. Telomerase in HPV-Associated Cancers
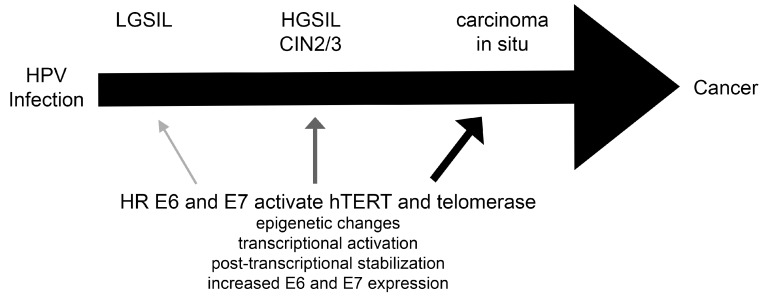
During cervical cancer initiation and progression, the expression of hTERT and the activity of telomerase parallels worsening disease [94,95,96,97]. Approximately half of HPV positive squamous intraepithelial lesions and cervical intraepithelial grade III lesions have detectable telomerase activity and that increases to over 90% in HPV positive cervical cancer samples [94,98]. The level of hTERT expression and telomerase activity found in cervical lesions is proportional to the pathologic severity of disease detected [94,96,98]. In HPV-positive cancers, telomerase is universally expressed (modeled in Figure 1) [34]. Telomerase is increasingly identified as having both canonical and non-canonical functions, and each is important to HPV-induced cellular immortalization and oncogenesis [99].
Figure 1.
HPV infection and telomerase induction. Telomerase, and its rate determining catalytic subunit, hTERT, is normally not expressed in somatic cells. With a HR HPV infection, E6 and E7 activate the hTERT gene. With disease progression, hTERT activation and telomerase activity increases (demonstrated by darker, larger arrows), and the expression of HR E6 and E7 also increases with the integration of HPV DNA into the host cell chromosomal DNA or loss of E2 regulation. LGSIL (low-grade squamous intraepithelial lesion) is typical for an active HPV infection. HGSIL (high-grade squamous intraepithelial lesion) is typical for a HR HPV infection with worsening cytologic changes and parallel greater histologic involvement, with multiple layers of the stratified squamous epithelium. CIN2/3 (cervical intraepithelial neoplasia 2 or 3) shows histologic changes due to an active HPV infection that involved most (2) or all (3) of the stratified squamous epithelium. Carcinoma in situ is the full thickness involvement of stratified squamous epithelium without breakdown of the basement membrane.
Interestingly, during the transition from HR HPV infection, to dysplasia, to frank cancer, HPV DNA typically no longer remains episomal. It becomes integrated in the cell’s chromosomes. This happens within the context of genomic instability, created and supported by the functions of HR E6 and E7 themselves. This, by definition, means HPV can no longer form infectious virions; it also means HPV gene expression itself is dysregulated. With HPV DNA integration, the HR E6 and E7 genes are universally preserved, but the regulatory E1 and E2 genes are often lost. Even in non-integrated HPV driven cancers, the binding sites for E2 often become methylated. These changes allow for greater expression of E6 and E7, as E2 moderates the expression level of these viral oncogenes [100,101]. Although not required, with increased E6 and E7, there is a parallel increase in telomerase activity [102]. During HR HPV infection and its associated cancer development and progression, HR E6, with E7, activates telomerase. This activation is augmented over time by changes that support cellular immortalization and growth and by the acceleration of viral and cellular genomic instability that was first initiated by HR E6 and E7.
High-risk HPV infections are associated with cancers in other sites besides the cervix [3]. These include vulvar, vaginal, anal, penile, and the head-and-neck. Each of these HPV-associated cancers are also associated with upregulated telomerase activity [34]. Therefore, the anatomic location of a HR HPV infection is not the singular instigator of immortalization—the commonality among these cancers is HR HPV, and HR E6 specifically, driving telomerase.
5. Conclusions
High-risk E6 hijacks host cell proteins from their usual function (E6AP, c-Myc, HDAC, HAT, mSin3A, NFX1-91, NFX1-123, PABPCs, and mRNA splicing factors) to activate hTERT and telomerase activity. This supports cellular immortalization. These viral-cellular protein partnerships primarily control the derepression of telomerase’s catalytic subunit, hTERT. They increase hTERT through the promoter’s cis and trans elements, the chromatin structure, the mRNA product, and associated RNA regulatory proteins. There are still many unanswered questions in the dysregulation of telomerase activation by HPV during infection and oncogenesis. However, its universality implies it is critical to the core function of HR HPV types and to induction of cancers caused by HPV.
Acknowledgments
The author would like to recognize all of the researchers’ work that was not recognized due to space limitations. This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M., Broker T.R., Stanley M.A. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J. The papillomavirus life cycle. J. Clin. Virol. 2005;32:S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.De Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herfs M., Yamamoto Y., Laury A., Wang X., Nucci M.R., McLaughlin-Drubin M.E., Munger K., Feldman S., McKeon F.D., Xian W., et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl. Acad. Sci. USA. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herfs M., Longuespee R., Quick C.M., Roncarati P., Suarez-Carmona M., Hubert P., Lebeau A., Bruyere D., Mazzucchelli G., Smargiasso N., et al. Proteomic signatures reveal a dualistic and clinically relevant classification of anal canal carcinoma. J. Pathol. 2017;241:522–533. doi: 10.1002/path.4858. [DOI] [PubMed] [Google Scholar]
- 6.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 7.Westrich J.A., Warren C.J., Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017;231:21–33. doi: 10.1016/j.virusres.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong S., Laimins L.A. Manipulation of the innate immune response by human papillomaviruses. Virus Res. 2017;231:34–40. doi: 10.1016/j.virusres.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowska A.K., Riemer A.B. The invisible enemy—How human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol. J. 2012;6:249–256. doi: 10.2174/1874357901206010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doorbar J., Egawa N., Griffin H., Kranjec C., Murakami I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015;25:2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V., Snijders P.J., Meijer C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 12.Hawley-Nelson P., Vousden K.H., Hubbert N.L., Lowy D.R., Schiller J.T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger K., Phelps W.C., Bubb V., Howley P.M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells S.I., Francis D.A., Karpova A.Y., Dowhanick J.J., Benson J.D., Howley P.M. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21CIP-dependent pathways. EMBO J. 2000;19:5762–5771. doi: 10.1093/emboj/19.21.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis D.A., Schmid S.I., Howley P.M. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J. Virol. 2000;74:2679–2686. doi: 10.1128/JVI.74.6.2679-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyson N., Howley P.M., Munger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 17.Boyer S.N., Wazer D.E., Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 18.Huibregtse J.M., Scheffner M., Howley P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheffner M., Huibregtse J.M., Vierstra R.D., Howley P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 20.Kiyono T., Hiraiwa A., Fujita M., Hayashi Y., Akiyama T., Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa S., Huibregtse J.M. Human scribble (vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 2000;20:8244–8253. doi: 10.1128/MCB.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.S., Glaunsinger B., Mantovani F., Banks L., Javier R.T. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 2000;74:9680–9693. doi: 10.1128/JVI.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson R.A., Thomas M., Banks L., Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J. Cell Sci. 2003;116:4925–4934. doi: 10.1242/jcs.00809. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen M.L., Nguyen M.M., Lee D., Griep A.E., Lambert P.F. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J. Virol. 2003;77:6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cech T.R. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/S0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt J.C., Cech T.R. Human telomerase: Biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015;29:1095–1105. doi: 10.1101/gad.263863.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 28.Counter C.M., Hahn W.C., Wei W., Caddle S.D., Beijersbergen R.L., Lansdorp P.M., Sedivy J.M., Weinberg R.A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Counter C.M., Meyerson M., Eaton E.N., Ellisen L.W., Caddle S.D., Haber D.A., Weinberg R.A. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 30.Meyerson M., Counter C.M., Eaton E.N., Ellisen L.W., Steiner P., Caddle S.D., Ziaugra L., Beijersbergen R.L., Davidoff M.J., Liu Q., et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/S0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S.B., Graham M.E., Lovrecz G.O., Bache N., Robinson P.J., Reddel R.R. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 32.Ulaner G.A., Hu J.F., Vu T.H., Giudice L.C., Hoffman A.R. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- 33.Nandakumar J., Cech T.R. Finding the end: Recruitment of telomerase to telomeres. Nat. Rev. Mol. Cell Biol. 2013;14:69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shay J.W., Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 35.Levy M.Z., Allsopp R.C., Futcher A.B., Greider C.W., Harley C.B. Telomere end-replication problem and cell aging. J. Mol. Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 36.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 37.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 38.Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S., Wright W.E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 39.Plug-DeMaggio A.W., Sundsvold T., Wurscher M.A., Koop J.I., Klingelhutz A.J., McDougall J.K. Telomere erosion and chromosomal instability in cells expressing the HPV oncogene 16E6. Oncogene. 2004;23:3561–3571. doi: 10.1038/sj.onc.1207388. [DOI] [PubMed] [Google Scholar]
- 40.Gabet A.S., Accardi R., Bellopede A., Popp S., Boukamp P., Sylla B.S., Londono-Vallejo J.A., Tommasino M. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 2008;22:622–632. doi: 10.1096/fj.07-8389com. [DOI] [PubMed] [Google Scholar]
- 41.Verdun R.E., Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 42.Janknecht R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 2004;564:9–13. doi: 10.1016/S0014-5793(04)00356-4. [DOI] [PubMed] [Google Scholar]
- 43.Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 44.Kiyono T., Foster S.A., Koop J.I., McDougall J.K., Galloway D.A., Klingelhutz A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 45.Liu X., Roberts J., Dakic A., Zhang Y., Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375:611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong S.E., Jung K.H., Jae L.C., Tae K.H., Seong H.E. The role of HPV oncoproteins and cellular factors in maintenance of hTERT expression in cervical carcinoma cells. Gynecol. Oncol. 2004;94:40–47. doi: 10.1016/j.ygyno.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 47.Van Doorslaer K., Burk R.D. Association between hTERT activation by HPV E6 proteins and oncogenic risk. Virology. 2012;433:216–219. doi: 10.1016/j.virol.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gewin L., Myers H., Kiyono T., Galloway D.A. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veldman T., Horikawa I., Barrett J.C., Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh S.T., Kyo S., Laimins L.A. Telomerase activation by human papillomavirus type 16 E6 protein: Induction of human telomerase reverse transcriptase expression through Myc and GC-Rich Sp1 binding sites. J. Virol. 2001;75:5559–5566. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldman T., Liu X., Yuan H., Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X., Dakic A., Zhang Y., Dai Y., Chen R., Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc. Natl. Acad. Sci. USA. 2009;106:18780–18785. doi: 10.1073/pnas.0906357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James M.A., Lee J.H., Klingelhutz A.J. HPV-16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int. J. Cancer. 2006;119:1878–1885. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Yuan H., Fu B., Disbrow G.L., Apolinario T., Tomaic V., Kelley M.L., Baker C.C., Huibregtse J., Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J. Biol. Chem. 2005;280:10807–10816. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- 55.Kelley M.L., Keiger K.E., Lee C.J., Huibregtse J.M. The global transcriptional effects of the human papillomavirus E6 protein in cervical carcinoma cell lines are mediated by the E6AP ubiquitin ligase. J. Virol. 2005;79:3737–3747. doi: 10.1128/JVI.79.6.3737-3747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handa K., Yugawa T., Narisawa-Saito M., Ohno S., Fujita M., Kiyono T. E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein. J. Virol. 2007;81:1379–1389. doi: 10.1128/JVI.01712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas M., Laura R., Hepner K., Guccione E., Sawyers C., Lasky L., Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- 58.Jung H.M., Phillips B.L., Chan E.K. miR-375 activates p21 and suppresses telomerase activity by coordinately regulating HPV E6/E7, E6AP, CIP2a, and 14–3-3ζ. Mol. Cancer. 2014;13:80. doi: 10.1186/1476-4598-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klingelhutz A.J., Foster S.A., McDougall J.K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 60.Cong Y.S., Wen J., Bacchetti S. The human telomerase catalytic subunit hTERT: Organization of the gene and characterization of the promoter. Hum. Mol. Genet. 1999;8:137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 61.Wick M., Zubov D., Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/S0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 62.Takakura M., Kyo S., Kanaya T., Hirano H., Takeda J., Yutsudo M., Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 63.Fujimoto K., Kyo S., Takakura M., Kanaya T., Kitagawa Y., Itoh H., Takahashi M., Inoue M. Identification and characterization of negative regulatory elements of the human telomerase catalytic subunit (hTERT) gene promoter: Possible role of MZF-2 in transcriptional repression of hTERT. Nucleic Acids Res. 2000;28:2557–2562. doi: 10.1093/nar/28.13.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh S., Song Y.H., Yim J., Kim T.K. Identification of Mad as a repressor of the human telomerase (hTERT) gene. Oncogene. 2000;19:1485–1490. doi: 10.1038/sj.onc.1203439. [DOI] [PubMed] [Google Scholar]
- 65.Gunes C., Lichtsteiner S., Vasserot A.P., Englert C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 2000;60:2116–2121. [PubMed] [Google Scholar]
- 66.Horikawa I., Cable P.L., Mazur S.J., Appella E., Afshari C.A., Barrett J.C. Downstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription: Evidence for an endogenous mechanism of transcriptional repression. Mol. Biol. Cell. 2002;13:2585–2597. doi: 10.1091/mbc.E01-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Won J., Yim J., Kim T.K. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J. Biol. Chem. 2002;277:38230–38238. doi: 10.1074/jbc.M206064200. [DOI] [PubMed] [Google Scholar]
- 68.Renaud S., Loukinov D., Bosman F.T., Lobanenkov V., Benhattar J. CTCF binds the proximal exonic region of hTERT and inhibits its transcription. Nucleic Acids Res. 2005;33:6850–6860. doi: 10.1093/nar/gki989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Racek T., Mise N., Li Z., Stoll A., Putzer B.M. C-terminal p73 isoforms repress transcriptional activity of the human telomerase reverse transcriptase (hTERT) promoter. J. Biol. Chem. 2005;280:40402–40405. doi: 10.1074/jbc.C500193200. [DOI] [PubMed] [Google Scholar]
- 70.Lebel R., McDuff F.O., Lavigne P., Grandbois M. Direct visualization of the binding of c-Myc/Max heterodimeric b-HLH-LZ to E-box sequences on the hTERT promoter. Biochemistry. 2007;46:10279–10286. doi: 10.1021/bi700076m. [DOI] [PubMed] [Google Scholar]
- 71.Xu M., Katzenellenbogen R.A., Grandori C., Galloway D.A. An unbiased in vivo screen reveals multiple transcription factors that control HPV E6-regulated hTERT in keratinocytes. Virology. 2013;446:17–24. doi: 10.1016/j.virol.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., Dakic A., Chen R., Disbrow G.L., Zhang Y., Dai Y., Schlegel R. Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc. J. Virol. 2008;82:11568–11576. doi: 10.1128/JVI.01318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang J.T., Yang H.T., Wang T.C., Cheng A.J. Upstream stimulatory factor (USF) as a transcriptional suppressor of human telomerase reverse transcriptase (hTERT) in oral cancer cells. Mol. Carcinog. 2005;44:183–192. doi: 10.1002/mc.20129. [DOI] [PubMed] [Google Scholar]
- 74.McMurray H.R., McCance D.J. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J. Virol. 2003;77:9852–9861. doi: 10.1128/JVI.77.18.9852-9861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gewin L., Galloway D.A. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-Myc. J. Virol. 2001;75:7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekaric P., Cherry J.J., Androphy E.J. Binding of human papillomavirus type 16 E6 to E6AP is not required for activation of hTERT. J. Virol. 2008;82:71–76. doi: 10.1128/JVI.01776-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shai A., Pitot H.C., Lambert P.F. E6-associated protein is required for human papillomavirus type 16 E6 to cause cervical cancer in mice. Cancer Res. 2010;70:5064–5073. doi: 10.1158/0008-5472.CAN-09-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu M., Luo W., Elzi D.J., Grandori C., Galloway D.A. NFX1 interacts with mSin3a/histone deacetylase to repress hTERT transcription in keratinocytes. Mol. Cell. Biol. 2008;28:4819–4828. doi: 10.1128/MCB.01969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schutze D.M., Kooter J.M., Wilting S.M., Meijer C.J., Quint W., Snijders P.J., Steenbergen R.D. Longitudinal assessment of DNA methylation changes during HPVE6E7-induced immortalization of primary keratinocytes. Epigenetics. 2015;10:73–81. doi: 10.4161/15592294.2014.990787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Wild J., Kooter J.M., Overmeer R.M., Claassen-Kramer D., Meijer C.J., Snijders P.J., Steenbergen R.D. hTERT promoter activity and CpG methylation in HPV-induced carcinogenesis. BMC Cancer. 2010;10:271. doi: 10.1186/1471-2407-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang J., Zhao L.J., Zhao C., Zhang G., Zhao Y., Li J.R., Li X.P., Wei L.H. Hypomethylated CpG around the transcription start site enables TERT expression and HPV16 E6 regulates TERT methylation in cervical cancer cells. Gynecol. Oncol. 2012;124:534–541. doi: 10.1016/j.ygyno.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 82.Zinn R.L., Pruitt K., Eguchi S., Baylin S.B., Herman J.G. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67:194–201. doi: 10.1158/0008-5472.CAN-06-3396. [DOI] [PubMed] [Google Scholar]
- 83.Oikonomou P., Messinis I., Tsezou A. DNA methylation is not likely to be responsible for hTERT expression in premalignant cervical lesions. Exp. Biol. Med. 2007;232:881–886. [PubMed] [Google Scholar]
- 84.Schutze D.M., Snijders P.J., Bosch L., Kramer D., Meijer C.J., Steenbergen R.D. Differential in vitro immortalization capacity of eleven, probable high-risk human papillomavirus types. J. Virol. 2014;88:1714–1724. doi: 10.1128/JVI.02859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilian A., Bowtell D.D., Abud H.E., Hime G.R., Venter D.J., Keese P.K., Duncan E.L., Reddel R.R., Jefferson R.A. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum. Mol. Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 86.Katzenellenbogen R.A., Vliet-Gregg P., Xu M., Galloway D.A. Nfx1-123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes. J. Virol. 2009;83:6446–6456. doi: 10.1128/JVI.02556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cerezo A., Kalthoff H., Schuermann M., Schafer B., Boukamp P. Dual regulation of telomerase activity through c-Myc-dependent inhibition and alternative splicing of hTERT. J. Cell Sci. 2002;115:1305–1312. doi: 10.1242/jcs.115.6.1305. [DOI] [PubMed] [Google Scholar]
- 88.Mole S., McFarlane M., Chuen-Im T., Milligan S.G., Millan D., Graham S.V. RNA splicing factors regulated by HPV16 during cervical tumour progression. J. Pathol. 2009;219:383–391. doi: 10.1002/path.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katzenellenbogen R.A., Egelkrout E.M., Vliet-Gregg P., Gewin L.C., Gafken P.R., Galloway D.A. Nfx1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16E6 expressing cells. J. Virol. 2007;81:3786–3796. doi: 10.1128/JVI.02007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grishin N.V. The R3H motif: A domain that binds single-stranded nucleic acids. Trends Biochem. Sci. 1998;23:329–330. doi: 10.1016/S0968-0004(98)01258-4. [DOI] [PubMed] [Google Scholar]
- 91.Mangus D.A., Evans M.C., Jacobson A. Poly(A)-binding proteins: Multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katzenellenbogen R.A., Vliet-Gregg P., Xu M., Galloway D.A. Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes. J. Virol. 2010;84:12934–12944. doi: 10.1128/JVI.01377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bedard K.M., Underbrink M.P., Howie H.L., Galloway D.A. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J. Virol. 2008;82:3894–3902. doi: 10.1128/JVI.01818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mutirangura A., Sriuranpong V., Termrunggraunglert W., Tresukosol D., Lertsaguansinchai P., Voravud N., Niruthisard S. Telomerase activity and human papillomavirus in malignant, premalignant and benign cervical lesions. Br. J. Cancer. 1998;78:933–939. doi: 10.1038/bjc.1998.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ley C., Bauer H.M., Reingold A., Schiffman M.H., Chambers J.C., Tashiro C.J., Manos M.M. Determinants of genital human papillomavirus infection in young women. J. Natl. Cancer Inst. 1991;83:997–1003. doi: 10.1093/jnci/83.14.997. [DOI] [PubMed] [Google Scholar]
- 96.Branca M., Giorgi C., Ciotti M., Santini D., Di B.L., Costa S., Benedetto A., Bonifacio D., Di B.P., Paba P., et al. Upregulation of telomerase (hTERT) is related to the grade of cervical intraepithelial neoplasia, but is not an independent predictor of high-risk human papillomavirus, virus persistence, or disease outcome in cervical cancer. Diagn. Cytopathol. 2006;34:739–748. doi: 10.1002/dc.20554. [DOI] [PubMed] [Google Scholar]
- 97.Nachajova M., Brany D., Dvorska D. Telomerase and the process of cervical carcinogenesis. Tumour Biol. 2015;36:7335–7338. doi: 10.1007/s13277-015-3976-z. [DOI] [PubMed] [Google Scholar]
- 98.Snijders P.J., van Duin M., Walboomers J.M., Steenbergen R.D., Risse E.K., Helmerhorst T.J., Verheijen R.H., Meijer C.J. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: Strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human papillomavirus DNA. Cancer Res. 1998;58:3812–3818. [PubMed] [Google Scholar]
- 99.Miller J., Dakic A., Chen R., Palechor-Ceron N., Dai Y., Kallakury B., Schlegel R., Liu X. HPV16 E7 protein and hTERT proteins defective for telomere maintenance cooperate to immortalize human keratinocytes. PLoS. Pathog. 2013;9:e1003284. doi: 10.1371/journal.ppat.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeon S., Lambert P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reuschenbach M., Huebbers C.U., Prigge E.S., Bermejo J.L., Kalteis M.S., Preuss S.F., Seuthe I.M., Kolligs J., Speel E.J., Olthof N., et al. Methylation status of HPV16 E2-binding sites classifies subtypes of HPV-associated oropharyngeal cancers. Cancer. 2015;121:1966–1976. doi: 10.1002/cncr.29315. [DOI] [PubMed] [Google Scholar]
- 102.Baege A.C., Berger A., Schlegel R., Veldman T., Schlegel R. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 2002;160:1251–1257. doi: 10.1016/S0002-9440(10)62552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]