Abstract
The flagella of the Gram-negative bacterium Pseudomonas aeruginosa serve not only for motility but also to bind bacteria to the host cell glycolipid asialoGM1 (ASGM1) through the protein flagellin. This interaction triggers defensive responses in host cells. How this response occurs is unclear because ASGM1 lacks transmembrane and cytoplasmic domains and there is little information about the downstream effectors that connect ASGM1 ligation to the initiation of host defense responses. Here, we show that ASGM1 ligation promotes ATP release from the host cell, followed by autocrine activation of a nucleotide receptor. This response links ASGM1 to cytoplasmic signaling molecules and results in activation of phospholipase C, Ca2+ mobilization, phosphorylation of a mitogen-activated protein kinase (Erk 1/2), and activation of mucin transcription. These results indicate that bacterial interaction with host cells can trigger autocrine nucleotide signaling and suggest that agents affecting nucleotide receptors may modulate host responses to bacteria.
The protein flagellin is a major structural component of bacterial flagella, organelles required for chemotaxis, motility, and nutrition (1). Because flagella are a feature of many strains of bacteria, it is not surprising that host organisms have developed the means to recognize this protein and respond defensively. It has been shown, for example, that flagellin from Pseudomonas aeruginosa (2) and Salmonella typhimurium (3) stimulates epithelial cells to produce IL-8, a cytokine that acts as a chemoattractant for neutrophils. The latter not only phagocytose bacteria, but also release antimicrobial agents including lactoferrin, lysozyme, defensin, and oxygen radicals.
We show in this report that, in addition to stimulating epithelial production of IL-8, flagellin also stimulates the production of mucin. In general, mucin benefits the host by forming a protective barrier against bacteria; when overproduced in the lung, however, it can compromise respiratory function. Flagellin–epithelial cell interactions may therefore represent a target for potential therapeutic intervention once they are adequately understood.
Although it has been shown that P. aeruginosa flagellin can elicit host cell responses through binding to a glycolipid receptor, asialoGM1 (ASGM1) (4), it is unclear how this process occurs because ASGM1 lacks transmembrane and intracellular domains and is therefore incapable of direct contact with cytoplasmic signaling molecules. Here, we use an in vitro system to investigate cellular signaling mechanisms by which ASGM1 ligation stimulates transcription of the mucin MUC 2. Results indicate that this signaling pathway involves the release of ATP extracellularly followed by activation of cell surface ATP receptors, Ca2+ mobilization, and extracellular signal-regulated kinase (Erk) 1/2 phosphorylation.
Materials and Methods
Reagents.
All tissue culture media and antibiotics were obtained from Life Technologies. All chemical inhibitors were purchased from CalBiochem, except pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt (PPADS) and Reactive Blue 2, which came from Research Biochemicals (Natick, MA) and Sigma, respectively. All nucleotide agonists were purchased from Research Biochemicals, except ATP and UTP, which came from Life Technologies. Anti-asialoGM1 antibody (α-ASGM1; rabbit polyclonal) was purchased from Wako Biochemicals (Osaka). Phosphospecific anti-Pyk2 [pY402], [pY579], [pY580], and [pY881] (rabbit polyclonal) antibodies were purchased from BioSource International (Camarillo, CA). Phosphospecific anti-Erk 1/2 (mouse monoclonal) was purchased from Cell Signaling Technology (Beverly, MA). All other antibodies were purchased from Santa Cruz Biotechnology. LipofectAMINE was purchased from Life Technologies. All other reagents were purchased from Sigma.
Bacterial Strains.
The P. aeruginosa strain used was PAO1, a well characterized prototypic strain (2). Flagellin was prepared as described (4). Pure (single band) flagellin was eluted from a 10% acrylamide gel and used in our experiments.
Ribonuclease Protection Assay (RPA).
RPA experiments were carried out by using an RNA probe containing a MUC 2-specific sequence as described (5).
Cell Culture and Transfection.
Human HM3 cells stably transfected with a MUC 2 promoter-driven luciferase reporter gene were maintained in DMEM supplemented with 10% FBS, penicillin, and streptomycin (100 μg/ml). Unless otherwise noted, cells were grown in 48-well plates until they reached 70–80% confluence. The medium was then changed to serum-free DMEM, and cells were incubated with either the appropriate stimulus (i.e., purified flagellin or α-ASGM1) or serum-free medium control for 4–6 h. Cells were lysed by using 100 μl Reporter Lysis Buffer (Promega), and relative light units of luciferase activity were read by using 150 μl luciferase substrate (Roche Molecular Biochemicals).
For transient transfections, HM3 cells grown in 24-well plates were transfected with 0.5 μg of pGL2 basic vector containing a 2.8-kb construct of the 5′-flanking region of human MUC 2 DNA using LipofectAMINE reagent. For cotransfections using dominant-negative mutants, 0.5 μg of both MUC 2 and the dominant-negative mutant were used per well. Cells were transfected for 6 h and lysed after 42 h. Empty expression vector was added when necessary to equalize the total amount of DNA transfected.
Immunoblotting.
Cell lysates were prepared by adding 400 μl of 2× SDS sample buffer with β-mercaptoethanol directly on cells plated in a six-well plate. Equal amounts of lysates were heated at 100°C for 3 min, and proteins were resolved by SDS/PAGE. For immunoblot analysis, proteins were transferred to nitrocellulose by using the Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell. Immunostaining was by routine methods as instructed by the manufacturer. Baseline levels of Erk 1/2 were visualized by stripping blots and reprobing with anti-Erk 1/2 antibody (rabbit polyclonal).
Calcium Imaging.
For real-time recording of [Ca]i, cells were grown in a monolayer on 10-mm2 polyornithine-coated chambered glass coverslips (Applied Scientific, San Francisco). HEK293 cells cotransfected with green fluorescent protein (GFP) and P2Y2 were plated at 8 × 105 cells/cm2 in the center of the coverslip and served as ATP “sensor” cells because they do not mobilize [Ca]i in response to stimulation with flagellin. HM3 or NCI 292 cells plated at 4 × 105 cells/cm2 in coculture with transfected “sensor” cells served as ATP “releasing” cells. Cells were loaded with Fura-2 (30 min at 37°C) in calcium imaging buffer (CIB) containing (in mM) 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 MgCl2, 1.2 NaHCO3, 10 glucose, 10 Hepes (pH 7.45), with 1 μM Fura-2 acetoxymethyl ester and 0.01% pleuronic acid (Molecular Probes), then rinsed twice with CIB. Images were collected with a Nikon Diaphot fluorescence microscope equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and an intensified charge-coupled device (CCD) camera (Hamamatsu, Middlesex, NJ). Dual images (340 and 380 nm excitation, 510 nm emission) were collected, and pseudocolor ratiometric images were monitored during the experiment (metafluor software, Universal Imaging, Media, PA). Cells were initially imaged in 100 μl of CIB, after which 100 μl CIB containing a 1:40 dilution of α-ASGM1 or 10 μg/ml flagellin was added. GFP-labeled cells in one high-power field were selected and overlayed on the ratiometric image to identify calcium-positive “sensor” cells. An amount equal to 100 μl of CIB containing 100 μM ATP was added after stimulation with the agonist to show a calcium response in the P2Y2-transfected “sensor” cells. Experiments using “sensor” cells transfected with GFP alone and cocultured with ATP “releasing” cells were also performed to rule out gap-junction communication leading to calcium mobilization in adjacent cells.
ATP Bioluminescence Assay.
ATP release into the medium was monitored by using the luciferin/luciferase-based Enlighten ATP assay system (Promega). Briefly, HM3 cells were grown in 48-well plates to 70% confluence and then serum-starved for 16 h. Because mechanical stimulation can cause ATP release from living cells, the culture dish was placed directly into the recording chamber of the Tropix (Bedford, MA) TR717 microplate luminometer and allowed 10 min to equilibrate before adding the agonist antibody. Medium was replaced with the agonist antibody and 100 μl of luciferase reagent without touching or tilting the cell monolayer. Luminescence was monitored for 35 min. Serum-free medium containing the luciferase reagent alone was placed in the control wells.
Results
ASGM1 Ligation Activates Mucin Transcription in a Ca2+-Dependent Manner.
The model used for these studies consists of homogeneous cultures of epithelial cells from human bronchus (NCIH292; ref. 6) or colon (HM3; ref. 7). In their responses to bacteria, both cell lines mimic bronchial cells in organ culture (8, 9). To conveniently monitor responses to bacterial stimuli, we stably transfected HM3 (7) with a construct consisting of the MUC 2 mucin 5′ upstream flank (10) ligated to the luciferase gene as a reporter (HM3MUC2 cells). Flagellin purified from P. aeruginosa and α-ASGM1 (referred to below as “agonist antibody”) stimulated MUC 2-luciferase activity in a dose-dependent manner up to ≈15- and ≈40-fold, respectively (Fig. 1 A and B). The endogenous MUC 2 gene responded similarly (Fig. 1C). In the following sections, epithelial cell exposures were performed with both agonist antibody and flagellin. Sensitivity to inhibitors was identical for the two agents. Agonist antibody data are shown to represent responses to both stimuli.
Figure 1.
Mucin (MUC 2) transcription is stimulated by ligation of the flagellin receptor, ASGM1. In A and B, HM3 cells stably transfected with a MUC 2 promoter-driven luciferase reporter were studied. Promoter activity is reported as the fold increase observed in stimulated cells vis-a-vis cells exposed to serum-free medium. (A) Stimulation with flagellin purified from strain PAO1. Proteolysis followed by boiling abolished activity. (B) Stimulation with an antibody directed against the flagellin receptor, asialoGM1 (α-ASGM1). Stimulation was for 4 h before cell lysis. Exposure of cells to an approximately equal concentration of an unrelated polyclonal antibody [CCAAT/enhancer binding protein α (α-c/ebp)] had no effect. (C) RNase protection assay was performed by using a MUC 2 gene-specific probe and a control cyclophilin probe to show MUC 2 mRNA up-regulation by purified flagellin (Flag) and ASGM1 antibody (α-ASGM1). SFM, serum-free medium.
To dissect the signaling pathway mediating responses to ASGM1 ligation, we first monitored the activity of signaling molecules implicated in host defensive responses studied previously. Elevations in intracellular Ca2+ have been shown to mediate both cytokine production¶ and phagocytosis (11) in cells exposed to bacteria or their products. As in ¶, HM3 cells loaded with the fluorescent Ca2+ indicator Fura-2 showed a transient increase in intracellular Ca2+ ≈20 s after administration of the agonist antibody (Fig. 2A) or flagellin (not shown). This finding raised the possibility that ASGM1-dependent mucin induction is mediated by an increase in intracellular Ca2+.
Figure 2.
ASGM1-mucin induction is calcium dependent. (A) Ratiometric calcium imaging of islands of HM3 cells loaded with Fura-2/AM shows increases in the 340/380-nm excitation fluorescence ratio (510 nm emission) after exposure to a (1:40) dilution of αASGM1. This increase in calcium mobilization peaks 20–24 s after antibody application. (B) HM3 cells stably transfected with a MUC 2 promoter-driven luciferase reporter gene were pretreated with BAPTA/AM (30 μM, 45 min) or thapsigargin (400 nM, 30 min) and then stimulated with a (1:100) dilution of ASGM1 antibody in the continued presence of the drug. After 4 h, cells were lysed and assayed for luciferase activity. Data are presented as the percentage of the mucin response, where 100% is assigned to cells treated with ASGM1 antibody in the presence of vehicle alone. Data are the mean ± SD of triplicate determinations from three to six separate experiments.
To test this hypothesis, we performed ASGM1 ligation experiments in which Ca2+ signaling was blocked by (i) 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester (BAPTA/AM), a Ca2+ chelator, or (ii) thapsigargin, which depletes Ca2+ stores by blocking Ca2+ ATPases in the endoplasmic reticulum. These agents inhibited mucin induction (Fig. 2B), indicating that an increase in intracellular free Ca2+ is required for activation of MUC 2 gene transcription after ASGM1 ligation.
MUC 2 Activation Is Erk 1/2-Dependent.
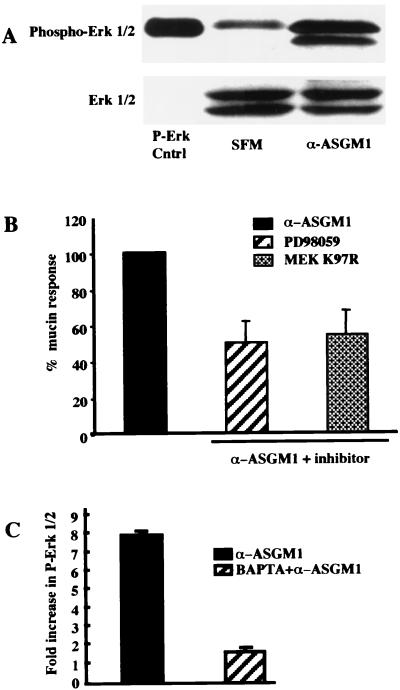
It has also been shown that certain host responses to bacteria are mediated by the mitogen-activated protein kinase (MAPK) Erk 1/2 (5). Using a phospho-Erk-specific antibody, we observed strong phosphorylation (activation) of both Erk 1 and 2 in agonist antibody-treated, but not control, cells (Fig. 3A). Because Erk 1/2 is the only substrate of MAPK/extracellular signal-regulated kinase kinase (MEK) 1/2, results showing that the MEK 1/2 inhibitor PD98059 and the MEK 1/2 dominant negative mutant MEK K97R inhibit mucin induction (Fig. 3B) indicate that Erk 1/2 is intrinsic to the ASGM1 response. Agonist antibody-induced phosphorylation of Erk 1/2 was almost completely blocked by BAPTA (Fig. 3C), indicating that elevated intracellular Ca2+ is required for, and therefore acts upstream of, Erk 1/2 phosphorylation. Notably, Ca2+ chelation almost completely blocked Erk phosphorylation (Fig. 3C) whereas Erk accounted for only about 50% of mucin induction (Fig. 3B), even when inhibitors were used at saturating concentrations. These data suggest that the ASGM-1 signaling pathway bifurcates after Ca2+ mobilization, giving rise to downstream events that are alternatively Erk dependent or independent.
Figure 3.
Ligation of flagellin receptor activates the MAP kinase cascade, which is required for mucin induction. (A) HM3 cells grown in six-well plates were lysed with SDS sample buffer 5 min after the addition of agonist antibody, α-ASGM1. Equal amounts of lysate were run on a 12% SDS gel, transferred to nitrocellulose, immunoblotted with a phospho-specific Erk 1/2 antibody, and visualized by chemiluminescence. Blots were stripped and reprobed with Erk 1/2 antibody to show equal loading. P-Erk Cntrl, positive control from vendor. (B) Effect of MEK 1/2 inhibitor PD98059 (37 μM, 30 min) and dominant negative mutant MEK K97R on MUC 2 promoter activity. (c) Inhibition of Erk phosphorylation by the calcium chelator, BAPTA/AM (30 μM, 45 min). Fold increase expressed as the ratio of phosphorylated Erk to baseline Erk 1/2 as determined by densitometry.
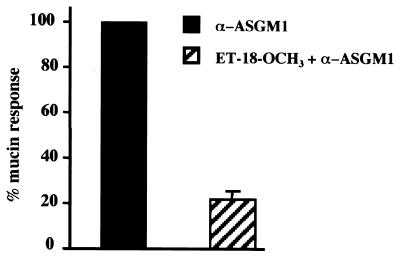
Mobilization of Ca2+ from intracellular stores is initiated by the phasic generation of inositol 1,4,5-triphosphate (IP3), a mediator that triggers Ca2+ release from the endoplasmic reticulum. The production of IP3 depends on the activity of phospholipase C (PLC), as is production of diacylglycerol. By directly measuring diacylglycerol and IP3 levels in stimulated cells vs, controls (not shown), we obtained evidence that PLC is activated by ASGM1 ligation. That the PLC inhibitor ET-18-OCH3 blocked mucin induction (Fig. 4)) supports the view that the Ca2+ mobilization illustrated in Fig. 2 stems from PLC activation.
Figure 4.
ASGM1 mucin induction depends on PLC. Effect of the phosphatidylinositol-specific phospholipase C (PI-PLC) inhibitor ET-18-OCH3 (30 μM, 45 min) on HM3 cells stably transfected with a MUC 2 promoter-driven luciferase reporter and stimulated with agonist antibody.
ATP Release and an ATP Receptor Link ASGM1 with Ca2+ Signaling.
We next asked how ligation of ASGM1, a membrane glycolipid, leads to activation of PLC, a cytoplasmic enzyme. PLC isoforms act downstream of both G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs), and we therefore asked whether mucin induction requires signaling through one of these two major receptor subtypes. Although genistein, an agent that blocks signaling through RTKs, had little or no effect (not shown), GPCR inhibitors suramin and GDPβS strongly inhibited mucin induction (Fig. 5A), suggesting that GPCRs are required for the response. Among the most extensively studied GPCRs known to exist on epithelial cells are receptors of the P2Y family that are activated by extracellular nucleotides (12–14). We observed that P2Y antagonists Reactive Blue 2 and Acid Blue 129 (15, 16) strongly inhibited mucin gene expression whereas inhibitors of P2X nucleotide-gated ion channels (PPADS and NF023) did not (Fig. 5A). This result reinforced the view that GPCRs are required for the response and more specifically implicated P2Y nucleotide receptors.
Figure 5.
ASGM1/mucin induction depends on a P2Y nucleotide receptor. (A) Involvement of a nucleotide receptor in the flagellin/mucin response demonstrated using the GPCR inhibitor suramin (250 μM, 45 min, in the presence of Triton X-100, 0.01%), the G-protein antagonist GDPβS (1 mM, 60 min, in the presence of Triton X-100, 0.01%), P2Y inhibitors [Reactive Blue-2 (100 μM, 45 min) and Acid Blue 129 (100 μM, 45 min)], and P2X inhibitors [PPADS (100 μM, 45 min) and NF023 (300 μM, 45 min)]. (B) Measurement of ATP in the extracellular medium of HM3 cells grown to 70% confluence and stimulated with 1:40 dilution of α-ASGM1 either alone or in the presence of 2.5 units/ml apyrase. (C) Luciferase reporter assay. HM3 MUC 2 cells pretreated with ATP permeability inhibitors, gadolinium (200 μM, 45 min), or glybenclamide (100 μM, 45 min) were then stimulated with α-ASGM1 antibody (1:100) for 4 h. Data are the mean ± SD values of triplicate determinations from three to six separate experiments.
One possible mode of interaction between ASGM1 and P2Y receptors would be through ASGM1's stimulating the extracellular release of ATP, followed by ATP binding to P2Y receptors on the same or adjacent cells. Indeed, direct measurement with a luciferin-luciferase ATP assay system showed that ATP is released extracellularly in response to agonist antibody (Fig. 5B). That this not only occurs, but also is required for mucin induction, was indicated by experiments in which gadolinium and glybenclamide, which are known to inhibit ATP release (17–19), inhibited the mucin response (Fig. 5C).
Consistent with the autocrine release of ATP, exogenous nucleotides showed additive or greater than additive effects with agonist antibody (not shown). The nucleotides alone stimulated mucin induction with a rank order efficacy consistent with that of receptors P2Y1 (20, 21) and P2Y11 (22). Involvement of P2Y1 can be excluded based on insensitivity of the response to the P2Y1-specific antagonist adenosine 3-phosphothio 5-phosphate (not shown). P2Y11 is a viable candidate, but we cannot rule out the participation of a yet unidentified P2Y receptor.
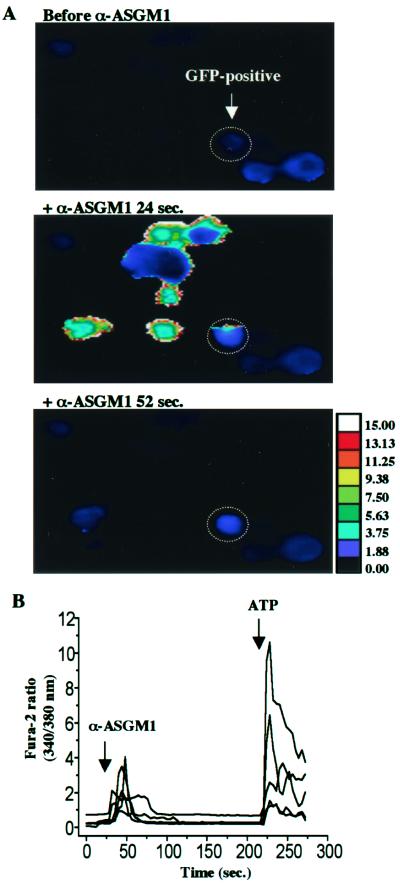
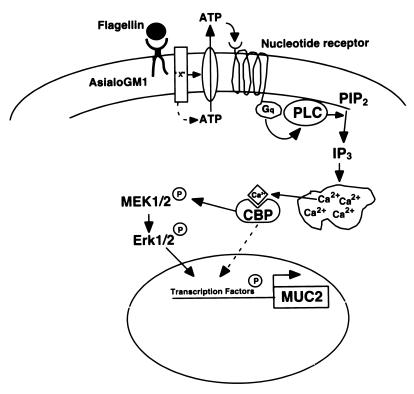
Taken together, the data in Fig. 5 suggest a novel mechanism of pathogenesis in which the ligation of a receptor lacking a transmembrane domain (ASGM1) stimulates autocrine nucleotide signaling. We obtained additional evidence from experiments (Fig. 6) where HM3 or NCIH292 cells, which release ATP and mobilize Ca2+ in response to agonist antibody, were cocultured with HEK 293 cells that were not antibody responsive. The latter had been transfected with P2Y2, enabling them to mobilize intracellular Ca2+ in response to ATP, and with GFP, permitting them to be identified during the course of Ca2+ imaging (HEK-P2Y2-GFP cells). Although HEK-P2Y2-GFP cells did not show Ca2+ mobilization in response to either flagellin or agonist antibody when grown by themselves, they displayed clear Ca2+ responses when grown with ATP-releasing cells (Fig. 6 A and B). These results provide further support for the view that ASGM1 stimulation leads to the release of ATP extracellularly and that autocrine/paracrine activation of nucleotide receptors provides the link between ASGM1 and intracellular signaling mechanisms. The proposed model is shown in Fig. 7.
Figure 6.
Fura-2 measurements of [Ca2+]i in cocultured cells. (A) Pseudocolor images of HEK293 cells cotransfected with GFP and P2Y2 (HEK-P2Y2-GFP, ATP “sensors”) in coculture with NCI H292 cells (ATP “releasers”) at three different time points. Representative HEK-P2Y2-GFP cell identified retrospectively by its GFP label is circled and shows an increase in intracellular Ca2+ 24 s after the addition of α-ASGM1 (1:40), which recovers by 52 s (typical of 28 cells, n = 5 experiments). (B) Fura-2 ratios showing increased [Ca2+]i 20 s after the addition of α-ASGM1 in ATP “sensing,” GFP-labeled HEK293 cells, followed by stimulation with 100 μM ATP as a control. Cells were imaged in the same high-power (×20) field while in coculture with ATP “releasers,” NCI H292 cells.
Figure 7.
Cartoon depicting events in flagellin-triggered host cell signaling. Flagellin binds to asialoGM1, a membrane glycolipid, which causes the extracellular release of ATP, which then binds to a nucleotide receptor. Downstream events include G-protein activation, the cleavage of PIP2 by PLC, the formation of IP3, Ca2+ mobilization, the phosphorylation of MEK 1/2 and Erk 1/2 [via an unknown calcium binding protein (CBP)], and mucin (MUC 2) transcription. Flagellin-induced signaling bifurcates after Ca2+ mobilization, with ≈50% of the response giving rise to downstream events that are Erk dependent whereas the remaining 50% is Erk independent.
Discussion
We have shown that the stimulation of host gene expression by P. aeruginosa flagellin or an ASGM1 agonist antibody is mediated by an autocrine mechanism involving ATP release and the binding of ATP to a nucleotide receptor. Downstream events including PLC activation and Ca2+ mobilization are consistent with previously described functions of nucleotide receptors (18, 23–25). A model for the proposed mechanisms is shown in Fig. 7. Our results are significant both in demonstrating a role for nucleotide receptors in bacterial pathogenesis and in explaining how the glycolipid bacterial receptor ASGM1 is able to engage cytoplasmic signaling networks.
Precedent for nucleotide signaling in host defense can be found in earlier work showing effects of extracellular ATP on mast cells, macrophages, and lymphocytes (26–28). Of particular interest are the results of experiments showing that ATP released from mast cells during degranulation can activate P2 receptors on adjacent cells, thereby causing further degranulation through mobilization of intracellular calcium (24). This is analogous to results in Fig. 6 showing that epithelial cells exposed to a bacterial stimulus also release nucleotide that can activate neighboring cells to mobilize intracellular Ca2+. This phenomenon could play a major role in the initiation of defensive responses throughout a mucosal epithelium, even when only a small fraction of the surface is directly exposed to pathogen. That autocrine ATP signaling also mediates regulatory volume decrease (RVD) in cells that have swelled in response to hypotonic stress (29) suggests that such signaling may be a common mediator of defensive responses in general.
Although it is clear that ATP resides in some secretory granules (e.g., ref. 24) and that exocytosis is one mechanism by which ATP is released extracellularly, it is unlikely that this constitutes the source of ATP in our studies. Although both HM3 and NCIH292 cells have some properties of adenocarcinoma (exocrine-like) cells and are capable of mucin production, the extensive literature on mucin does not describe its corelease with ATP, nor is this likely because both molecules are negatively charged. Thus, the means by which ATP is released from HM3 and NCIH292 cells (and mucosal epithelial cells in general) is unknown. Some reports suggest that ATP is pumped out of cells by members of the ABC transporter family, at least one member of which, the cystic fibrosis transmembrane conductance regulator (CFTR) is present on mucosal epithelial cells (30, 31). Other reports (32) dispute this conclusion, however, leaving the question of how ATP exits cells in a nonexocytotic manner still unresolved.
Another question requiring further investigation is that of how Ca2+ mobilization leads to Erk 1/2 activation. Although Ca2+ has been reported to activate MAPK signaling via the Ca2+-dependent tyrosine kinase Pyk2 in PC12 cell (33, 34), we found no evidence for flagellin-induced Pyk2 phosphorylation in immunoblots, and no effect of the Pyk2 dominant negative mutant, Pyk2-KM (the generous gift of Axel Ullrich), on mucin induction. Similarly, a protein kinase C (PKC)-dependent mechanism linking P2Y receptors with Erk 1/2(MAPK) in epithelial cells (35) does not appear applicable to the present results because mucin induction by ASGM1 is not sensitive to the PKC inhibitor bisindoleamide (not shown). These differences with respect to other systems testify to the complexity and context-dependency of Ca2+-dependent signaling pathways noted earlier (35).
Within the specific context of mucin production, however, it is possible to note significant common themes. For example, the signaling pathway by which ASGM1 stimulates mucin transcription is similar to that stimulated by the Gram-negative endotoxin, lipopolysaccharide (LPS; ref. 5) as well as by the Gram-positive cell wall component, lipoteichoic acid (LTA; H. Lemjabbar and C.B., unpublished results) in that it requires activation of Erk 1/2. Convergence of these bacterial recognition pathways on Erk 1/2 is consistent with the idea that mucin activation pathways evolved from a single phylogenetically ancient network (36).
In summary, our results reveal the existence of an autocrine nucleotide loop responsible for mediating a host cell response to P. aeruginosa flagellin. Although our endpoint is transcription of the host defense gene mucin, involvement of the MAP kinase Erk 1/2 suggests that the consequences of flagellin-induced nucleotide signaling may be much more general, affecting multiple genes. Thus, the modulation of nucleotide receptor signaling could play an important role in optimizing the host response to bacterial infection.
Acknowledgments
We thank Alice Prince, M.D. for a sample of purified flagellin and instructions regarding its purification as well as helpful discussion. We thank Marianne Gallup for technical advice. This work was supported by National Institutes of Health Grants RO1 HL43762 and PO1 H L24136 (to C.B.), K23 EY00371 (to N.M.) and GM 44298 (to D.J.).
Abbreviations
- ASGM1
asialoGM1
- α-ASGM1
anti-ASGM1 antibody
- MAPK
a mitogen-activated protein kinase
- MUC 2
mucin MUC 2 gene
- ERK
extracellular signal-regulated kinase
- PPADS
pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt
- GFP
green fluorescent protein
- MEK
MAPK/extracellular signal-regulated kinase kinase
- PLC
phospholipase C
- GPCR
G protein-coupled receptor
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
Footnotes
Bryan, R., Ratner, A., Heyer, G., Nguyen, B., Davis, M. B., Heath, M., Cheung, A. A., Weiser, J. & Prince, A. Ninety-ninth General Meeting of the American Society of Microbiology, June 1, 1999, Chicago, IL, abstr. B/D-245, p. 77.
References
- 1.Shapiro L. Cell. 1995;80:525–527. doi: 10.1016/0092-8674(95)90505-7. [DOI] [PubMed] [Google Scholar]
- 2.DiMango E, Zar H J, Bryan R, Prince A. J Clin Invest. 1995;96(5):2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gewirtz A T, Simon P O, Jr, Schmitt C K, Taylor L J, Hagedorn C H, O'Brien A D, Neish A S, Madara J L. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J D, Feng W, Gallup M, Kim J H, Gum J, Kim Y, Basbaum C. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine S J, Larivee P, Logun C, Angus C W, Ognibene F P, Shelhamer J H. Am J Respir Cell Mol Biol. 1995;12:196–204. doi: 10.1165/ajrcmb.12.2.7865217. [DOI] [PubMed] [Google Scholar]
- 7.Kuan S F, Byrd J C, Basbaum C B, Kim Y S. Cancer Res. 1987;47:5715–5724. [PubMed] [Google Scholar]
- 8.Li Q, Schachter J B, Harden T K, Nicholas R A. Biochem Biophys Res Commun. 1997;236:455–460. doi: 10.1006/bbrc.1997.6984. [DOI] [PubMed] [Google Scholar]
- 9.Dohrman A, Miyata S, Gallup M, Li J D, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Biochim Biophys Acta. 1998;1406:251–259. doi: 10.1016/s0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 10.Gum J R, Hicks J W, Kim Y S. Biochem J. 1997;325, Pt. 1:259–267. doi: 10.1042/bj3250259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilsson A, Lundqvist H, Gustafsson M, Stendahl O. J Leukocyte Biol. 1996;59:902–907. doi: 10.1002/jlb.59.6.902. [DOI] [PubMed] [Google Scholar]
- 12.Cressman V L, Lazarowski E, Homolya L, Boucher R C, Koller B H, Grubb B R. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 13.Homolya L, Watt W C, Lazarowski E R, Koller B H, Boucher R C. J Biol Chem. 1999;274:26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- 14.North R A, Barnard E A. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 15.Rice W R, Singleton F M. Br J Pharmacol. 1989;97:158–162. doi: 10.1111/j.1476-5381.1989.tb11937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuluc F, Bultmann R, Glanzel M, Frahm A W, Starke K. Naunyn-Schmiedebergs Arch Pharmakol. 1998;357:111–120. doi: 10.1007/pl00005144. [DOI] [PubMed] [Google Scholar]
- 17.Schwiebert E M, Egan M E, Hwang T H, Fulmer S B, Allen S S, Cutting G R, Guggino W B. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A L, Kudlow B A, Marrs K L, Gruenert D C, Guggino W B, Schwiebert E M. Am J Physiol. 1998;275, 5 Pt. 1:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- 19.Roman R M, Feranchak A P, Davison A K, Schwiebert E M, Fitz J G. Am J Physiol. 1999;277, 6 Pt. 1:G1222–G1230. doi: 10.1152/ajpgi.1999.277.6.G1222. [DOI] [PubMed] [Google Scholar]
- 20.Webb T E, Simon J, Krishek B J, Bateson A N, Smart T G, King B F, Burnstock G, Barnard E A. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 21.Henderson D J, Elliot D G, Smith G M, Webb T E, Dainty I A. Biochem Biophys Res Commun. 1995;212:648–656. doi: 10.1006/bbrc.1995.2018. [DOI] [PubMed] [Google Scholar]
- 22.Communi D, Govaerts C, Parmentier M, Boeynaems J M. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- 23.Soltoff S P, McMillian M K, Lechleiter J D, Cantley L C, Talamo B R. Ann NY Acad Sci. 1990;603:76–90. doi: 10.1111/j.1749-6632.1990.tb37663.x. [DOI] [PubMed] [Google Scholar]
- 24.Osipchuk Y, Cahalan M. Nature (London) 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K, Suzuki M, Furukawa M, Takasaka T. Cell Calcium. 1995;18:89–99. doi: 10.1016/0143-4160(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 26.Wiley J S, Chen J R, Snook M B, Jamieson G P. Br J Pharmacol. 1994;112:946–950. doi: 10.1111/j.1476-5381.1994.tb13172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambon A, Bronte V, Di Virgilio F, Hanau S, Steinberg T H, Collavo D, Zanovello P. Cell Immunol. 1994;156:458–467. doi: 10.1006/cimm.1994.1190. [DOI] [PubMed] [Google Scholar]
- 28.Di Virgilio F. Immunol Today. 1995;16(11):524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 29.Light D B, Capes T L, Gronau R T, Adler M R. Am J Physiol. 1999;277, 3 Pt. 1:C480–C491. doi: 10.1152/ajpcell.1999.277.3.C480. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E H, Prat A G, Gerweck L, Seneveratne T, Arceci R J, Kramer R, Guidotti G, Cantiello H F. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reisin I L, Prat A G, Abraham E H, Amara J F, Gregory R J, Ausiello D A, Cantiello H F. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- 32.Reddy M M, Quinton P M, Haws C, Wine J J, Grygorczyk R, Tabcharani J A, Hanrahan J W, Gunderson K L, Kopito R R. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 33.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 34.Dikic I, Dikic I, Schlessinger J. J Biol Chem. 1998;273:14301–14308. doi: 10.1074/jbc.273.23.14301. [DOI] [PubMed] [Google Scholar]
- 35.Short S M, Boyer J L, Juliano R L. J Biol Chem. 2000;275:12970–12977. doi: 10.1074/jbc.275.17.12970. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R, Janeway C A., Jr Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]