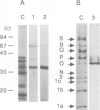
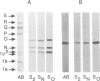
Abstract
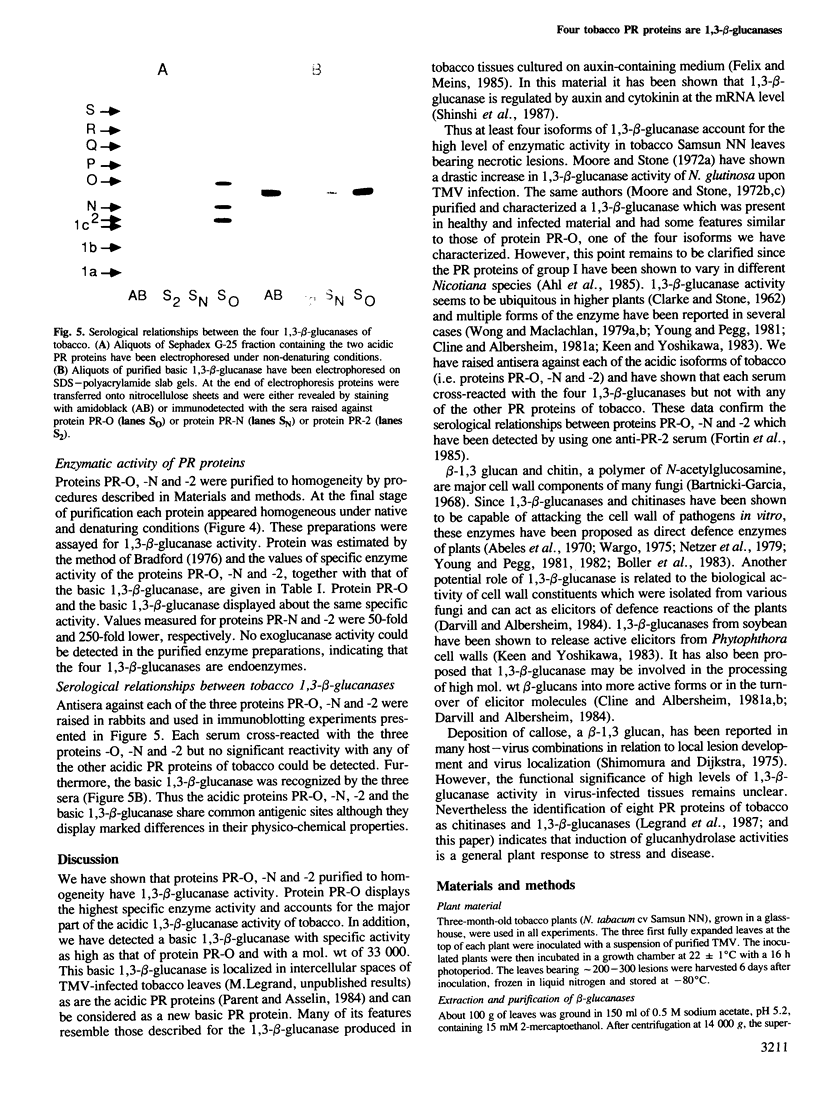
Three of the ten acidic `pathogenesis-related' (PR) proteins known to accumulate in Nicotiana tabacum cv Samsun NN reacting hypersensitively to tobacco mosaic virus, namely −O, −N and −2, have been shown to have 1,3-β-glucanase (EC 3.2.1.39) activity. By using sera raised against each protein purified to homogeneity close serological relationships have been demonstrated between the three proteins. The same specific sera cross-reacted with a basic protein which is also a 1,3-β-glucanase induced by virus infection and which can be considered as a new basic pathogenesis-related protein of tobacco. Protein PR-O and the basic 1,3-β-glucanase display about the same specific enzymatic activity, i.e. 50-fold and 250-fold higher than specific activities of proteins PR-N and -2 respectively.
Keywords: 1,3-β-glucanases; purification; specific activities; antibodies; serological relationships
Full text
PDF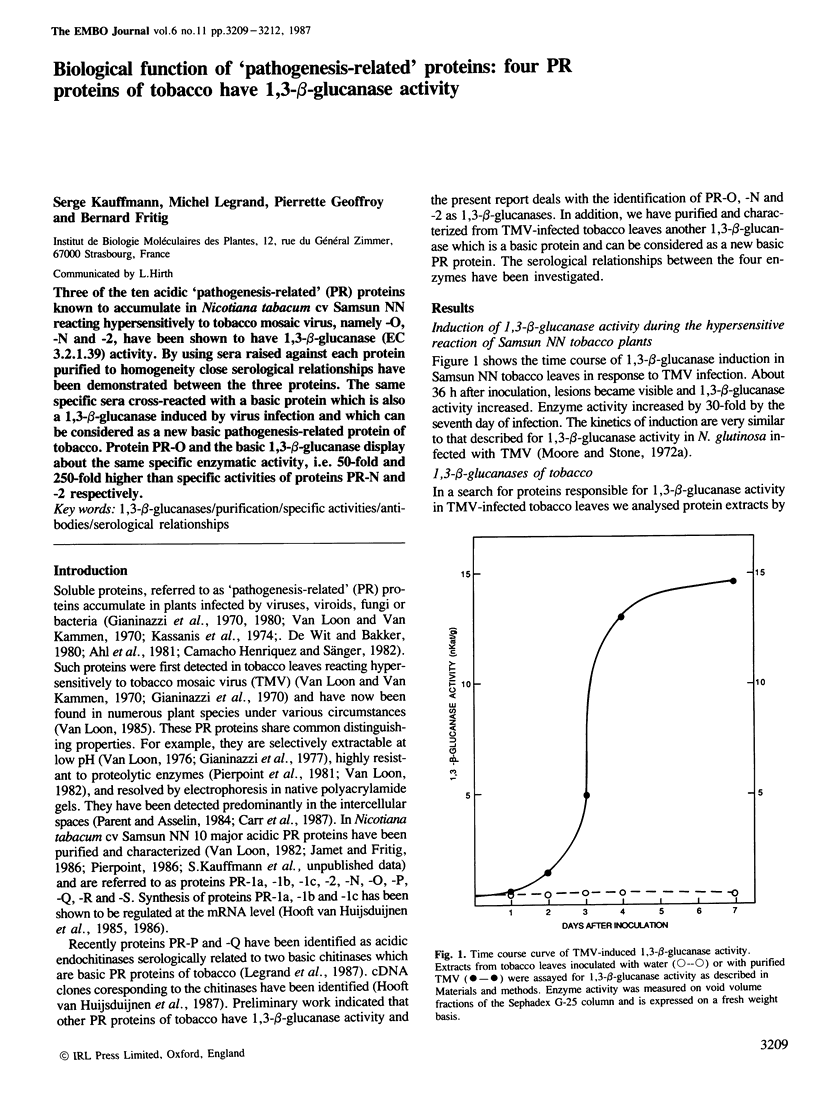
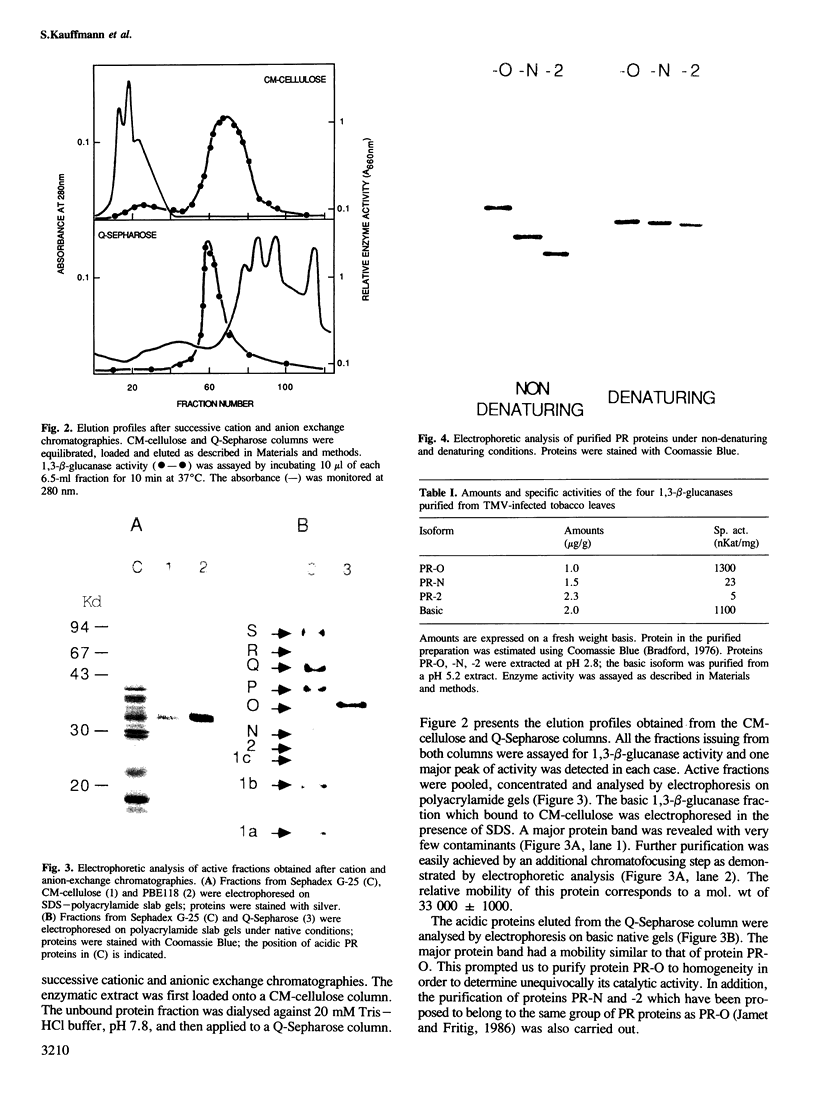

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Bosshart R. P., Forrence L. E., Habig W. H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971 Jan;47(1):129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camacho Henriquez A., Sänger H. L. Analysis of acid-extractable tomato leaf proteins after infection with a viroid, two viruses and a fungus and partial purification of the "pathogenesis-related" protein p 14. Arch Virol. 1982;74(2-3):181–196. doi: 10.1007/BF01314711. [DOI] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Nikolau B. J., Voelkerding K. V., Klessig D. F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987 Apr;7(4):1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Albersheim P. Host-Pathogen Interactions : XVII. HYDROLYSIS OF BIOLOGICALLY ACTIVE FUNGAL GLUCANS BY ENZYMES ISOLATED FROM SOYBEAN CELLS. Plant Physiol. 1981 Jul;68(1):221–228. doi: 10.1104/pp.68.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Albersheim P. Host-Pathogen Interactions: XVI. PURIFICATION AND CHARACTERIZATION OF A beta-GLUCOSYL HYDROLASE/TRANSFERASE PRESENT IN THE WALLS OF SOYBEAN CELLS. Plant Physiol. 1981 Jul;68(1):207–220. doi: 10.1104/pp.68.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S., Martin C., Vallée J. C. Hypersensibilité aux virus, température et protéines soubles chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 11;270(19):2383–2386. [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., Cornelissen B. J., van Loon L. C., van Boom J. H., Tromp M., Bol J. F. Virus-induced synthesis of messenger RNAs for precursors of pathogenesis-related proteins in tobacco. EMBO J. 1985 Sep;4(9):2167–2171. doi: 10.1002/j.1460-2075.1985.tb03911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. A -I,3-glucan hydrolase from Nicotiana glutinosa. I. Extraction, purification and physical properties. Biochim Biophys Acta. 1972 Jan 20;258(1):238–247. doi: 10.1016/0005-2744(72)90982-5. [DOI] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. A -I,3-glucan hydrolase from Nicotiana glutinosa. II. Specificity, action pattern and inhibitor studies. Biochim Biophys Acta. 1972 Jan 20;258(1):248–264. doi: 10.1016/0005-2744(72)90983-7. [DOI] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. Effect of infection with TMV and other viruses on the level of a -1,3-glucan hydrolase in leaves of Nicotiana glutinosa. Virology. 1972 Dec;50(3):791–798. doi: 10.1016/0042-6822(72)90433-3. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Maclachlan G. A. 1,3-beta-D-glucanases from Pisum sativum seedlings. I. Isolation and purification. Biochim Biophys Acta. 1979 Dec 7;571(2):244–255. doi: 10.1016/0005-2744(79)90095-0. [DOI] [PubMed] [Google Scholar]
- Wong Y. S., Maclachlan G. A. 1,3-beta-D-glucanases from Pisum sativum seedlings. II. Substrate specificities and enzymic action patterns. Biochim Biophys Acta. 1979 Dec 7;571(2):256–269. doi: 10.1016/0005-2744(79)90096-2. [DOI] [PubMed] [Google Scholar]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]