Abstract
Parabens, benzophenone-3 and triclosan are common ingredients used as preservatives, ultraviolet radiation filters and antimicrobial agents, respectively. Human exposure occurs through consumption of processed food and use of cosmetics and consumer products. The aim of this study was to provide a preliminary characterisation of exposure to selected personal care product chemicals in the general Australian population. De-identified urine specimens stratified by age and sex were obtained from a community-based pathology laboratory and pooled (n = 24 pools of 100). Concentrations of free and total (sum of free plus conjugated) species of methyl, ethyl, propyl and butyl paraben, benzophenone-3 and triclosan were quantified using isotope dilution tandem mass spectrometry; with geometric means 232, 33.5, 60.6, 4.32, 61.5 and 87.7 ng/mL, respectively. Age was inversely associated with paraben concentration, and females had concentrations approximately two times higher than males. Total paraben and benzophenone-3 concentrations are significantly higher than reported worldwide, and the average triclosan concentration was more than one order of magnitude higher than in many other populations. This study provides the first data on exposure of the general Australian population to a range of common personal care product chemical ingredients, which appears to be prevalent and warrants further investigation.
Keywords: Biomonitoring, Urine, Parabens, Personal care products, Population monitoring, Children
1. Introduction
The type, concentration and use of chemical ingredients in personal care products are many and varied. Parabens are alkyl esters (e.g., methyl (MeP), ethyl (EtP), propyl (PrP), butyl (BuP)) of p-hydroxybenzoic acid, used widely as antimicrobial preservatives in cosmetics, pharmaceuticals and processed food products (Eriksson et al., 2008; Shen et al., 2007; Soni et al., 2005) due to their stability, high water solubility, and low cost. Exposure occurs primarily via dermal absorption (Soni et al., 2005) and after metabolism, excretion occurs largely via urine. Results from in vitro and in vivo experiments suggest estrogenic activity of MeP, EtP, PrP and BuP (Boberg et al., 2010; Darbre and Harvey, 2008), but several orders of magnitude lower than that of oestrogen. Adverse reproductive effects of BuP and PrP have been reported in some animal studies (Oishi, 2002a,b) but not others (Hoberman et al., 2008), with some association with sperm damage (Meeker et al., 2011) and altered thyroid hormones (Koeppe et al., 2013) in humans.
Benzophenone-3 is a broadband ultraviolet radiation filter used as a sunscreen and photostabiliser in various cosmetic products worldwide; dermal contact is the dominant exposure pathway (Kim and Choi, 2014; Liao and Kannan, 2014). Urinary benzophenone-3 is used as the primary biomarker of exposure (Wang and Kannan, 2013). Benzophenone-3 exhibits slight estrogenic potential, and there is evidence for influence on reproduction and sex hormone signalling in rodents (Kim and Choi, 2014), and for influence on hormone-dependent diseases and adverse birth outcomes in humans (Kunisue et al., 2012; Wolff et al., 2008).
Triclosan is a synthetic, broad spectrum antimicrobial agent used in a wide range of personal care products and other consumer items (Rodricks et al., 2010; Witorsch and Thomas, 2010), and exposure mainly occurs through dermal application or oral use of consumer products containing triclosan. Triclosan is rapidly metabolised and excreted as conjugated urinary metabolites. The endocrine disrupting potential of triclosan is under debate (Huang et al., 2014; Jung et al., 2012; Lee et al., 2014; Witorsch, 2014; Witorsch and Thomas, 2010), and recent evidence suggests liver carcinogenicity (Yueh et al., 2014). Furthermore, concern has been raised as to the development of triclosan-resistant pathogens due to widespread use (Aiello and Larson, 2003; Levy, 2001).
Human exposure to chemicals used in personal care products occurs as a result of the frequent and complex use of such products, and biomonitoring is regarded as the gold standard for exposure assessment (Sexton et al., 2004). Biomonitoring data of the prevalence of exposures to chemicals used in personal care products exists for Northern European (Den Hond et al., 2013; Dewalque et al., 2014; Frederiksen et al., 2014; Moos et al., 2014; Pirard et al., 2012), North American (Calafat et al., 2008a,b, 2010; CDC, 2015, Health Canada, 2013; Meeker et al., 2013; Wang et al., 2013) and South East Asian (Kim et al., 2011; Ma et al., 2013; Shirai et al., 2013) populations, but the extent of Australians' exposure to personal care product chemicals is unknown. As biomonitoring is expensive pooling is an affordable alternative. The suitability of pooled biological samples for monitoring temporal and spatial trends in exposure has been demonstrated (Heffernan et al., 2013, 2014a,b). The aim of this study was to provide a preliminary characterisation of exposure to selected personal care product chemicals in the general Australian population using pooled urine specimens.
2. Materials and methods
2.1. Study population and sample collection
De-identified urine specimens were obtained from a community-based pathology laboratory (Sullivan Nicolaides Pathology, Taringa, QLD, Australia) from surplus stored urine that had been collected and analysed as part of routine testing throughout the state of Queensland, Australia. Urine specimens were collected from November 2012 to November 2013 in sterile polyethylene urine specimen containers, refrigerated for up to three days, and then frozen. As this was a pre-existing, convenience population no specific sampling protocols were employed. This work was approved by the University of Queensland ethics committee (approval number 2013000397). The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
2.2. Pooling protocol
Descriptive information about each specimen was limited to date of sample collection, sex, and date of birth of the individual. Before pooling, samples were stratified by age and sex into the following strata: 0–4, 5–14, 15–29, 30–44, 45–59, >60 years. The mean age of each pool was calculated from the average age of the individuals making up that pool. A total of 2400 individual specimens were combined into 24 pools, with 100 individual specimens contributing to each pool; there was a replicate pool for each of the 12 demographic groups. Specimens were pooled based on volume, where each individual in the pool contributed the same volume, thus the concentration measured in each pool is equivalent to the arithmetic mean of the concentration in each individual sample contributing to the pool (Caudill, 2010; Mary-Huard, 2007). During pooling, individual urine specimens were thawed, homogenised and aliquoted, after which the pooled sample was well-mixed, divided into smaller aliquots and frozen until analysis. A synthetic urine sample was included as a procedural blank (Calafat and Needham, 2009). No measures of creatinine or specific gravity were available for individual samples.
2.3. Chemical analysis
Concentrations of the free and total (sum of free and conjugated) species of MeP, EtP, PrP and BuP and benzophenone-3 and triclosan in urine were measured at the CDC (Atlanta, USA) using online solid phase extraction-high performance liquid chromatography isotope dilution tandem mass spectrometry as described previously (Ye et al., 2005,2006). Concentrations of free species were measured using the same methodology, but omitting the enzymatic hydrolysis. To monitor for accuracy and precision, each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentrations. The limits of detection (LOD) were 1 ng/mL (MeP, EtP, triclosan), 0.2 ng/mL (benzophenone-3), and 0.1 ng/mL (PrP and BuP). We did not detect the target compounds in the synthetic urine sample.
2.4. Statistical analysis
The influence of age (in years) and sex on chemical concentration was assessed via linear regression on ln-transformed urinary concentration, as follows:
| (1) |
An interaction term between age and sex was included in the models where significant. We summed the concentrations of the four parabens to create a summary measure (Σ paraben). All analyses were conducted using IBM SPSS Statistics, version 22 for Windows, (IBM, New York, USA, www.ibm.com). Criteria for significance were set as p < 0.05. Outliers in the ln-transformed values were identified using the outlier labelling rule (Hoaglin and Iglewicz, 1987; Hoaglin et al., 1986).
3. Results
3.1. Parabens
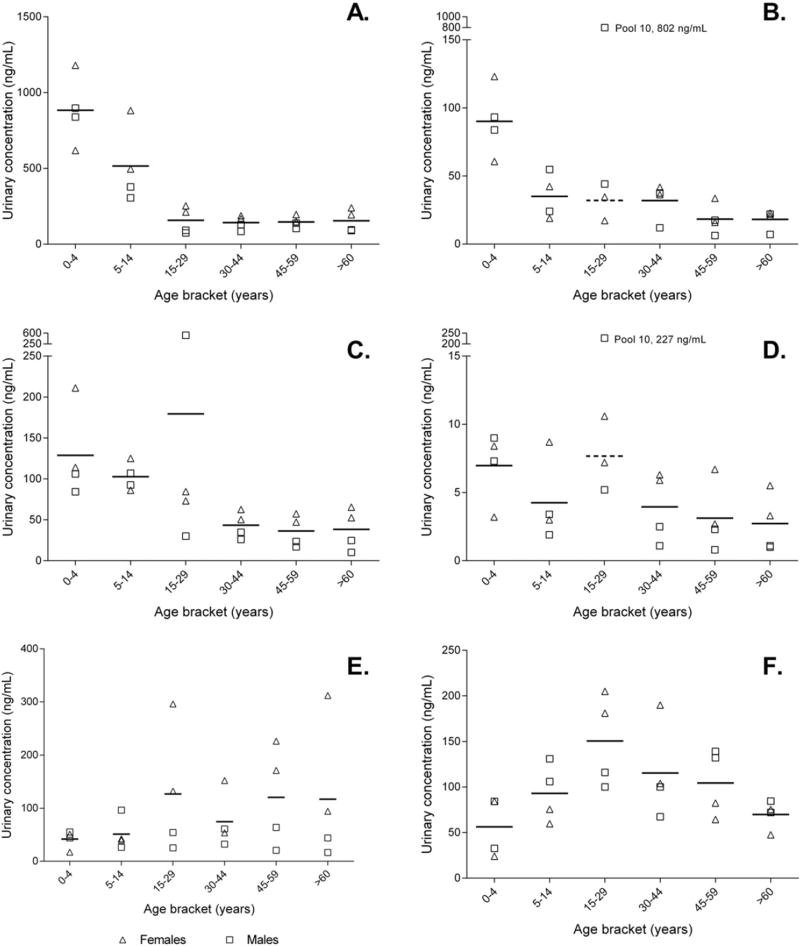
Results for MeP, EtP, PrP, BuP and Σ paraben concentrations for samples pooled by age and sex (n = 24) are summarised in Table 1. MeP, EtP, PrP and BuP were detected in all samples predominantly in their conjugated form (78–100%, Table SI-1) at total concentrations ranging from 74.4–1180, 6.3–802, 10.2–530 and 0.8–227 ng/mL, respectively; geometric means (GM) were 232 ng/mL (MeP), 33.5 ng/mL (EtP), 60.6 ng/mL (PrP), and 4.3 ng/mL (BuP). Σ paraben total concentration ranged from 114 to 1650 ng/mL, with GM 356 ng/mL. One pooled sample (pool 10, Table 1) had relatively high total concentrations of EtP (802 ng/mL), PrP (530 ng/mL) and BuP (227 ng/mL) compared with other pools in the same age strata. These values were identified as statistical outliers for EtP and BuP, but not for PrP. Omission of the outliers yields GMs of 29.1 ng/mL and 3.64 ng/mL for EtP and BuP, respectively..
Table 1.
Summary of pool characteristics and chemical concentration (ng/mL) per strata. Each pool represents 100 individuals.
| Pool # | Age strata (years) | Av. age (years) | Urinary total concentration (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Benzophenone-3 | Triclosan | MeP | EtP | PrP | BuP | Σ paraben | |||
| 1 | 0–4 | 2.93 | 55.3 | 84.2 | 898 | 93.2 | 106 | 7.3 | 1110 |
| 2 | 2.74 | 44.0 | 32.6 | 839 | 83.8 | 84.3 | 9 | 1020 | |
| 3 | 3.33 | 51.0 | 84.3 | 1180 | 123 | 211 | 8.4 | 1520 | |
| 4 | 3.24 | 17.2 | 24.1 | 618 | 60.6 | 114 | 3.2 | 796 | |
| 5 | 5–14 | 8.83 | 26.2 | 106 | 306 | 24.1 | 92.5 | 1.9 | 425 |
| 6 | 9.21 | 96.2 | 131 | 378 | 54.8 | 107 | 3.4 | 543 | |
| 7 | 8.74 | 39.8 | 75.5 | 882 | 18.9 | 125 | 8.7 | 1040 | |
| 8 | 9.54 | 42.3 | 59.8 | 496 | 42.3 | 86.1 | 3 | 627 | |
| 9 | 15–29 | 24.3 | 54.1 | 116 | 74.4 | 44.2 | 30.1 | 5.2 | 154 |
| 10 | 24.0 | 25.3 | 100 | 91.9 | 802* | 530 | 227* | 1650 | |
| 11 | 24.0 | 132 | 205 | 253 | 34.6 | 84.5 | 10.6 | 383 | |
| 12 | 23.4 | 296 | 181 | 212 | 17.2 | 73.1 | 7.2 | 310 | |
| 13 | 30–44 | 37.8 | 32.2 | 100 | 129 | 12 | 34.8 | 2.5 | 178 |
| 14 | 37.3 | 60.9 | 67.4 | 84.6 | 37.5 | 26 | 1.1 | 149 | |
| 15 | 36.7 | 152 | 190 | 188 | 41.8 | 62.6 | 6.3 | 299 | |
| 16 | 36.8 | 53.6 | 104 | 168 | 36.3 | 50.4 | 5.9 | 261 | |
| 17 | 45–59 | 52.9 | 20.7 | 139 | 139 | 17.5 | 17 | 0.8 | 174 |
| 18 | 53.2 | 63.7 | 132 | 104 | 6.3 | 23.6 | 2.3 | 136 | |
| 19 | 53.3 | 226 | 82.3 | 196 | 33.6 | 57.4 | 6.7 | 294 | |
| 20 | 53.0 | 171 | 64.4 | 149 | 15.9 | 47.2 | 2.7 | 215 | |
| 21 | >60 | 73.7 | 44.0 | 84.4 | 90.1 | 22 | 24.8 | 1.1 | 138 |
| 22 | 71.9 | 16.5 | 72.1 | 95.4 | 7.1 | 10.2 | 1 | 114 | |
| 23 | 75.1 | 312 | 47.5 | 194 | 20.6 | 52.6 | 3.3 | 271 | |
| 24 | 76.1 | 94.2 | 75.4 | 240 | 22.9 | 65.5 | 5.5 | 334 | |
| Total | 21.4 | 61.5 | 87.7 | 232 | 33.5 | 60.6 | 4.32 | 356 | |
Italicized data represent females pools. Asterisk indicates statistical outlier.
There was a small but significant inverse association between age and total concentration for MeP (p = 0.0001), EtP (p = 0.008) and PrP (p = 0.0004), but not BuP (Table 2). When the outlier was removed, the strength of the association increased, and age became a significant predictor of BuP concentration (p = 0.007) (Table SI-2). Female pools had MeP total concentrations 1.8 times higher than male pools. There were no significant differences between male and female pools for EtP and PrP. For BuP sex was significant only if the outlier was omitted, with total concentrations in the female pools being 2.3 times higher than in male pools.
Table 2.
Regression parameters (β (95% CI)) for log-transformed urinary total concentrations (ng/mL) of methyl paraben (MeP), ethyl paraben (EtP), propyl paraben (PrP), butyl paraben (BuP); and sum paraben (Σ paraben) (sum of MeP, EtP, PrP and BuP) (n = 24 pooled samples).
| MeP | EtP | PrP | BuP | Σ paraben | |
|---|---|---|---|---|---|
| Intercept | 6.499 (6.029 to 6.969) | 4.198 (3.462 to 4.933) | 5.076 (4.556 to 5.597) | 2.262 (1.368 to 3.156) | 6.861 (6.403 to 7.318) |
| Age (years) | −0.023*** (−0.033 to −0.013) | −0.021** (−0.037 to −0.006) | −0.022*** (−0.032 to −0.011) | −0.017 (0.035 to 0.001) | −0.024*** (−0.033 to −0.014) |
| Sex | 0.576* (−1.056 to −0.097) | 0.053 (−0.697 to 0.803) | −0.499 (−1.030 to 0.031) | −0.461 (−1.373 to 0.451) | −0.355 (−0.821 to 0.112) |
| R2 | 0.589 | 0.291 | 0.503 | 0.184 | 0.585 |
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Benzophenone-3 and triclosan
Benzophenone-3 was detected in all pooled samples; total concentrations ranged from 16.5 ng/mL to 312 ng/mL (GM = 61.5 ng/mL) across all strata (Table 1). There was a significant interaction between age and sex in the multivariate model (p = 0.007, Table SI-3), with the highest total concentrations found in older females' pools. When the male and female data were examined separately, there was a small effect of age on concentration for female pools (p = 0.019) but not for male pools.
Triclosan was detected in all samples at total concentrations ranging from 24.1 ng/mL to 205 ng/mL (GM = 87.7 ng/mL, Table 1). Triclosan was not linearly associated with age (Fig. 1), with the highest total concentrations (100–205 ng/mL) found in the 15–29 years age strata pools. There were no significant differences between male and female pools.
Fig. 1.
Urinary total concentration (ng/mL) versus age (years) for methyl-, ethyl-, propyl- and butyl paraben; and benzophenone-3 and triclosan (A to F, respectively). Triangles denote female pools, squares denotemale pools. Horizontal line indicates mean concentration of four pools in each age strata. Dashed horizontal line indicates mean concentration for age strata with outliers removed. Outliers labelled on ethyl- and butyl paraben plots.
4. Discussion
4.1. Parabens
Paraben total concentration was inversely associated with age, with a significant decrease across strata from 0 to 4 to 15–29 years (Fig. 1), followed by a plateau from 16 to >60 years for MeP, EtP and PrP (BuP did not appear to follow this trend). Total concentrations of MeP and PrP were higher than EtP and BuP across all age strata (Table 1), consistent with the more prevalent use of MeP and PrP in personal care products and food processing (Shen et al., 2007; Soni et al., 2005; Wang and Zhou, 2013). The highest MeP and PrP total concentrations were found in pools from children aged 5–14 years (Fig. 1) at GM552 and 107 ng/mL, respectively. These concentrations were substantially higher than GMs of MeP and PrP, respectively, in children from China (9–10 years, n = 70, 5.28 and 1.89 ng/mL) (Wang et al., 2013), Belgium (1–6 years, n = 23, median = 34.8 and 2.1 ng/mL) (Dewalque et al., 2014), India (2–14 years, n = 76, 6.77 and 0.86 ng/mL) (Xue et al., 2015) and the USA (3–10 years, n = 40, 62.4 and 0.92 ng/mL (Wang and Kannan, 2013) and 6–11 years, n = 415, 33.9 and 3.28 ng/mL (CDC, 2015). Similarly, GM total concentrations in these general Australian population pools were higher than adult populations in the USA (CDC, 2015), China (Wang et al., 2013), Belgium (Dewalque et al., 2014), Denmark (Frederiksen et al., 2011) and Greece (Asimakopoulos et al., 2014) (Table SI-4). Urinary total concentrations for MeP, PrP and BuP were approximately two times higher in female pools than in male pools (Table 2). This sex-related difference in paraben concentrations has also been reported in the US general population (Calafat et al., 2010; CDC, 2015), and is likely reflective of the more frequent use of paraben-containing cosmetic products by women than men. There was no significant interaction between age and sex for any parabens.
The age trend with paraben total concentration is clear, particularly for MeP and PrP, and is suggestive of higher exposures to parabens in Australian children than in adults. This may be the result of behavioural or physiological differences between children and adults, such as mouthing behaviours (Tulve et al., 2002; Xue et al., 2007); a larger surface area-to-bodyweight ratio, increasing the potential for exposure via dermal absorption; increased ventilation rate relative to lung surface area for greater exposure via inhalation; or increased energy and water intake combined with enhanced retention and absorption of nutrients for increased exposure via food (Miller et al., 2002; WHO, 2011). The opposite relationship is observed in the United States, where adults have higher paraben concentrations than children (CDC, 2015), suggesting that the observed age trend in the Australian population is most likely due to child-specific exposure sources such as baby products (wipes, lotions etc.). Although this was a convenience population and no specific sampling strategies were employed, the fact that the parabens were measured predominantly in their conjugated form (78–100%, Table SI-1) rules out systematic exogenous contamination of the samples with the target parabens..
4.2. Benzophenone-3
Benzophenone-3 was associated with age for females, but not for males in the general Australian pools. Increased total concentrations of benzophenone-3 in older females is most likely from frequent sunscreen use, and consistent with the generally more positive attitude towards skin care in females than in males, and in adults compared with adolescents (Abroms et al., 2003; Manová et al., 2013; Potente et al., 2011). This trend was observed in the NHANES 2009–10 population, where adult females had GM concentrations more than double those of males (CDC, 2015), but this is not seen in all studies (Chen et al., 2012; Gao et al., 2015; Zhang et al., 2013). Benzophenone-3 concentrations were similar in both sexes for school aged children (<15 years, Fig. 1), and may be the result of “SunSmart” sun safety policies in place in Australian schools, where sunscreen application is mandatory (Montague et al., 2001; Shih et al., 2009).
A recent review by Kim and Choi (2014) summarises human biomonitoring results for benzophenone-3 in urine. The Australian population pools showed substantially higher urinary concentrations (GM 61.5 ng/mL) than adult populations in China (GM: 0.62, n = 106) (Gao et al., 2015); Denmark (GM 1.73–4.25, n = 1003) (Frederiksen et al., 2014); France (median: 1.3 ng/mL, n = 191) (Philippat et al., 2012); Spain (median: 3.4 ng/mL, n = 120) (Casas et al., 2011); and the USA (GM 23.3 ng/mL, n = 2749 (CDC, 2015) and 6.1 ng/mL, n = 625 (Kunisue et al., 2012), respectively). Similarly, concentrations in Australian children (pool mean 17.2–96.2 ng/mL, n = 8 pools, 0–14 years) were considerably higher than in children from India (GM: 0.91 ng/mL, n = 76, 2–14 years) (Xue et al., 2015) and China (GM: 9.97 ng/mL, n = 38, 3–10 years) (Wang and Kannan, 2013) (Table SI-4), which may be attributed to lower sunscreen usage in India and China compared to Australia. Results orders of magnitude higher (>2 mg/mL) than reported in our study have been reported in some studies (Calafat et al., 2008a; Wolff et al., 2008), but pooled samples are unable to capture this variance. Of note, Australia and New Zealand have the highest rate of skin cancer in the world, approximately 12 times the global average (Cancer Australia, 2008; Ferlay et al., 2013). The significantly higher mean benzophenone-3 total concentrations in the Australian pools across the population compared to other countries globally is most likely reflective of the frequent sun safety public health campaigns and much higher sunscreen use in Australia. Further, samples were collected from Queensland, Australia, colloquially referred to as the ‘Sunshine State’. Located in the North East of Australia, Queensland has the highest average maximum temperatures of any state and the highest rate of skin cancers in Australia, and thus, in the world (AACR, 2012). The combination of these factors means that one would expect considerable sunscreen use and thus greater benzophenone-3 concentrations in residents of Queensland than in other Australian states. Similar regional variability has been reported in the USA, with significantly higher benzophenone-3 levels in women from California than those from Utah (Kunisue et al., 2012). Consequently, the benzophenone-3 results from the present study may not be representative of average Australians' exposure considering climate and average sun exposure.
4.3. Triclosan
Exposure of the Australian population to triclosan has previously been demonstrated using blood (Allmyr et al., 2008) and breast milk (Toms et al., 2011), but not urine. The highest urinary total concentrations of triclosan were found in females aged 16–45 years (pool means ranging from 104 to 205 ng/mL), and may be the result of increased use of antibacterial products among this demographic. Similarly, Den Hond et al. (2013) report higher triclosan levels in 14–15 year old Flemish adolescent females compared with males, but in NHANES there was no significant differences between US males and females (CDC, 2015). In the present study there was no association between urinary triclosan and age or sex, and these variables accounted for only 1% of the observed variance, consistent with a previous Australian study of individual human milk samples showing high variability in triclosan concentrations (Toms et al., 2011). This suggests that there are factors other than age and sex that contribute to measured triclosan concentrations, most likely exposure source - specifically the type and frequency of triclosan-containing products (e.g. personal care products and consumer products treated with antimicrobials, such as socks and kitchenware (Adolfsson-Erici et al., 2002)) used by a given individual. For example, in a study of Flemish adolescents triclosan levels were associated with the use of day/night cream and haircare products (Den Hond et al., 2013), and the use of liquid soap was significantly associated with triclosan levels in Puerto Rican pregnant women (Meeker et al., 2013).
The average triclosan total concentration in these Australian pools (pool mean: 83.0 ng/mL) was more than one order of magnitude higher than those reported in Flemish adolescents (GM 2.19 ng/mL, n = 193) (Den Hond et al., 2013), and adults from the Korean National Human Biomonitoring (GM 1.68 ng/mL, n = 1860) (Kim et al., 2011); and significantly higher than concentrations in Greece (GM 8.0 ng/mL, n = 100) (Asimakopoulos et al., 2014), the United States 2009–10 NHANES (14.5 ng/mL, n = 2749) (CDC, 2015), and pregnant women from Puerto Rico (GM 29.9 ng/mL, n =105) (Meeker et al., 2013) and several of the continental United States and Hawaii (GM 19.0 ng/mL, n = 506) (Mortensen et al., 2014) (Table SI-4). Triclosan urinary total concentrations in the Australian pools were comparable to those found in Danish mothers (GM 66 ng/mL, n = 145) and children (GM 43 ng/mL, n =143) (Frederiksen et al., 2013b).
4.4. Limitations
A number of assumptions have been made that must be considered when interpreting the results of the study: (1) pathology specimens do not introduce significant bias into the study population; (2) pooled samples provide an accurate measurement of mean concentration; and (3) spot samples provide a reasonable estimate of internal exposure over a given time frame.
4.4.1. The use of pooled, pathology specimens
The study population consisted of convenience samples collected during the course of routine pathology testing. The samples are not statistically representative of the Australian population as a whole, but there is no reason to expect exposures to the target analytes to be different in this community pathology-sourced population than in the general Australian population (except perhaps for benzophenone-3 and this would be largely related to the geographical location of the community pathology laboratory). The use of pooled specimens is advantageous as it saves significantly on analytical costs, reduces the time and resources required for recruitment, and may avoid ethical difficulties associated with reporting individual results (reviewed in Heffernan et al. (2014b)). Pooled pathology specimens were used successfully in previous studies to measure urinary bisphenol A, another ubiquitous short half-life environmental chemical (Heffernan et al., 2013,2014a). As discussed above, because this was a pre-existing, convenience population no specific sampling protocols were employed. This includes strategies related to sample collection containers and protocol, and sample storage. However, synthetic urine was collected, stored and processed under conditions simulating real sample conditions with all results <LOD, and all of the target analytes were measured predominately in their conjugated form. Together these findings suggest that there was no systematic contamination resulting from the sampling and pooling protocols. No creatinine or specific gravity data were available for the samples used in this study. However, for the interpretation of pooled measurements as representative measures of average concentration, variation in individual sample hydration status is expected to be averaged out and not introduce significant bias to the estimated average concentrations and excretion rates.
4.4.2. Variability in short half-life chemicals and the use of spot samples
For mainly episodic exposures to short half-life chemicals with rapid elimination kinetics, a large degree of within- individual variability is expected (Aylward et al., 2012), sometimes up to 3 orders of magnitude (Koch et al., 2014; Preau et al., 2010), and thus the time of sample collection relative to the exposure event will strongly impact the urinary concentration for any given person. Intraclass correlation coefficients (ICCs) for benzophenone-3 (0.57–0.81), triclosan (0.55–0.93) and EtP (0.48–0.76) (Koch et al., 2014; Lassen et al., 2013; Philippat et al., 2013) suggest that a spot sample provides a reasonable estimate of urinary concentration over days to months. For MeP and PrP ICCs are lower (0.2–0.56 and 0.29–0.51, respectively) (Engel et al., 2014; Koch et al., 2014; Meeker et al., 2013; Philippat et al., 2013; Smith et al., 2012) and thus measurements have higher uncertainty. The use of pooled samples is likely to mitigate this effect to some extent, as persons having extreme high or low concentrations will be averaged out by the large number of individuals contributing to the pool.
This study provides the first data on exposure among the general Australia population to a range of common personal care product chemical ingredients. Parabens and triclosan total concentrations in these pooled samples are significantly higher than the concentrations reported in similar populations worldwide, and age was a significantly inversely associated with paraben concentration. Benzophenone-3 total concentrations were significantly higher than reported values elsewhere, likely reflective of the prevalent sunscreen use in Australia; but may be elevated in Queensland compared with other Australian states.
Supplementary Material
Acknowledgments
The authors wish to thank Soumini Vijayasarathy, Andrew Banks, Beatrix Fletcher, Nhung Dang and the staff at Sullivan Nicolaides Pathology Taringa for assistance with sample collection and pooling. We also gratefully acknowledge Xiaoliu Zhou, Tao Jia, and Joshua Kramer for technical assistance in measuring the urinary concentrations of the phenols and parabens. LMLT is funded by an ARC DECRA (DE120100161). JFM is funded by an ARC Future Fellowship. The authors would like to thank the Australian Government Department of the Environment for their financial support, and for allowing access to the submitted report entitled “Chemical Monitoring Initiative: Australian human blood sample collection and chemical testing”. Entox is a joint venture of the University of Queensland and the Queensland Department of Health. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the views of the Australian Department of the Environment.
Abbreviations
- MeP
methyl paraben
- EtP
ethyl paraben
- PrP
propyl paraben
- BuP
butyl paraben
- CDC
Centers for Disease Control and Prevention, United States
- LOD
limit of detection
- ng/mL
nanograms per millilitre
- GM
geometric mean
- NHANES
National Health and Nutritional Examination Survey
- ICC
intraclass correlation coefficient
Footnotes
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2015.09.001.
References
- AACR (Australasian Association of Cancer Registries) Cancer Series no. 74. Cat. No. CAN 70. Australian Institute of Health and Welfare; Canberra: 2012. Cancer in Australia: an overview, 2012. [Google Scholar]
- Abroms L, Jorgensen CM, Southwell BG, Geller AC, Emmons KM. Gender differences in young adults' beliefs about sunscreen use. Health Educ. Behav. 2003;30(1):29–43. doi: 10.1177/1090198102239257. http://dx.doi.org/10.1177/1090198102239257. [DOI] [PubMed] [Google Scholar]
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46(9–10):1485–1489. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Larson E. Antibacterial cleaning and hygiene products as an emerging risk factor for antibiotic resistance in the community. Lancet Infect. Dis. 2003;3(8):501–506. doi: 10.1016/s1473-3099(03)00723-0. http://dx.doi.org/10.1016/S1473-3099(03)00723–0. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Harden F, Toms LM, Mueller JF, McLachlan MS, Adolfsson-Erici M, et al. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci. Total Environ. 2008;393(1):162–167. doi: 10.1016/j.scitotenv.2007.12.006. http://dx.doi.org/10.1016/j.scitotenv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Thomaidis NS, Kannan K. Widespread occurrence of bisphenol a diglycidyl ethers, p-hydroxybenzoic acid esters (parabens), benzophenone type-UV filters, triclosan, and triclocarban in human urine from Athens, Greece. Sci. Total Environ. 2014;470–471:1243–1249. doi: 10.1016/j.scitotenv.2013.10.089. http://dx.doi.org/10.1016/j.scitotenv.2013.10.089. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Kirman CR, Adgate JL, McKenzie LM, Hays SM. Interpreting variability in population biomonitoring data: role of elimination kinetics. J. Expo. Sci. Environ. Epidemiol. 2012;22(4):398–408. doi: 10.1038/jes.2012.35. http://dx.doi.org/10.1038/jes.2012.35. [DOI] [PubMed] [Google Scholar]
- Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010;30(2):301–312. doi: 10.1016/j.reprotox.2010.03.011. http://dx.doi.org/10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Needham LL. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ. Health Perspect. 2009;117(10):1481–1485. doi: 10.1289/ehp.0901108. http://dx.doi.org/10.1289/ehp.0901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect. 2008a;116(7):893–897. doi: 10.1289/ehp.11269. http://dx.doi.org/10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010;118(5):679–685. doi: 10.1289/ehp.0901560. http://dx.doi.org/10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ. Health Perspect. 2008b;116(3):303–307. doi: 10.1289/ehp.10768. http://dx.doi.org/10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Australia. Cancer series 43. Cat. no. CAN 39. Australian Insitute for Health and Welfare; Canberra, ACT: 2008. Non-melanoma skin cancer: general practice consultations, hospitalisation and mortality; pp. 1–63. [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 2011;37(5):858–866. doi: 10.1016/j.envint.2011.02.012. http://dx.doi.org/10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Caudill SP. Characterizing populations of individuals using pooled samples. J. Expo. Sci. Environ. Epidemiol. 2010;20:29–37. doi: 10.1038/jes.2008.72. [DOI] [PubMed] [Google Scholar]
- CDC (United States Centers for Disease Control) U.S. Department of Health and Human Services; Atlanta, GA, USA: 2015. Feb, Fourth national report on human exposure to environmental chemicals. 2015. Updated Tables. ( www.cdc.gov/exposurereport) [Google Scholar]
- Chen M, Zhu P, Xu B, Zhao R, Qiao S, Chen X, et al. Determination of nine environmental phenols in urine by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Anal. Toxicol. 2012;36(9):608–615. doi: 10.1093/jat/bks072. http://dx.doi.org/10.1093/jat/bks072. [DOI] [PubMed] [Google Scholar]
- Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008;28(5):561–578. doi: 10.1002/jat.1358. http://dx.doi.org/10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- Den Hond E, Paulussen M, Geens T, Bruckers L, Baeyens W, David F, et al. Biomarkers of human exposure to personal care products: results from the Flemish Environment and Health Study (FLEHS 2007–2011) Sci. Total Environ. 2013;463–464:102–110. doi: 10.1016/j.scitotenv.2013.05.087. http://dx.doi.org/10.1016/j.scitotenv.2013.05.087. [DOI] [PubMed] [Google Scholar]
- Dewalque L, Pirard C, Charlier C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. Biomed. Res. Int. 2014;2014:649314. doi: 10.1155/2014/649314. http://dx.doi.org/10.1155/2014/649314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LS, Buckley JP, Yang G, Liao LM, Satagopan J, Calafat AM, et al. Predictors and variability of repeat measurements of urinary phenols and parabens in a cohort of Shanghai women and men. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1306830. http://dx.doi.org/10.1289/ehp.1306830. [DOI] [PMC free article] [PubMed]
- Eriksson E, Andersen HR, Ledin A. Substance flow analysis of parabens in Denmark complemented with a survey of presence and frequency in various commodities. J. Hazard. Mater. 2008;156(1–3):240–259. doi: 10.1016/j.jhazmat.2007.12.022. http://dx.doi.org/10.1016/j.jhazmat.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- Frederiksen H, Jensen TK, Jørgensen N, Kyhl HB, Husby S, Skakkebæk NE, et al. Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. Reproduction. 2014;147(4):555–565. doi: 10.1530/REP-13-0522. http://dx.doi.org/10.1530/rep-13-0522. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Jorgensen N, Andersson AM. Parabens in urine, serum and seminal plasma from healthy Danishmen determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J. Expo. Sci. Environ. Epidemiol. 2011;21(3):262–271. doi: 10.1038/jes.2010.6. http://dx.doi.org/10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Nielsen JK, Morck TA, Hansen PW, Jensen JF, Nielsen O, et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int. J. Hyg. Environ. Health. 2013b;216(6):772–783. doi: 10.1016/j.ijheh.2013.02.006. http://dx.doi.org/10.1016/j.ijheh.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Gao CJ, Liu LY, Ma WL, Zhu NZ, Jiang L, Li YF, et al. Benzonphenone-type UV filters in urine of Chinese young adults: concentration, source and exposure. Environ. Pollut. 2015;203:1–6. doi: 10.1016/j.envpol.2015.03.036. http://dx.doi.org/10.1016/j.envpol.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Health Canada. Results of the Canadian Health Measures Survey Cycle 2 (2009–2011) Health Canada; Ottawa, Ontario: 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada; pp. 1–444. [Google Scholar]
- Heffernan A, Sly P, Toms L, Hobson P, Mueller J. Bisphenol A exposure is not associated with area-level socioeconomic index in Australian children using pooled urine samples. Environ. Sci. Pollut. Res. Int. 2014a;21(15):9344–9355. doi: 10.1007/s11356-014-2882-z. http://dx.doi.org/10.1007/s11356-014-2882-z. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Aylward LL, Toms LM, Eaglesham G, Hobson P, Sly PD, et al. Age-related trends in urinary excretion of bisphenol A in Australian children and adults: evidence from a pooled sample study using samples of convenience. J. Toxic. Environ. Health A. 2013;76(18):1039–1055. doi: 10.1080/15287394.2013.834856. http://dx.doi.org/10.1080/15287394.2013.834856. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Aylward LL, Toms LM, Sly PD, Macleod M, Mueller JF. Pooled biological specimens for human biomonitoring of environmental chemicals: opportunities and limitations. J. Expo. Sci. Environ. Epidemiol. 2014b;24(3):225–232. doi: 10.1038/jes.2013.76. http://dx.doi.org/10.1038/jes.2013.76. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B. Fine-Tuning Some Resistant Rules for Outlier Labeling. J Am Stat Assoc. 1987;82(400):1147–1149. http://dx.doi.org/10.2307/2289392. [Google Scholar]
- Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1986;81(396):991–999. http://dx.doi.org/10.2307/2289073. [Google Scholar]
- Hoberman AM, Schreur DK, Leazer T, Daston GP, Carthew P, Re T, et al. Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2008;83(2):123–133. doi: 10.1002/bdrb.20153. http://dx.doi.org/10.1002/bdrb.20153. [DOI] [PubMed] [Google Scholar]
- Huang H, Du G, Zhang W, Hu J, Wu D, Song L, et al. The in vitro estrogenic activities of triclosan and triclocarban. J. Appl. Toxicol. 2014;34(9):1060–1067. doi: 10.1002/jat.3012. http://dx.doi.org/10.1002/jat.3012. [DOI] [PubMed] [Google Scholar]
- Jung EM, An BS, Choi KC, Jeung EB. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol. Lett. 2012;208(2):142–148. doi: 10.1016/j.toxlet.2011.10.017. http://dx.doi.org/10.1016/j.toxlet.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Kim K, Park H, Yang W, Lee JH. Urinary concentrations of bisphenol a and triclosan and associations with demographic factors in the Korean population. Environ. Res. 2011;111(8):1280–1285. doi: 10.1016/j.envres.2011.09.003. http://dx.doi.org/10.1016/j.envres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014;70c:143–157. doi: 10.1016/j.envint.2014.05.015. http://dx.doi.org/10.1016/j.envint.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos R, Cocker J, et al. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: personal care product ingredients. Toxicol. Lett. 2014 doi: 10.1016/j.toxlet.2014.06.023. http://dx.doi.org/10.1016/j.toxlet.2014.06.023. [DOI] [PubMed]
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci. Total Environ. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. http://dx.doi.org/10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisue T, Chen Z, Buck Louis GM, Sundaram R, Hediger ML, Sun L, et al. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012;46(8):4624–4632. doi: 10.1021/es204415a. http://dx.doi.org/10.1021/es204415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebaek NE, et al. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environ. Res. 2013;126:164–170. doi: 10.1016/j.envres.2013.07.001. http://dx.doi.org/10.1016/j.envres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem. Res. Toxicol. 2014;27(5):834–842. doi: 10.1021/tx5000156. http://dx.doi.org/10.1021/tx5000156. [DOI] [PubMed] [Google Scholar]
- Levy SB. Antibacterial household products: cause for concern. Emerg. Infect. Dis. 2001;7(3 Suppl):512–515. doi: 10.3201/eid0707.017705. http://dx.doi.org/10.3201/eid0707.010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: an assessment of human exposure. Environ. Sci. Technol. 2014;48(7):4103–4109. doi: 10.1021/es405450n. http://dx.doi.org/10.1021/es405450n. [DOI] [PubMed] [Google Scholar]
- Ma WL, Wang L, Guo Y, Liu LY, Qi H, Zhu NZ, et al. Urinary concentrations of parabens in Chinese young adults: implications for human exposure. Arch. Environ. Contam. Toxicol. 2013;65(3):611–618. doi: 10.1007/s00244-013-9924-2. http://dx.doi.org/10.1007/s00244-013-9924-2. [DOI] [PubMed] [Google Scholar]
- Manová E, von Goetz N, Keller C, Siegrist M, Hungerbühler K. Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int. J. Environ. Res. Public Health. 2013;10(7):2778–2798. doi: 10.3390/ijerph10072778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary-Huard T. Biases induced by pooling samples in microarray experiments. Bioinformatics. 2007;23:i313–i318. doi: 10.1093/bioinformatics/btm182. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ. Sci. Technol. 2013;47(7):3439–3447. doi: 10.1021/es400510g. http://dx.doi.org/10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect. 2011;119(2):252–257. doi: 10.1289/ehp.1002238. http://dx.doi.org/10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Marty MA, Arcus A, Brown J, Morry D, Sandy M. Differences between children and adults: implications for risk assessment at California EPA. Int. J. Toxicol. 2002;21(5):403–418. doi: 10.1080/10915810290096630. http://dx.doi.org/10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- Montague M, Borland R, Sinclair C. Slip! Slop! Slap! and SunSmart, 1980–2000: skin cancer control and 20 years of population-based campaigning. Health Educ. Behav. 2001;28(3):290–305. doi: 10.1177/109019810102800304. http://dx.doi.org/10.1177/109019810102800304. [DOI] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Bruning T, Koch HM. Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int. J. Hyg. Environ. Health. 2014;217(8):845–853. doi: 10.1016/j.ijheh.2014.06.003. http://dx.doi.org/10.1016/j.ijheh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Mortensen ME, Calafat AM, Ye X, Wong LY, Wright DJ, Pirkle JL, et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ. Res. 2014;129:32–38. doi: 10.1016/j.envres.2013.12.004. http://dx.doi.org/10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S. Effects of butyl paraben on the male reproductive system in mice. Arch. Toxicol. 2002a;76(7):423–429. doi: 10.1007/s00204-002-0360-8. http://dx.doi.org/10.1007/s00204-002-0360-8. [DOI] [PubMed] [Google Scholar]
- Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002b;40(12):1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ. Health Perspect. 2012;120(3):464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ. Health Perspect. 2013;121(10):1225–1231. doi: 10.1289/ehp.1206335. http://dx.doi.org/10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirard C, Sagot C, Deville M, Dubois N, Charlier C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ. Int. 2012;48:78–83. doi: 10.1016/j.envint.2012.07.003. http://dx.doi.org/10.1016/j.envint.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Potente S, Coppa K, Williams A, Engels R. Legally brown: using ethnographic methods to understand sun protection attitudes and behaviours among young Australians ‘I didn't mean to get burnt–it just happened! ’. Health Educ. Res. 2011;26(1):39–52. doi: 10.1093/her/cyq066. http://dx.doi.org/10.1093/her/cyq066. [DOI] [PubMed] [Google Scholar]
- Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ. Health Perspect. 2010;118(12):1748–1754. doi: 10.1289/ehp.1002231. http://dx.doi.org/10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010;40(5):422–484. doi: 10.3109/10408441003667514. http://dx.doi.org/10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Sexton K, Needham L, Pirkle J. Human biomonitoring of environmental chemicals. Am. Sci. 2004;92(1):38–45. [Google Scholar]
- Shen HY, Jiang HL, Mao HL, Pan G, Zhou L, Cao YF. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. J. Sep. Sci. 2007;30(1):48–54. doi: 10.1002/jssc.200600215. [DOI] [PubMed] [Google Scholar]
- Shih ST-F, Carter R, Sinclair C, Mihalopoulos C, Vos T. Economic evaluation of skin cancer prevention in Australia. Prev. Med. 2009;49(5):449–453. doi: 10.1016/j.ypmed.2009.09.008. http://dx.doi.org/10.1016/j.ypmed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Shirai S, Suzuki Y, Yoshinaga J, Shiraishi H, Mizumoto Y. Urinary excretion of parabens in pregnant Japanese women. Reprod. Toxicol. 2013;35:96–101. doi: 10.1016/j.reprotox.2012.07.004. http://dx.doi.org/10.1016/j.reprotox.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ. Health Perspect. 2012;120(11):1538–1543. doi: 10.1289/ehp.1104614. http://dx.doi.org/10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of phydroxybenzoic acid (parabens) Food Chem. Toxicol. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. http://dx.doi.org/10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Toms LM, Allmyr M, Mueller JF, Adolfsson-Erici M, McLachlan M, Murby J, et al. Triclosan in individual human milk samples from Australia. Chemosphere. 2011;85(11):1682–1686. doi: 10.1016/j.chemosphere.2011.08.009. http://dx.doi.org/10.1016/j.chemosphere.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Tulve NS, Suggs JC, McCurdy TR, Cohen Hubal EA, Moya J. Frequency of mouthing behaviour in young children. J. Expo. Anal. Environ. Epidemiol. 2002;12(4):259–264. doi: 10.1038/sj.jea.7500225. [DOI] [PubMed] [Google Scholar]
- Wang L, Kannan K. Characteristic profiles of benzonphenone-3 and its derivatives in urine of children and adults from the United States and China. Environ. Sci. Technol. 2013;47(21):12532–12538. doi: 10.1021/es4032908. http://dx.doi.org/10.1021/es4032908. [DOI] [PubMed] [Google Scholar]
- Wang L, Wu Y, Zhang W, Kannan K. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ. Sci. Technol. 2013;47(4):2069–2076. doi: 10.1021/es304659r. http://dx.doi.org/10.1021/es304659r. [DOI] [PubMed] [Google Scholar]
- Wang PG, Zhou W. Rapid determination of parabens in personal care products by stable isotope GC-MS/MS with dynamic selected reaction monitoring. J. Sep. Sci. 2013;36(11):1781–1787. doi: 10.1002/jssc.201201098. http://dx.doi.org/10.1002/jssc.201201098. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit. Rev. Toxicol. 2014;44(6):535–555. doi: 10.3109/10408444.2014.910754. http://dx.doi.org/10.3109/10408444.2014.910754. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: a critical review of the literature. Crit. Rev. Toxicol. 2010;40(Suppl. 3):1–30. doi: 10.3109/10408444.2010.515563. http://dx.doi.org/10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. http://dx.doi.org/10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organisation) Summary of Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals in Children's Environmental Health. World Health Organization; Geneva, Switzerland: 2011. pp. 1–58. [Google Scholar]
- Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol a diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. 2015;137:120–128. doi: 10.1016/j.envres.2014.12.007. http://dx.doi.org/10.1016/j.envres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian VG, Moya J, Freeman N, Beamer P, Black K, et al. A meta-analysis of children's hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007;27(2):411–420. doi: 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006;844(1):53–59. doi: 10.1016/j.jchromb.2006.06.037. http://dx.doi.org/10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Ye XY, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. http://dx.doi.org/10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Yueh M-F, Taniguchi K, Chen S, Evans RM, Hammock BD, Karin M, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc. Natl. Acad. Sci. 2014 doi: 10.1073/pnas.1419119111. http://dx.doi.org/10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed]
- Zhang T, Sun H, Qin X, Wu Q, Zhang Y, Ma J, et al. Benzophenone-type UV filters in urine and blood from children, adults, and pregnant women in China: partitioning between blood and urine as well as maternal and fetal cord blood. Sci. Total Environ. 2013;461–462:49–55. doi: 10.1016/j.scitotenv.2013.04.074. http://dx.doi.org/10.1016/j.scitotenv.2013.04.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.