Abstract
The counterclockwise brain torque, defined as a larger right prefrontal and left parietal-occipital lobe, is a consistent brain asymmetry. Reduced or reversed lobar asymmetries are markers of atypical cerebral laterality and have been found in adults who stutter. It was hypothesized that atypical brain torque would be more common in children who stutter. MRI-based morphology measures were completed in boys who stutter (n=14) and controls (n=14), ages 8–13. The controls had the expected brain torque configurations whereas the boys who stutter were atypical. These results support the hypothesis that developmental stuttering is associated with atypical prefrontal and parietal-occipital lobe asymmetries.
Keywords: stuttering, children, brain torque, hemispheric specialization, neuroimaging
INTRODUCTION
The cerebral hemispheres of the human brain are functionally and anatomically asymmetric. Anatomical asymmetries have been found at the global (whole brain or regional), local (regionally specific), and cellular level (Toga & Thompson, 2003). The direction and magnitude of inter-hemispheric asymmetries have been studied using many different methods including post-mortem and in vivo neuroimaging imaging approaches (Jancke & Steinmetz, 2003). One global anatomical asymmetry that has shown a consistent directional asymmetry at the population level is the counterclockwise skull-based brain torque with the resultant enlargement of the right prefrontal and left occipital-parietal regions. Brain torque was discovered over a century ago (a.k.a. Yakovlevian anticlockwise torque) by noticing the regional impressions (petalias) on the inner surface of the skull. These protrusions are evolutionary conserved (Hopkins & Marino, 2000; LeMay, 1976) and are associated with a greater horizontal extension of the lateral Sylvian fissure in the left hemisphere and a shortening of the post-central Sylvian fissure in the right hemisphere (Foundas, 2001; Geschwind & Galaburda, 1985; Leonard & Eckert, 2008). This typical brain asymmetry occurs in about 70 to 75 percent of the healthy adult population defined as individuals with no family or personal history of a developmental or psychiatric disorder (Koff, Naeser, Pieniadz, Foundas, & Levine, 1986; LeMay, 1976; Pieniadz, Naeser, Koff, & Levine, 1983; Weinberger, Luchins, Morihisa, & Wyatt, 1982). These directional asymmetries at the population level are considered typical and any deviation from this configuration in magnitude or direction is considered atypical (Foundas, et al., 2003; Geschwind & Galaburda, 1985; Hopkins & Rilling, 2000). Atypical brain anatomy is a marker of an increased risk for atypical hemispheric specialization (Foundas, 2001; Geschwind & Galaburda, 1985; Jancke & Steinmetz, 2003).
In 1927 Orton proposed that atypical hemispheric specialization, particularly for language, was the underlying defect that put individuals at increased risk for the development of stuttering. Functional neuroimaging studies have shown that adults who stutter have different task specific blood flow changes relative to fluent, matched controls (Brown, Ingham, Ingham, Laird, & Fox, 2005; Fox, et al., 1996). Results across studies implicate a wide network of anomalous hemodynamic activation and deactivation in specific cortical, subcortical, and cerebellar regions that mediate the selection and activation of lexical-semantic functions, motor planning, and speech production (Brown, et al., 2005). A recent meta-analysis of functional neuroimaging studies found more areas of activation and a wider distribution of these areas for stutterers relative to controls when performing the same tasks. The areas that showed the greatest difference in people who stutter included: right>left over-activation of the frontal operculum extending into the anterior insula cortex, bilateral deactivation in the auditory temporal cortex, and activation in the cerebellar vermis.
Supporting the functional imaging results, neuroanatomical anomalies have been found in some of these same regions in individuals who stutter. The planum temporale, a portion of the auditory temporal cortex, was found to be larger in the right and left hemispheres with a reduced inter-hemispheric asymmetry in adults who stutter (Foundas, Bollich, Corey, Hurley, & Heilman, 2001). These results have been replicated in voxel-based morphometry studies with one study showing an increase in gray matter bilaterally (Beal, Gracco, Lafaille, & De Nil, 2007), and two other studies showing increased right hemisphere gray (Kikuchi, et al., 2011) and white matter volumes (Jancke, Hanggi, & Steinmetz, 2004) in the superior temporal gyrus of adults who stutter. These findings were not replicated in a sample of children who stutter (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008). Atypical perisylvian sulcal morphology has been found bilaterally (Foundas, et al., 2001), and in the right hemisphere (Cykowski, et al., 2008) in adults who stutter suggesting that there may also be minor gyral anomalies within these perisylvian regions. Finally, atypical rightward planum temporale asymmetry has been associated with increased stuttering severity and fluency induced with delayed auditory feedback in a sample of adults who stutter (Foundas, et al., 2004). Since anatomy and functional representations are more variable in left-handers, it’s important to note that the Foundas studies (2001, 2004) included right handed men and women and left handed men, in contrast to most of the other anatomical studies which were limited to right-handed males.
Anomalies in white matter pathways that interconnect speech-language and motor networks have also been found in people who stutter. Using diffusion tensor imaging, decreased white matter integrity of the left rolandic operculum deep to the oromotor region has been found in adults who stutter (Kell, et al., 2009; Sommer, Koch, Paulus, Weiller, & Buchel, 2002; Watkins, Smith, Davis, & Howell, 2008) and in children who stutter (Chang, et al., 2008), suggesting that this defect occurs early in the developmental process. A recent study that compared structural differences in white matter using diffusion tensor imaging with functional imaging data found diminished white matter integrity associated with decreased hemodynamic activation in the ventral motor area in adults and adolescents who stutter (Watkins, et al., 2008). Since white matter connections develop throughout childhood and well into adulthood (Bartzokis, et al., 2001; Giedd, et al., 1996), it is unclear whether some of these white matter irregularities are the result of developmental events that may be associated with pre-existing anatomical anomalies that produce aberrant cortical-cortical connections, or may be compensatory changes associated with a persistent dysfluent speech disorder.
The perisylvian speech-language regions discussed above constitute localized brain regions that may be anomalous in individuals who stutter and may represent a “core” localized defect. There is also evidence that adults who stutter may have a more global anatomical anomaly (brain torque) of the prefrontal and parietal-occipital lobes (Foundas, et al., 2003; Strub, Black, & Naeser, 1987). In a recent study a striking deviation from the typical brain torque distribution was found in a sample of adults who stutter (Foundas, et al., 2003). The control group had the expected 70 percent typical distribution of a right prefrontal and left parietal-occipital configuration. In contrast, 70 percent of the adults who stutter had atypical brain torque configurations with a more leftward prefrontal and rightward occipital-parietal configuration. Given that stuttering is a developmental disorder that begins during childhood, it is important to determine whether children who stutter display the same global anatomical irregularities found in adults who stutter. The current study was designed to study developmental effects by examining prefrontal and parietal-occipital lobar volume and asymmetry patterns in right-handed boys who stutter, ages 8 to 13, compared to a matched group of controls. Several investigators have postulated that the typical brain torque configuration represents an “idealized” template for the development of localized brain asymmetries that contribute to lateralized functional representations and to the development of white matter interconnections of functionally related neural circuits (Foundas, et al., 2003; Geschwind & Galaburda, 1985; Hopkins & Rilling, 2000; LeMay, 1976, 1977). In contrast, it has been hypothesized that atypical brain torque configurations lead to the development of aberrant cerebral architecture that predisposes to functional disruption (Chance, Esiri, & Crow, 2005; Zadina, et al., 2006). Since left hand preference and the female sex are associated with more variation in anatomy and function, our sample was limited to right-handed boys. We hypothesized that the majority of the right-handed boys who stutter would have an atypical brain torque configuration compared to controls, and that these differences would be found in both the prefrontal and parietal-occipital regions. Since some studies found bilateral differences while other studies have found defects in one cerebral hemisphere in people who stutter, we did not predict a priori whether group differences would be found in the right or the left cerebral hemisphere, or bilaterally. We also were not entirely sure whether gray matter and or white matter volumes would differ between groups, and if so, in what direction. Previous studies which investigated brain torque in developmental populations found correlations between structural anomalies and language processing abilities (Foundas, et al., 2003; Zadina, et al., 2006). Therefore, a second aim of this study was to determine whether stuttering severity and language functions correlated with specific anatomical measures in childhood stuttering.
METHODOLOGY
Participants
Participants were boys, 8 to 13 years of age, including boys who stutter (n = 14) and fluent controls (n = 14). Groups were matched for age (boys who stutter = 10.1 ± 2.0, fluent controls = 10.2 ± 1.6) and years of education (boys who stutter = 3.7 ± 1.5, fluent controls = 3.9 ± 1.8). All participants were native English speakers and based on the Edinburgh Handedness Inventory (Oldfield, 1971) classified as right handed (boys who stutter = 79.2 ± 11.9, fluent controls = 87.8 ± 16.8). Participants and legal guardians gave written informed consent. Participants were recruited and imaged from two different recruitment sites (Site 1 (boys who stutter = 14, fluent controls = 6) and Site 2 (fluent controls = 8)).
The boys who stutter met the following criteria: (1) parental judgment of the child as exhibiting stuttering with a rating of mild or greater, (2) speech-language pathologist’s judgment of the current presence of stuttering with a rating of mild or greater, (3) current speech containing 3 stuttering-like dysfluencies (SLD) per 100 syllables (monosyllabic word repetitions, part-word repetitions, blocks and prolongations), (4) stuttering continually present for at least three years from onset, and (5) no frank neuropathology. No boys who stutter were taking any medication to treat stuttering or any medication that could result in stuttering. Six of the fourteen boys who stutter had a family history of stuttering. Controls included: (1) healthy children, (2) regarded by parents and/or a speech-language clinician to exhibit normally fluent speech, (3) exhibited fewer than three instances of SLD or within-word dysfluencies, (4) had no history or family history of stuttering, and (5) had a negative history of neuropsychiatric conditions.
Stuttering Severity
Stuttering severity was determined using the Stuttering Severity Instrument, 3rd Ed. (SSI-3) and by evaluating the number of SLDs in a 500 syllable conversational speech sample (Riley, 1994). The SSI-3 provides a categorical measure of stuttering severity (very mild, mild, moderate, severe, and very severe) based on the frequency of SLDs, duration of SLDs, and presence of concomitant behaviors. The number of SLDs was summed and divided by the total number of spoken syllables to arrive at a frequency score. The length of SLDs was based on the average of the three longest SLDs. Measures of associated movements were also recorded and quantified. Speech samples were transcribed from videotaped sessions. Mean SSI-3 score for the boys who stutter was 22.3 (7.5) with seven participants classified as mild, two as moderate, three as severe and one as very severe. Stuttering severity could not be determined in one boy who stutters because the video recorder did not record the session.
To quantify stuttering severity independent of concomitant behaviors and duration of stuttering events, an average SLD rate ([ # SLD / # of syllables spoken] * 100) was also obtained. The average SLD rate for the boys who stutter was 6.77 (5.4) (range 3.3 – 20.8).
Cognitive Measures
General Cognition
The Wechsler Abbreviated Scale of Intelligence (WASI) (Site 1) (Wechsler, 1999) and the Wechsler Intelligence Scale for Children-4th Edition (WISC-IV) (Site 2) (Wechsler, 2003) were administered to measure intelligence. The WASI and WISC-IV full scale IQ scores have a validity correlation of 0.86, which allows for a direct comparison between the two tests.
Language Abilities
The Oral and Written Language Scales (OWLS) (Carrow-Woolfolk, 1996) were administered to the participants from Site 1. The Woodcock-Johnson Cognitive Abilities Battery (WJ-III) (Woodcock, McGrew, & Mather, 2001), the Test of Word Reading Efficiency (TOWRE) (Torgesen, Wagner, & Rashotte, 1999) and the Gray Oral Reading Test-4th Edition (GORT-4) (Wiederhollt & Bryant, 2001) were administered to the participants for Site 2. To compare the participants from Site 1 and Site 2 on language abilities (Reading Comprehension and Oral Expression), specific subtest scores were converted to z-scores to derive an overall Reading Comprehension and Oral Expression score. For the Site 1 participants the Reading Comprehension score consisted of the listening comprehension subtest (OWLS) while the average z-scores from the passage comprehension (WJ-III-Reading Section), oral comprehension (GORT-4) and overall reading ability (GORT-4) made up the Reading Comprehension score for the Site 2 participants. The Oral Expression score for the Site 1 participants consisted of the oral expression subtest (OWLS) while the fluency score (rate + accuracy) (GORT-4) was used for the Site 2 participants.
Neuroanatomical Measures
MRI Acquisition
Volumetric MRI scans were acquired for Site 1 on a General Electric 1.5 Tesla Signa scanner (St. Louis, MO) with a T1-weighted sequence, as a gapless series of 124 contiguous sagittal images with the following technical factors: 1.5-mm slice thickness, field of view of 240 mm, 20° flip angle, one excitation, and 256 × 256 pixel matrix. The volumetric MRI scans for Site 2 were acquired on 1.5 Tesla Signa LX (Signa CVi, GE Medical Systems, Milwaukee, WI) using a similar image acquisition sequence (3D SPGR, 1 echo, minimum TE, 15° flip angle, two excitations, 256 × 256 acquisition matrix, 124 sagittal slices, 0.94 × 0.94 mm in-plane, 1.2 mm slice thickness). The two MRI machines used in this study are both from the same manufacturer and used a similar image acquisition protocol, therefore the images obtained from both MRI machines are reliable and comparable (Reig, et al., 2009). Also, to quantitatively determine if any volumetric differences existed within the control group due to recruitment site, separate univariate ANOVAs were used with a specific ROI as the dependent variable and recruitment site as the fixed factor. No significant effects of recruitment site were observed for any ROI.
All MRIs were aligned in the sagittal, axial, and coronal planes to correct for head rotation. One-half of the MRIs were randomly selected and hemispheres were flipped to reverse the right and left hemispheres. These formatting procedures ensured that the rater was blind to group and hemisphere. Intra-rater and inter-rater reliability (between first author and graduate student) were computed for all methods and regions of interest (ROIs) in five randomly selected MRIs (10 hemispheres). The average mean absolute error across all ROI volumes was 1.1 cm3 and the intra-class correlation was greater than 0.93 for all ROIs (SPSS, 2001).
Anatomical Regions of Interest
For brain torque to be quantified, the following ROIs were measured in each hemisphere: prefrontal (total, superior and inferior subregions) and parietal-occipital (total, superior and inferior subregions). Hemisphere (left, right) and total brain volumes were also measured. Each ROI was segmented into gray matter (GM) and white matter (WM) compartments.
The prefrontal ROI was defined as the portion of the frontal lobe rostral to the precentral sulcus comprised of heteromodal association cortex. The prefrontal ROI included prefrontal cortex anterior to a vertical line defined by the most rostral extension of the corpus callosum. Based on this boundary, the prefrontal ROI excluded a small portion of orbitofrontal cortex (retrogenual) and included a small portion of the anterior cingulate gyrus. The prefrontal ROI was subdivided into superior and inferior subregions with these areas including the inferior frontal gyrus and dorsolateral prefrontal cortex respectively.
The parietal-occipital ROI included portions of the parietal, occipital, and posterior temporal cortices. Similar to the posterior boundary for the prefrontal volume, the anterior boundary for the parietal-occipital ROI was defined based on the corpus callosum and a vertical line at the most caudal extension of this internal landmark. The parietal-occipital ROI was also subdivided into superior and inferior subregions that included the inferior parietal lobule and posterior temporal cortex respectively in addition to the cuneus and precuneus. The boundaries of these gross brain regions were defined based on these internal landmarks and have been used in other studies (Foundas, et al., 2003; Gilmore, et al., 2007; Weinberger, et al., 1982; Zadina, et al., 2006).
Three programs, ANALYZE (Mayo Clinic, 1986), Brainsuite (Shattuck & Leahy, 2002), and MRIcro (Rorden, 1999), were used to quantify total, gray matter (GM) and white matter (WM) volumes for each ROI. Five steps were used as described below.
Step 1. ROI boundaries using Coordinates
The ANALYZE program was used to define the midline in the sagittal view to divide the total brain into hemispheres. This midline image was excluded from the left and right hemisphere ROI. Midline coordinates were identified and used to divide each hemisphere into prefrontal and parietal-occipital regions. The boundaries of the prefrontal and parietal-occipital ROIs defined by the corpus callosum were found by using the anterior most point or the posterior most point of the corpus callosum respectively. This specific coordinate was defined as the first anterior or posterior coronal slice where the corpus callosum was completely connected across the two hemispheres. On this coronal image, the inferior and superior most point of the corpus callosum was identified and the difference between these two coordinates was used to find the center of the genu or splenium. If this difference was an odd number, the middle coordinate was considered the center. If the difference was an even number, the lower coordinate was considered the center. All coordinates were identified before any volumetric MRI scans were stripped and prior to any segmentation of brain tissue into GM or WM. Coordinates were determined using the ANALYZE Cartesian coordinate system.
Step 2. Skull and Dura Stripping
ANALYZE was used to strip the brain manually of the skull, dura, brainstem and cerebellum.
Step 3. Classifying brain tissue
The Bias-field correction module of Brainsuite was used to correct for any inhomogeneity in the magnetic fields in order to classify tissue based on image intensity. The semi-automated Partial Volume Classification module of Brainsuite was then used to parcel out and convert the MRI voxels into WM, GM, cerebrospinal fluid or dura. The Partial Volume Classification module default setting was used for all images (Shattuck & Leahy, 2002). After tissue classification, each MRI was divided into ROIs.
Step 4. Defining ROIs
Using the midline image, the total brain was divided into hemispheres. For each hemisphere, the coordinates from Step 1 were used to divide the hemispheres into prefrontal and parietal-occipital regions, and each lobe into superior and inferior ROIs. Due to the possibility that the contralateral homologous ROI could crossed midline and be counted in the wrong ROI, any brain matter that was not connected to the ROI was deleted.
Step 5. Calculating volumes
ROI volumes were computed using the ROI filter module in MRIcro (Rorden, 1999). The ROI filter module computed pixel counts for each ROI. The ROI filter module allowed for the calculation of total (GM + WM), WM, and GM pixel counts for each ROI. Volumes (cm3) for each ROI were then computed from the pixel counts using the voxel dimensions described above. Furthermore, to control for individual differences in brain volume, ROI volumes were converted to percent of total hemisphere volume (i.e., R% = 100*(Right ROI Volume / Right Hemisphere Volume) and L% = 100*(Left ROI Volume / Left Hemisphere Volume).
Step 6. Calculating Brain Torque
Brain torque was quantified by determining inter-hemispheric asymmetry patterns using total volume (GM + WM) in the prefrontal and parietal-occipital regions. Anatomical symmetries were measured via an asymmetry quotient (AQ), computed as AQ = (L–R) / 0.5*(L+R), where L and R are non-normalized ROI volumes measured in the left and right hemispheres, respectively. AQs were defined as rightward (AQ < −0.025), symmetrical (−0.025 ≤ AQ ≤ 0.025), or leftward (AQ > 0.025) (Foundas, et al., 2001; Foundas, et al., 2003). Each participant’s brain torque was classified as “typical” (R>L prefrontal and L>R parietal-occipital) or “atypical” (any other configuration).
Statistical Analysis
Statistical analyses were conducted using SPSS 13.0 (SPSS, 2001) with a p < 0.05 significance level. Partial eta-squared (partial η2) was reported for all volumetric and AQ data.
Cognitive Measures
Cognitive measures and language abilities were tested separately using a one-way between-groups analysis of variance (ANOVA) with Group (Controls, boys who stutter) as the single grouping factor.
Brain Torque
Chi-square test of association was used to assess group differences in brain torque and chi-square goodness-of-fit tests were used to compare the distribution of brain torque in each group to the expected distribution derived from the literature (i.e., 70% typical, 30% atypical) (LeMay, 1976; Weinberger, et al., 1982). With 14 participants, the expected distribution of atypical brain torque would be 4.2 out of 14.
Volumetric Measurements
Total (GM + WM), GM and WM volumes for total brain and each hemisphere were analyzed separately by one-way ANOVA with Group as the single grouping factor. The frontal and posterior regions were analyzed separately as percent of hemisphere volumes via two-way split-plot ANOVA with Group entered as a grouping factor and Hemisphere (left, right) as a repeated-measures variable.
Since the prefrontal and parietal-occipital ROIs were subdivided by tissue type (GM, WM) and location (superior and inferior), a large number of possible pairwise comparisons are possible. Type I error rate was controlled via orthogonal contrasts using predetermined hierarchical levels (Level 1: Total volume (GM + WM), Level 2: GM and WM volumes, Level 3: inferior and superior volumes specific to GM or WM). For example, total ROI volumes were tested first and significant effects were followed by orthogonal decomposition of the ROI into gray and white matter volumes. GM and WM significant effects were followed by further decomposition into superior-inferior subregions. If no significant effects were observed, no further analyses were conducted.
Asymmetry Quotients
AQs were analyzed by one-way ANOVA with Group as the single grouping factor using the same hierarchical levels as described above to control for Type I error rate.
Structure-Function Relationships
Pearson correlation (r) was used to assess relationships between anatomical measures and average SLD rate in the boys who stutter, and language measures in both groups.
RESULTS
Cognitive Measures
General Cognition
No group differences in IQ (Full scale, Verbal or Spatial) scores were detected (F(1,26) = 0.01, p = 0.93; boys who stutter = 109.8 ± 24.6, controls = 108.6 ± 11.9)
Language Abilities
No group differences were found for Reading Comprehension (F(1,26) = 0.41, p = 0.53; boys who stutter = 0.15 ± 1.44, fluent controls = −0.14 ± 0.93) or Oral Expression (F(1,26) = 1.49, p = 0.23; boys who stutter = −0.13 ± 2.00, controls = 0.56 ± 0.73).
Neuroanatomical Measures
Brain Torque
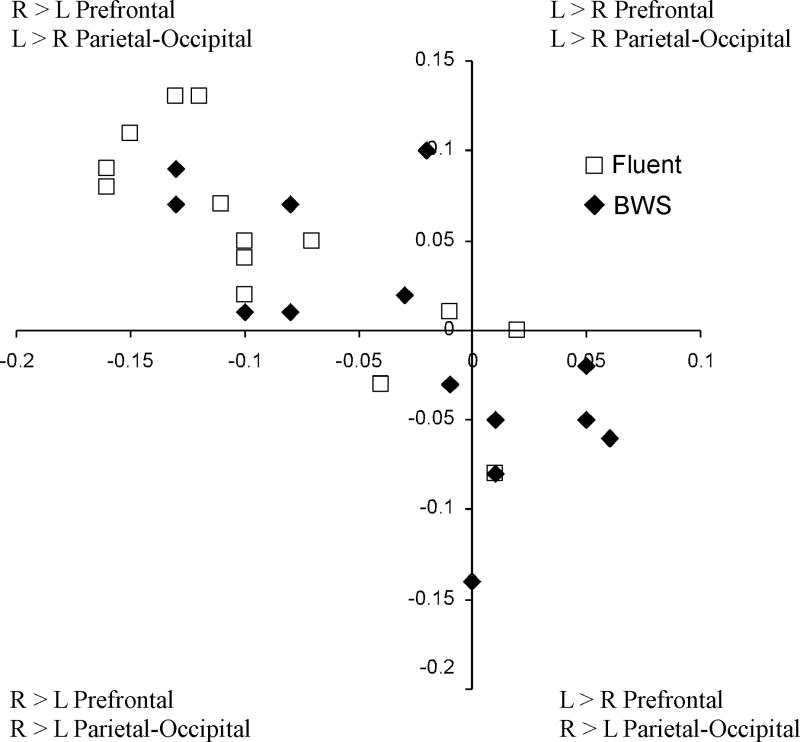
Atypical brain torque was more common among boys who stutter (11 of 14, 79%) compared to controls (5 of 14, 36%; χ2(1) = 5.3, p = 0.022). This increased frequency of atypical brain torque differed from the expected distribution in the boys who stutter (χ2(1) = 15.7, p = 0.00007). The frequency of atypical brain torque did not differ from the expected distribution in the control group (χ2(1) = 0.22, p = 0.68). Interestingly, atypical brain torque configurations where both the prefrontal and parietal-occipital lobes had L > R (0 out of 28) or R > L (1 out of 28) asymmetry pattern were rare in the sample. Individual brain torque distributions by Group are depicted in Figure 1 and the distribution of the nine possible brain torque configurations separated by Group are shown in Table 1. Means (SD) by Group for each ROI volume and AQ are presented in Table 2 with prefrontal and parietal-occipital volumes expressed as percent of hemisphere volume.
FIGURE 1. Brain Torque.
The relationship of the prefrontal to parietal-occipital asymmetry pattern for each participant is depicted by group. x-axis = prefrontal asymmetry quotient, y–axis = parietal-occipital asymmetry quotient, BWS = boys who stutter, R = Right, L = Left.
Table 1. Distribution of Brain Torque Configurations.
The number of individuals in each of the nine possible brain torque configurations (prefrontal, parietal-occipital) separated by group.
| Typical Brain Torque |
Atypical Brain Torque
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| R>L, L>R | R>L, R=L | R>L, R>L | R=L, R=L | R=L, R>L | R=L, L>R | L>R, R>L | L>R, R=L | L>R, L>R | |
| Fluent | 9 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 |
| BWS | 3 | 3 | 0 | 0 | 4 | 1 | 2 | 1 | 0 |
BWS = boys who stutter, R = right, L = left.
TABLE 2. Volumetric and Asymmetry Quotient Data.
Means and standard deviations (SD) for volumes (total, gray, white) and asymmetry quotients (AQ) are shown for all measures. Hemisphere volumes are expressed in cm3 and prefrontal and parietal-occipital volumes are expressed as percent of hemisphere. p-values for group and group × hemisphere (G × H) interactions are shown for volumes, and p-values for group are shown for AQs.
| Left Hemisphere
|
Right Hemisphere
|
Asymmetry Quotient
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluent
|
BWS
|
Fluent
|
BWS
|
Fluent
|
BWS
|
||||||||||||
| Region | Subregion | Brain Matter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | PGroup | PG × H | Mean | SD | Mean | SD | PGroup |
| Hemisphere | Total | 599.85 | 46.43 | 631.33 | 43.79 | 606.77 | 49.60 | 640.14 | 43.21 | n/s | n/s | −0.01 | 0.01 | −0.01 | 0.02 | n/s | |
| Gray | 381.14 | 27.18 | 392.49 | 32.53 | 384.90 | 30.25 | 398.94 | 31.48 | n/s | n/s | −0.01 | 0.03 | −0.02 | 0.03 | n/s | ||
| White | 218.71 | 23.59 | 238.84 | 26.58 | 221.87 | 24.77 | 241.20 | 23.81 | 0.043 | n/s | −0.01 | 0.04 | −0.01 | 0.03 | n/s | ||
|
| |||||||||||||||||
| Prefrontal % | Total | 14.30 | 1.22 | 14.09 | 1.21 | 15.43 | 1.25 | 14.32 | 1.32 | n/s | 0.008 | −0.08 | 0.06 | −0.02 | 0.07 | 0.021 | |
| Gray | 9.96 | 0.74 | 9.62 | 0.97 | 10.58 | 0.80 | 9.68 | 1.03 | n/s | 0.014 | −0.07 | 0.06 | −0.02 | 0.06 | 0.040 | ||
| White | 4.34 | 0.57 | 4.47 | 0.57 | 4.85 | 0.59 | 4.64 | 0.61 | n/s | 0.031 | −0.12 | 0.09 | −0.05 | 0.10 | 0.045 | ||
| Superior | Gray | 5.77 | 0.46 | 5.66 | 0.64 | 6.13 | 0.45 | 5.82 | 0.74 | n/s | n/s | −0.06 | 0.06 | −0.03 | 0.06 | n/s | |
| White | 2.44 | 0.37 | 2.45 | 0.43 | 2.74 | 0.35 | 2.57 | 0.49 | n/s | n/s | −0.12 | 0.11 | −0.05 | 0.14 | n/s | ||
| Inferior | Gray | 4.19 | 0.43 | 3.97 | 0.46 | 4.44 | 0.57 | 3.87 | 0.46 | 0.024 | 0.014 | −0.06 | 0.11 | 0.03 | 0.07 | 0.027 | |
| White | 1.90 | 0.25 | 2.02 | 0.29 | 2.11 | 0.35 | 2.06 | 0.24 | n/s | n/s | −0.10 | 0.14 | −0.02 | 0.10 | n/s | ||
|
| |||||||||||||||||
| Parietal-Occipital % | Total | 31.99 | 2.74 | 30.27 | 1.83 | 30.20 | 3.13 | 29.94 | 1.42 | n/s | n/s | 0.06 | 0.06 | 0.01 | 0.07 | 0.045 | |
| Gray | 21.10 | 1.91 | 19.23 | 1.46 | 20.26 | 2.22 | 19.56 | 1.10 | --- | --- | 0.03 | 0.06 | −0.03 | 0.07 | 0.015 | ||
| White | 10.89 | 1.04 | 11.04 | 1.04 | 9.94 | 1.62 | 10.38 | 0.87 | --- | --- | 0.09 | 0.10 | 0.05 | 0.09 | n/s | ||
| Superior | Gray | 11.35 | 0.85 | 10.32 | 1.43 | 10.96 | 1.42 | 10.59 | 1.33 | --- | --- | 0.03 | 0.08 | −0.03 | 0.08 | 0.028 | |
| White | 5.84 | 0.54 | 5.55 | 0.94 | 5.55 | 1.15 | 5.54 | 0.79 | --- | --- | 0.60 | 0.13 | −0.02 | 0.11 | --- | ||
| Inferior | Gray | 9.75 | 1.28 | 8.91 | 0.84 | 9.30 | 1.39 | 8.97 | 0.87 | --- | --- | 0.05 | 0.09 | −0.01 | 0.09 | n/s | |
| White | 5.05 | 0.81 | 5.49 | 0.72 | 4.39 | 0.86 | 4.84 | 0.86 | --- | --- | 0.13 | 0.15 | 0.12 | 0.12 | --- | ||
BWS = boys who stutter, n/s = nonsignificant, --- = not analyzed due to Type I error control.
Prefrontal Volumes
A Hemisphere effect was observed across groups with the right prefrontal volume larger than the left (F(1,26) = 18.9, p = 0.0002, partial η2 = 0.42). The Hemisphere effect was due to a larger right prefrontal GM volume (F(1,26) = 10.2, p = 0.004, partial η2 = 0.28) and WM (F(1,26) = 20.1, p = 0.0001, partial η2 = 0.44). When this ROI was divided into superior and inferior subregions, the Hemisphere effect was detected for superior GM (F(1,26) = 17.8, p = 0.0003, partial η2 = 0.41), inferior WM (F(1,26) = 7.5, p = 0.011, partial η2 = 0.22) and superior WM volumes (F(1,26) = 14.3, p = 0.001, partial η2 = 0.35).
A Group × Hemisphere interaction in the prefrontal region was found (F(1,26) = 8.4, p = 0.008, partial η2 = 0.24). The boys who stutter had a reduced right prefrontal volume compared to controls. This group difference in right prefrontal volume was due to a reduction in prefrontal GM (F(1,26) = 7.0, p = 0.014, partial η2 = 0.21) and WM volumes (F(1,26) = 5.2, p = 0.031, partial η2 = 0.17). The reduced right prefrontal GM volume in the boys who stutter was driven by a reduction of GM in the inferior subregion (F(1,26) = 6.86, p = 0.014, partial η2 = 0.21) which includes the inferior frontal gyrus. Also, an overall Group effect was found for the inferior GM volume ( F(1,26) = 5.8, p = 0.024, partial η2 = 0.18) indicating that the boys who stutter had reduced GM volumes in this ROI in both hemispheres. No other Group or Group × Hemisphere effects for WM volumes were found in the inferior or superior prefrontal subregions.
Prefrontal AQs
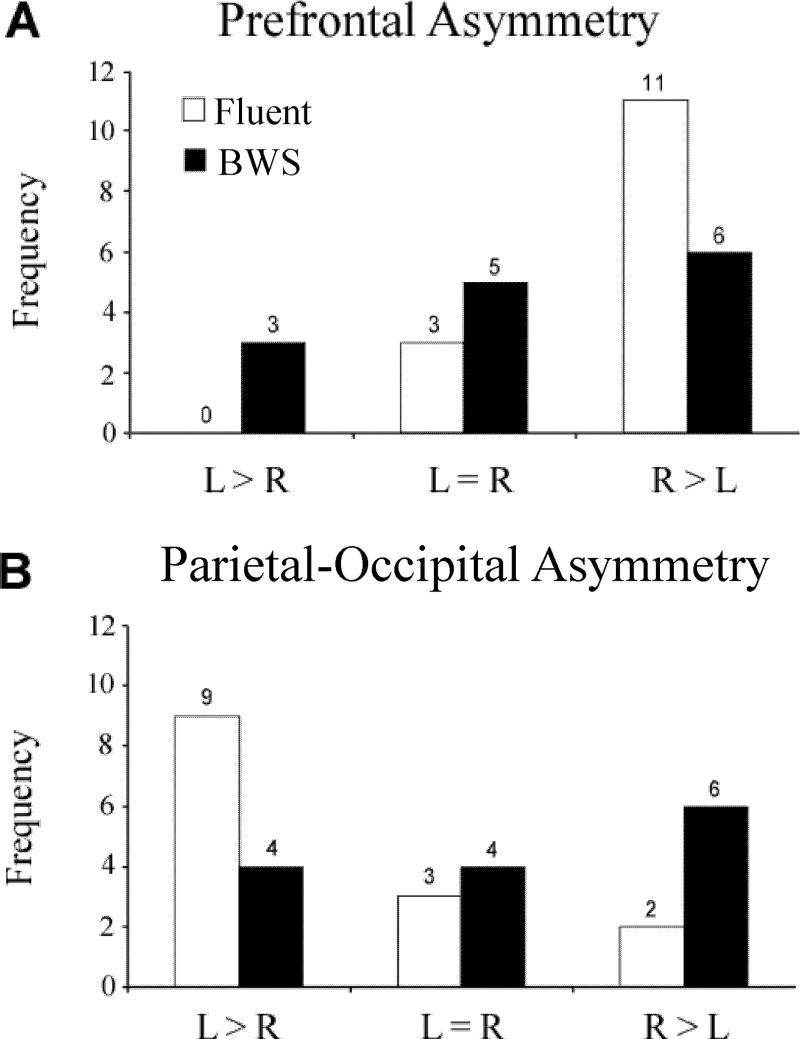
Analyses of prefrontal AQs yielded results consistent with the Group × Hemisphere effects described above. The boys who stutter displayed a reduced rightward prefrontal asymmetry relative to controls (F(1,26) = 6.0, p = 0.021, partial η2 = 0.19). Whereas 11 of 14 (79%) of the controls had the typical rightward prefrontal asymmetry, only 6 of 14 (43%) boys who stutter had a rightward asymmetry (Refer to Figure 2A). The boys who stutter also had a reduced prefrontal GM AQ (F(1,26) = 4.7, p = 0.040, partial η2 = 0.15) and WM AQ (F(1,26) = 4.4, p = 0.045, partial η2 = 0.15) relative to controls. The prefrontal inferior GM AQ (F(1,26) = 5.5, p = 0.027, partial η2 = 0.17) differed between groups; the boys who stutter showed a leftward (atypical) asymmetry while the controls had a rightward (typical) asymmetry.
FIGURE 2. Asymmetry Distributions.
Distribution of (A) prefrontal and (B) parietal-occipital asymmetry quotients depicted by group, BWS = boys who stutter.
Parietal-Occipital Volumes
Across groups a Hemisphere effect (F(1,26) = 8.2, p = 0.008, partial η2 = 0.24) was found with the left parietal-occipital volume larger than the right. Only parietal-occipital WM volume (F(1,26) = 16.3, p = 0.0004, partial η2 = 0.39) was larger in the left hemisphere. When subdivided into superior and inferior subregions, a Hemisphere effect was found with the inferior WM volume larger in the left hemisphere (F(1,26) = 26.7, p = 0.00002, partial η2 = 0.51). For total parietal-occipital volume, the group or Group × Hemisphere interaction (p = 0.060) did not reach significance.
Parietal-Occipital AQs
The parietal-occipital AQ differed between Groups (F(1,26) = 4.4, p = 0.045, partial η2 = 0.15) with a leftward AQ in Controls and a reduced leftward asymmetry in the boys who stutter. Whereas 9 of 14 (64%) of the controls had the typical leftward asymmetry, only 4 of 14 (29%) boys who stutter had the typical configuration (Refer to Figure 2B). A group difference was found in GM AQ (F(1,26) = 6.7, p = 0.015, partial η2 = 0.21). The magnitude of the AQ was reduced (more symmetrical) in the boys who stutter compared to the controls who had a leftward AQ. Further analyses revealed that the superior GM AQ (F(1,26) = 5.4, p = 0.028, partial η2 = 0.17) differed between groups; the boys who stutter had a rightward (atypical) asymmetry while the controls had a leftward (typical) asymmetry.
Total Brain and Hemisphere Measures
There were no group differences in total brain volume (p = 0.056, boys who stutter = 1286.9 ± 86.4, fluent controls = 1218.1 ± 95.7). However, total brain WM volume was larger in the boys who stutter (481.3 ± 50.1) relative to controls (441.6 ± 47.7, F(1,26) = 4.6, p = 0.042, partial η2 = 0.15). No group differences were found for total GM volume (GMstutter = 805.6 ± 64.2; GMcontrol = 776.4 ± 57.1; p = 0.214).
When hemisphere volumes were analyzed, the right hemisphere was larger than the left (F(2,25) = 8.5, p = 0.002, partial η2 = 0.40). This Hemisphere effect was associated with increased GM (F(1,26) = 8.0, p = 0.009, partial η2 = 0.235 and WM volumes (F(1,26) = 4.6, p = 0.041, partial η2 = 0.15). A main effect of Group was found for Hemisphere WM volumes (F(1,26) = 4.6, p = 0.043, partial η2 = 0.15); the boys who stutter had increased WM in both hemispheres relative to controls.
Structure-Function Relationships
The following positive correlations were detected in the boys who stutter: average SLD rate was associated with raw volumes (but not proportional measures) of left (r = 0.58, p = 0.037) and right prefrontal WM (r = 0.62, p = 0.025). That is, increased SLDs were associated with increased left and right prefrontal WM volume. There were no significant relationships between cognitive or language abilities and anatomy.
DISCUSSION
In this study, MRI-based morphological measures of prefrontal and parietal-occipital lobe volume and asymmetry patterns were examined in right-handed boys with developmental stuttering compared to a group of age and IQ matched right-handed boys with fluent speech and no family history of stuttering. The a priori hypothesis was that the group of boys who stutter would differ from the fluent control group on lobar volume and asymmetry patterns, including group differences in overall brain torque. In a previous study group differences were found in prefrontal and parietal-occipital lobe volume and asymmetry configurations (i.e., atypical brain torque) in adults with persistent developmental stuttering (Foundas, et al., 2003). Thus, we postulated that if atypical brain torque is a neural risk for the development of stuttering, then similar brain anomalies should be found in children and adolescents with persistent developmental stuttering. Sex and hand preference were controlled in the current study, as we limited the sample to right-handed boys. The earlier study of adults included matched groups of right and left-handed men and right-handed women. Because the sample included in the current study was limited to right-handed boys and because of the group design without a longitudinal component, we cannot determine whether other factors, like sex-linked, handedness, or developmental brain changes may account for some differences between the results in the two studies. Similar to the previous study in adults who stutter (Foundas, et al., 2003), the brain scans were maintained in native space and the volume measures were referenced proportional to hemisphere volume. One methodological difference is that the current study included a segmentation of the volumes into gray and white matter compartments allowing for the direct examination of gray and white matter volumes by region and hemisphere.
There were three major findings. First, stuttering was associated with atypical prefrontal and parietal-occipital asymmetry patterns (atypical brain torque). Second, the boys who stutter had more white matter volume in the right and left hemispheres compared to controls. Third, there was a positive correlation between dysfluency rate and prefrontal white matter volume in the boys who stutter. Each of these findings will be discussed in turn within the context of brain development and theories about the etiology of stuttering.
Brain Torque Anatomy: Prefrontal and Parietal-Occipital Regions
Most of the boys with persistent stuttering had atypical brain torque with a reduced right prefrontal and left parietal-occipital asymmetry. Only 3 of 14 (21%) boys who stutter had the typical brain torque configuration. In contrast, the fluent boys displayed the expected ratio (70% typical, 30% atypical). These group differences were more pronounced in the parietal-occipital region relative to the frontal region, as 10 of the 14 (71%) boys who stutter had atypical parietal-occipital asymmetries and half had an atypical prefrontal asymmetry. In contrast, in the control group 11 of 14 (78%) boys had the typical right prefrontal configuration, and 9 of 14 (64%) had the typical left parietal-occipital configuration. When these ROIs were divided into superior and inferior subregions, there was a disassociation with an atypical asymmetry pattern in the inferior prefrontal and superior parietal-occipital areas in the boys who stutter. Despite these regional differences, the boys who stutter had more total brain white matter (increased by 8 %) relative to the fluent children.
When examining the distribution of all the brain torque configurations, certain patterns emerged. For instance, only one individual showed an asymmetry pattern with the prefrontal and parietal-occipital ROIs being larger in the same hemisphere (i.e. R>L prefrontal, R>L parietal-occipital or L>R prefrontal, L>R parietal-occipital). Also, every fluent boy with a typical L>R parietal-occipital asymmetry had a R>L prefrontal asymmetry. Of the four boys who stutter who displayed the typical L>R parietal-occipital asymmetry, three showed the typical R>L prefrontal asymmetry and one had R=L prefrontal symmetry. Therefore, we postulate through intra- and interhemispheric connections, the L>R parietal-occipital asymmetry might drive the development of the R>L prefrontal asymmetry. Further studies consisting of larger sample sizes and longitudinal data are needed to determine whether specific configurations have relevance to how speech and language develop.
There is evidence that these skull-based asymmetries are fixed (Hopkins & Marino, 2000; LeMay, 1976, 1977; Weinberger, et al., 1982). That is, these lobar asymmetries are evolutionary conserved, constrained by the bony configurations of the skull, do not change during development and do not vary throughout the lifecycle. One postmortem study found both prefrontal and parietal-occipital protrusions are present as early as 20 weeks gestational age (Weinberger, et al., 1982). Another study found similar results using computed tomography scans in infants under one year of age (LeMay, 1977). However, a volumetric MRI study of neonates found only the left parietal-occipital asymmetry was pronounced at birth (Gilmore, et al., 2007). The left hemisphere was expanded relative to the right resulting in a slight leftward prefrontal asymmetry, suggesting that the prefrontal volumes may change with development.
Despite the empiric evidence that skull-based asymmetries may be constrained through development, there are changes in both the prefrontal and parietal-occipital gray and white matter volumes throughout childhood. In one longitudinal study, the right frontal region showed both gray matter thinning and cortical expansion while the occipital lobes showed bilateral cortical gray matter thinning during normal childhood maturation (Sowell, et al., 2004). There is also a rapid increase in prefrontal white matter volume during childhood with a slower, but consistent increase in myelination through midlife (Bartzokis, et al., 2001; Giedd, et al., 1996). Therefore, longitudinal studies investigating prefrontal and parietal-occipital lobe development throughout life are needed to determine if specific localized developmental changes influence brain torque asymmetry patterns.
The parietal-occipital measure used in this study included portions of the inferior parietal lobule and the posterior temporal cortex (superior and middle temporal gyri). These brain regions are important in the perception, preparation, and production of speech (Okada & Hickok, 2006; Mock, Foundas, & Golob, 2011). Research suggests adults who stutter have atypical neuronal patterns during speech preparation and execution (Chang, Kenney, Loucks, & Ludlow, 2009; Lu, et al., 2009). These anomalous speech networks might be related to the hypothesis that dysfluent speech occurs in people who stutter because of an overreliance on auditory feedback (Civier, Tasko, & Guenther, 2010). These atypical language networks might also be related to slower phonological abilities in adults who stutter which show slower reaction times when asked to determine whether two visually presented words rhyme (Weber-Fox, Spencer, Spruill, & Smith, 2004). Boys who stutter make more errors, have increased reaction times and atypical late hemispheric electrophysiological responses compared to fluent children using the same visually presented rhyming judgment task (Weber-Fox, Spruill, Spencer, & Smith, 2008). Magnetoencephalography studies have revealed atypical temporal patterns of evoked-potentials in the frontal motor and temporal auditory areas during speech and pseudo-speech production (Salmelin, Schnitzler, Schmitz, & Freund, 2000; Salmelin, et al., 1998). Thus, an atypical brain torque configuration might predispose to an anomalous frontal-temporal-parietal network that may delay these rapid processes. These atypical neural networks may lead to a timing defect in speech perception, preparation and execution that may induce dysfluent overt speech.
Atypical brain torque and the underlying anatomical variants found in people who stutter may be associated with anomalous intra- and inter-hemispheric hemodynamic activation and deactivation patterns under task specific conditions. These aberrant cortical networks may be associated with atypical activations in subcortical or cortical motor areas (Giraud, et al., 2008; Watkins, et al., 2008). Hyperactivity has been consistently found in right hemispheric motor regions including dorsolateral primary and premotor areas, portions of the supplementary motor area, and the contralateral midline cerebellum (Braun, et al., 1997; Brown, et al., 2005; Fox, et al., 1996). This right hemisphere “shift” may be associated with a less efficient processing system that predisposes to stuttering-like behavior, which is a compelling explanation especially given our finding of a positive correlation with stuttering severity and right and left prefrontal white matter volume in our sample of boys who stutter. Somewhat contrary to our result, stuttering severity has been found to negatively correlate with right frontal operculum activation and right middle and superior temporal gyrus activation in adults who stutter (Preibisch et al., 2003; Fox et al., 2000). However, increased white matter clusters have been found in both of these right hemispheric areas in one study of adults who stutter (Jancke et al., 2004) raising questions about how white matter differences might be related to stuttering behavior in children compared to adults. Furthermore, a localized defect has been found in the white matter deep to oromotor regions in the left frontal operculum in adults who stutter (Cykowski, Fox, Ingham, Ingham, & Robin, 2010; Kell, et al., 2009; Sommer, et al., 2002; Watkins, et al., 2008) and in boys who stutter (Chang, et al., 2008). It may be that global anatomical anomalies (i.e. atypical brain torque), which might represent atypical hemispheric connections, predispose individuals who stutter to local brain anomalies in the auditory temporal cortex (e.g., planum temporale) (Beal, et al., 2007; Foundas, et al., 2001) that contribute to the development of aberrant frontal white matter pathways (Chang, et al., 2008; Cykowski, et al., 2010; Sommer, et al., 2002). The localized left frontal white matter defect could shift the more predominant leftward network activated in fluent individuals to the right prefrontal region in individuals who stutter (Kell, et al., 2009). It is unclear whether a cortical defect may drive significant cortical and/or subcortical reorganization, which may be modified by fluency shaping therapy (Giraud, et al., 2008).
Although the findings of atypical lobar volumes and asymmetries in adults (Foundas, et al., 2003) and in children who stutter are compelling, two recent studies did not find differences in prefrontal and parietal-occipital protrusions in a group of right-handed men and children who stutter (Chang, et al., 2008; Cykowski, et al., 2008). Different image processing and measurement techniques might explain these contradictory results. Cykowski et al. (2007) used a one-dimensional axial view to measure the length and width of the prefrontal and occipital protrusions, while Chang et al. (2008) used a voxel-based morphometry method. Furthermore, MRI images in both studies were globally and spatially normalized to a Talairach coordinate system. This type of warping of brain images into a standard space may obscure subtle, important MRI based morphological differences between groups, especially in children and in developmental disorders. Additional participant variables such as small sample sizes, degree of handedness, family history of stuttering, and stuttering severity could also account for the conflicting results.
Total Brain Volume: Left and Right Hemisphere Volumes
Total brain and right and left hemisphere volumes did not differ between the boys who stutter and the fluent controls. However, the boys who stutter did have increased hemispheric white matter volumes bilaterally compared to controls. Clusters of increased white matter have been found in the left and right hemispheres in adults who stutter (Beal, et al., 2007; Jancke, et al., 2004). These findings may have functional significance because increased leftward white matter concentrations have been found in language regions (Pujol, et al., 2002), associated with working memory and reading ability (Nagy, Westerberg, & Klingberg, 2004), and the learning of novel speech sounds (Golestani, Paus, & Zatorre, 2002). Also, the arcuate fasciculus is more robust in the left hemisphere (Takao, et al., 2010) and axonal myelination occurs earlier in the left hemisphere during development (Paus, 2005). Further study is needed to determine whether our results support the hypothesis that increased white matter volume may be associated with a predisposition to develop atypical (bilateral or rightward) language representations in right-handed boys who stutter. This study also found that the right hemisphere was larger than the left in both groups consistent with studies in adolescents and adults (Giedd, et al., 1996; Good, et al., 2001), although one recent study found that the left hemisphere was larger than the right in neonates (Gilmore, et al., 2007), supporting the postulate that some developmental changes may occur early in post-natal life.
Structure-Function Relationships
Atypical brain torque configurations have been associated with atypical language function and atypical cerebral laterality (Bear, Schiff, Saver, Greenberg, & Freeman, 1986; Bilder, et al., 1994; Zadina, et al., 2006). A previous study found language processing deficits to be associated with absolute prefrontal and parietal-occipital volume reduction in adults who stutter (Foundas, et al., 2003). There were also regional differences with reduced right superior prefrontal volume associated with lower scores on tests of listening comprehension and oral expression. In contrast, left and right parietal-occipital volume reductions were associated with impaired comprehension and not oral expression with these effects found in the inferior subregion. We did not find any of these effects in the boys who stutter. It is important to note that our sample of boys who stutter did not differ from controls on these language tests, whereas the adults who stutter did differ from controls on the language measures. In another study conducted on college age adults with and without dyslexia, similar correlations between anatomy and function were found (Zadina, et al., 2006). Reduced prefrontal superior volumes were found in the language impaired group. Perhaps future studies of boys who stutter with and without linguistic deficits may show similar relationships.
We conducted an exploratory analysis in the boys who stutter and used several measures of stuttering severity (SSI-score and percent stuttering like dysfluencies-SLD). A significant relationship was found in the boys who stutter with left and right prefrontal white matter volume positively correlated with stuttering severity (SLD rate). Functional imaging studies in adults who stutter have found stuttering severity negatively correlated with right frontal operculum activation and to right middle and superior temporal gyrus activation (Fox, et al., 2000; Preibisch, et al., 2003). A study also found an atypical planum temporale asymmetry (rightward) in adults who stutter was associated with fluency induced with delayed auditory feedback, especially in individuals with severe stuttering behavior (Foundas, et al., 2004). A previous study that investigated brain torque in adults who stutter using a similar methodology did not find a relationship between stuttering severity and anatomy (Foundas, et al., 2003). Perhaps these results reflect the fact that the adults who stutter were less severe compared to our sample of boys who stutter. It is important to continue to examine stuttering behavior with reference to MRI-based structural and functional measures because these measures may be modified by or may predict functional recovery.
Acknowledgments
Grants: Malcolm Fraser Foundation; NIH DC04957
Contributor Information
Jeffrey Ryan Mock, Louisiana State University Health Sciences Center, New Orleans, Louisiana.
Janet N. Zadina, Tulane University, New Orleans, Louisiana
David M. Corey, Tulane University, New Orleans, Louisiana
Jeremy D. Cohen, Xavier University, New Orleans, Louisiana
Lisa C. Lemen, University of Cincinnati, Cincinnati, Ohio
Anne L. Foundas, Louisiana State University Health Sciences Center, New Orleans, Louisiana
References
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil LF. Voxel-based morphometry of auditory and speech-related cortex in stutterers. Neuroreport. 2007;18(12):1257–1260. doi: 10.1097/WNR.0b013e3282202c4d. [DOI] [PubMed] [Google Scholar]
- Bear D, Schiff D, Saver J, Greenberg M, Freeman R. Quantitative analysis of cerebral asymmetries. Fronto-occipital correlation, sexual dimorphism and association with handedness. Arch Neurol. 1986;43(6):598–603. doi: 10.1001/archneur.1986.00520060060019. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, et al. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151(10):1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120(Pt 5):761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk E, editor. Oral and Written Language Scales. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- Chance SA, Esiri MM, Crow TJ. Macroscopic brain asymmetry is changed along the antero-posterior axis in schizophrenia. Schizophr Res. 2005;74(2–3):163–170. doi: 10.1016/j.schres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O, Tasko SM, Guenther FH. Overreliance on auditory feedback may lead to sound/syllable repetitions: simulations of stuttering and fluency-inducing conditions with a neural model of speech production. J Fluency Disord. 2010;35(3):246–279. doi: 10.1016/j.jfludis.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for impaired myelination. Neuroimage. 2010;52(4):1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov PV, Ingham RJ, Ingham JC, Mangin JF, Riviere D, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cereb Cortex. 2008;18(3):571–583. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- Foundas AL. The Anatomical Basis of Language. In: Bulter KG, editor. The Neural Basis of Language: Current Neuroimaging Perspectives. Vol. 21. Gaithersburg, MD: Aspen Publication; 2001. pp. 1–19. [Google Scholar]
- Foundas AL, Bollich AM, Corey DM, Hurley M, Heilman KM. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001;57(2):207–215. doi: 10.1212/wnl.57.2.207. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, et al. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology. 2004;63(9):1640–1646. doi: 10.1212/01.wnl.0000142993.33158.2a. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Corey DM, Angeles V, Bollich AM, Crabtree-Hartman E, Heilman KM. Atypical cerebral laterality in adults with persistent developmental stuttering. Neurology. 2003;61(10):1378–1385. doi: 10.1212/01.wnl.0000094320.44334.86. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382(6587):158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production. A PET performance-correlation analysis. Brain. 2000;123(Pt 10):1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology I-III: A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. 521–552, 634–654. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Neumann K, Bachoud-Levi AC, von Gudenberg AW, Euler HA, Lanfermann H, et al. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain Lang. 2008;104(2):190–199. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35(5):997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Marino L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38(4):493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Rilling JK. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: implication for the evolution of functional asymmetries. Behav Neurosci. 2000;114(4):739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Hanggi J, Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BMC Neurol. 2004;4(1):23. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H. Anatomical Brain Asymmetries and Their Relevance for Functional Asymmetries. In: Hugdahl K, Davidson R, editors. The Asymmetrical Brain. London: The MIT Press; 2003. pp. 187–229. [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132(Pt 10):2747–2760. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Ogata K, Umesaki T, Yoshiura T, Kenjo M, Hirano Y, et al. Spatiotemporal signatures of an abnormal auditory system in stuttering. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2010.12.083. [DOI] [PubMed] [Google Scholar]
- Koff E, Naeser MA, Pieniadz JM, Foundas AL, Levine HL. Computed tomographic scan hemispheric asymmetries in right- and left-handed male and female subjects. Arch Neurol. 1986;43(5):487–491. doi: 10.1001/archneur.1986.00520050059023. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- LeMay M. Asymmetries of the skull and handedness. Phrenology revisited. J Neurol Sci. 1977;32(2):243–253. doi: 10.1016/0022-510x(77)90239-8. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA. Asymmetry and dyslexia. Dev Neuropsychol. 2008;33(6):663–681. doi: 10.1080/87565640802418597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Chen C, Ning N, Ding G, Guo T, Peng D, et al. The neural substrates for atypical planning and execution of word production in stuttering. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic, M. ANALYZE (Version 5.0) Rochester, MN: BioMedical Imaging Resources; 1986. [Google Scholar]
- Mock JR, Foundas AL, Golob EJ. Modulation of sensory and motor cortex activity during speech preparation. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2010.07585.x. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G. Left posterior auditory-related cortices participate both in speech perception and speech production: Neural overlap revealed by fMRI. Brain Lang. 2006;98(1):112–117. doi: 10.1016/j.bandl.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pieniadz JM, Naeser MA, Koff E, Levine HL. CT scan cerebral hemispheric asymmetry measurements in stroke cases with global aphasia: atypical asymmetries associated with improved recovery. Cortex. 1983;19(3):371–391. doi: 10.1016/s0010-9452(83)80007-0. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, et al. Evidence for compensation for stuttering by the right frontal operculum. Neuroimage. 2003;20(2):1356–1364. doi: 10.1016/S1053-8119(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez-Sala A, Deus J, Cardoner N, Sebastian-Galles N, Conesa G, et al. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage. 2002;17(2):670–679. [PubMed] [Google Scholar]
- Reig S, Sanchez-Gonzalez J, Arango C, Castro J, Gonzalez-Pinto A, Ortuno F, et al. Assessment of the increase in variability when combining volumetric data from different scanners. Hum Brain Mapp. 2009;30(2):355–368. doi: 10.1002/hbm.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley GD. Stuttering severity instrument for children and adults. 3. Austin, TX: Pro-Ed; 1994. [DOI] [PubMed] [Google Scholar]
- Rorden C. MRIcro. 1999 (Version 1.37) [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123(Pt 6):1184–1202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Jancke L, Witte OW, Freund HJ. Functional organization of the auditory cortex is different in stutterers and fluent speakers. Neuroreport. 1998;9(10):2225–2229. doi: 10.1097/00001756-199807130-00014. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6(2):129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Buchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360(9330):380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows, Rel. 13.0. Chicago: SPSS Inc; 2001. [Google Scholar]
- Strub RL, Black FW, Naeser MA. Anomalous dominance in sibling stutterers: evidence from CT scan asymmetries, dichotic listening, neuropsychological testing, and handedness. Brain Lang. 1987;30(2):338–350. doi: 10.1016/0093-934x(87)90107-6. [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, et al. Gray and white matter asymmetries in healthy individuals aged 21–29 years: A voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Torgesen J, Wagner RK, Rashotte CA, editors. Test of Word Reading Efficiency. Austin, TX: Pro-ed; 1999. [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131(Pt 1):50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C, Spencer RM, Spruill JE, 3rd, Smith A. Phonologic processing in adults who stutter: electrophysiological and behavioral evidence. J Speech Lang Hear Res. 2004;47(6):1244–1258. doi: 10.1044/1092-4388(2004/094). [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, 3rd, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Dev Sci. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition: Administrative and scoring manual Table 5.14. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wechsler D, editor. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Weinberger DR, Luchins DJ, Morihisa J, Wyatt RJ. Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Ann Neurol. 1982;11(1):97–100. doi: 10.1002/ana.410110118. [DOI] [PubMed] [Google Scholar]
- Wiederhollt JL, Bryant BR. Gray Oral Reading Test. 4. Austin, Tx: Pro-ed; 2001. [Google Scholar]
- Woodcock R, McGrew K, Mather N. Woodcock-Johnson III Tests of Cognitive Ability. Itasca, Il: Riverside Publishing; 2001. [Google Scholar]
- Zadina JN, Corey DM, Casbergue RM, Lemen LC, Rouse JC, Knaus TA, et al. Lobar asymmetries in subtypes of dyslexic and control subjects. J Child Neurol. 2006;21(11):922–931. doi: 10.1177/08830738060210110201. [DOI] [PubMed] [Google Scholar]