Abstract
Integral membrane proteins contain a hydrophobic transmembrane domain and mainly locate in the plasma membrane lipid bilayer. The receptor tyrosine kinases (RTK) of the epidermal growth factor receptor (EGFR) superfamily, including ErbB-1, ErbB-2, ErbB-3, and ErbB-4, constitute an important group of such membrane proteins which have profound impact on cancer initiation, progression, and patient outcome. Although studies about their functions have conventionally focused on their membrane-associated forms, documented observations of the presence of these membrane receptors and their functioning partners in the nucleus have re-shaped the intracellular geography and highlight the need to modify the central dogma. The ErbB proteins in the membrane can translocate to the nucleus through different mechanisms. Nuclear RTKs regulate a variety of cellular functions, such as cell proliferation, DNA damage repair, and signal transduction, both in normal tissues and in human cancer cell. In addition, they play important roles in determining cancer response to cancer therapy. Nuclear presence of these ErbB proteins is emerging as an important marker in human cancers. An integrated picture of the RTK-centered signaling transduction network extending from the membrane-cytoplasm boundary to the nuclear compartment is looming in the foreseeable horizon for clinical application.
BACKGROUND
The epidermal growth factor receptor (EGFR) proteins
The EGFR proteins, which are arguably the most studied receptor tyrosine kinases (RTK), include EGFR/ErbB-1/HER-1, ErbB-2/HER-2, ErbB-3/HER-3, and ErbB-4/HER-4. The prototype RTK in the membrane contains an extracellular domain, a transmembrane domain, and an intracellular region, Except for ErbB-3, the intracellular region harbors a tyrosine kinase activity, which is vital for the signaling functions of these membrane molecules. Upon growth factor stimulation, these membrane receptors form homodimers or heterodimers, for which ErbB-2 is the most favorable dimerization partner. Receptor dimerization triggers conformational change and subsequent kinase activation, followed by receptor internalization (1). Activated ErbB receptors then triggers the activation of multiple signaling cascades, such as the pathways mediated by MAPK (mitogen-activated protein kinase), PI-3K (phosphatidylinositol-3 kinase), PLC (phospholipase C), and STAT (signal transducer and activator of transcription). Thus, the activated receptors regulate multiple cellular activities and the specific biological outcome caused by activated receptor tyrosine kinases depends on the crosstalk of these signaling pathways.
ErbB proteins in the Nucleus
Membrane receptors in the nucleus, or MRIN, a term we propose to refer to the unique biological process of membrane to nucleus translocation and the associated functions, has been documented by a substantial body of evidence accumulated in the past decades (2–6), which has called for a revisit to the central dogma that RTK resides and functions only in the lipid bilayer of the plasma membrane or extra-nuclear vesicles in the cytosol (7). Among the EGFR family members, EGFR (8, 9), ErbB-2 (10, 11), and ErbB-3 (12) can be detected in the nucleus as full-length receptors. Nuclear expression of these receptors has also been found in different normal tissues or primary cells (13–16). The primary mechanism of ErbB-4 nuclear trafficking is through the sequential proteolytic processing to produce an intracellular domain (ICD) which then translocates to the nucleus and functions as a transcriptional factor (5, 17), while detection of full-length ErbB-4 in the nucleus of normal cells has also been described (13, 15). Fragments derived from ErbB-2 by N-terminal truncation or alternative translation have also been found in the nucleus of cancer cells (18, 19). Interestingly, some of these fragments generated by alternative translational initiation still retain the transmembrane domain and therefore are likely to associate with the membrane. Thus their nuclear translocation would need to overcome the same energy barrier as the full-length receptor.
The mechanisms proposed for nuclear transport of membrane proteins include the activity of transmembrane domain-binding chaperones, endosome-mediated nuclear translocation, and retro-translocation by endoplasmic reticulum (ER)-associated trafficking machinery (6). Among them, the latter two mechanisms have received the most experimental supports. Upon activation by ligand stimulation, the membrane receptors are internalized through the clathrin-coated endocytotic vehicle formed by the GTPase dynamin (20). In the endosome-mediated nuclear translocation, the internalized endosome is directed by the nuclear transporter importin proteins, which recognize the nuclear localization signal (NLS) of the ErbB proteins to the nucleus through the nuclear pore complex (21–24). The retro-translocation model of nuclear translocation also involves the internalization of the surface receptor with the endosomal vehicle, which then merges with the endoplasmic reticulum (ER) through the Golgi. The ER-bound EGFR is retro-translocated into the cytosol with the aide of the Sec61 translocon, which forms a channel across the ER membrane for protein transport (25). The membrane protein in the cytosol is stabilized by the chaperone protein HSP70, and then directed by the importin proteins for nuclear entry (22). Another recent study described an alternative but related mechanism for nuclear transport of the integral membrane proteins located in the ER membrane (26, 27), in which the NLS motif presents in the cytoplasmic domain of these integral proteins mediated nuclear entry by the importin proteins through lateral diffusion. This is another example that the membrane-bound integral proteins can be recognized and directed to the nucleus through the NLS-mediated mechanism. It will be interesting to test whether and how this mechanism is involved in the nuclear translocation of plasma membrane proteins.
Nuclear expression of EGFR has been demonstrated in normal tissues with active cell proliferation, such as the liver tissue during regeneration (8), the uterus of pregnant mouse and the basal cells of normal mouth mucosa (9). These observations suggest a role of nuclear EGFR in normal biology. All the four ErbB proteins have been identified in the nucleus of the mouse lung type II epithelial cell, with ErbB-1, -2 and -3 predominantly in the nucleus while ErbB-4 diffusively distributed in the cytoplasm and the nucleus (14). The subnuclear localization and dimerization partner changed in response to the stimulation of the growth factor neuregulin, suggesting that the nuclear ErbB proteins have a role in normal lung epithelium. Upon NRG 1β stimulation, which is a potent mitogenic factor in Schwann cells, ErbB-2 and ErbB-3 form heterodimer and promote proliferation and survival of Schwann cells (28, 29). This growth stimulation is associated with significant nuclear translocation of NRG 1β as well as the two receptors, suggesting an intranuclear signaling of the ligand and the receptors in promoting neuronal growth (16). Nuclear expression of ErbB-2, -3, and -4 can have a biological function in brain development of primates as their nuclear presence was detected in the front cortex of juvenile and adult monkeys (15). Similar nuclear localization and co-localization of the ErbB proteins was identified in primary human umbilical venous endothelial cells (HUVEC) and arterial endothelial cells (HUAEC) derived from early fetal gestation (13).
Molecular Functions of the Nuclear ErbB Proteins
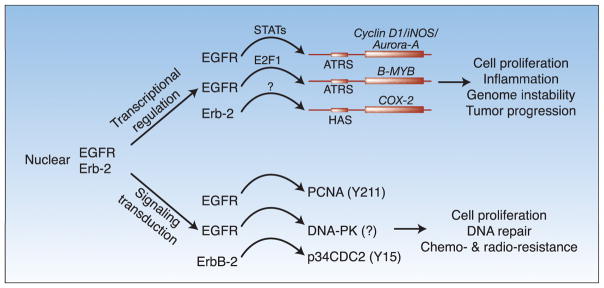
Two major functions of the nuclear ErbB proteins have been unraveled (fig 1).
Fig. 1.
A working model of RTK functions in the nucleus. EGFR and ErbB-2 can function in transcriptional regulation. EGFR transactivates cyclin D1, iNOS, and Aurora-A together with the transcriptional factor STAT3 and STAT5. EGFR and E2F1 can stimulate transcription of the B-MYB gene. The AT-rich sequence (ATRS) on the promoter is important for the EGFR-mediated transactivation. Nuclear ErbB-2 transactivate the COX-2 gene through a HER2-associated sequence (HAS). The transcriptional factors involved remain to be identified. Nuclear EGFR can also function as a kinase to phosphorylate PCNA and increases its stability on the chromatin. Nuclear EGFR interacts with DNA-PK and convey a resistance phenotype to ionizing irradiation. It remains to be determined whether EGFR phosphorylates DNA-PK. ErbB-2 has been shown to phosphorylate the cyclin-dependent kinase p34CDC2, which causes G2-M arrest in cancer cells and subsequently leads to resistance to taxol treatment.
Transcriptional regulation
The C-terminal regions of EGFR/ErbB-1, ErbB-2/HER-2/neu, and ErbB-4 displayed intrinsic transactivation activity (9–11, 17). Multiple gene promoters have been identified as the targets of the nuclear ErbB receptors. The cyclin D1 promoter is the first identified genomic target of nuclear RTK (9). Upon EGF stimulation, EGFR translocates to the nucleus and binds to an A/T-rich sequence (ATRS) in the proximal region of the cyclin D1 promoter and stimulates its expression in cancer cells, providing a direct link between nuclear EGFR function and cell proliferation. The other ATRS-containing promoters targeted by the nuclear EGFR include the iNOS (inducible nitric oxide synthase) gene (30). Nuclear EGFR is recruited to the ATRS motif of the iNOS promoter through its interaction with the transcriptional factor STAT3. Like other members of the EGFR family, EGFR lacks a DNA-binding domain. Therefore association with other DNA-binding factors such as STAT3 is expected to be required for chromatin recruitment and gene activation activity for nuclear EGFR. Similarly, EGFR was associated with STAT5 on the ATRS motif to transactivate the Aurora-A promoter (31). Increased expression of the Aurora-A gene induced centrosome amplification and microtubule disorder. EGFR has also been found to cooperate with E2F1 to transactivate the B-MYB gene in a cell cycle-dependent manner (32). Thus, B-MYB joins cyclin D1 as the transcriptional targets of nuclear EGFR to promote cell proliferation. The genomic targets of nuclear ErbB-2 were discovered by chromatin immunoprecipitation and molecular cloning to identify the HER-2/ErbB-2-associated sequence (HAS) (11). ErbB-2 transactivated the COX-2 (cyclooxygenase 2) gene promoter through a HAS motif in the promoter. COX-2 is important for cancer development as a promoting factor for cell survival, proliferation, metastasis, and angiogenesis and its expression is correlated with ErbB-2 in primary cancer tissues (33–36). ErbB-4 conveys its transcriptional function through its ICD domain. Heregulin (also known as neuregulin) stimulated nuclear co-translocation and association of the ErbB-4 ICD and STAT5A which in turn transactivated the β-casein gene promoter (37). Nuclear ErbB-4 ICD was also shown to be a co-activator of estrogen receptor α (ER-α) and enhanced sensitivity to tamoxifen in breast cancer cells (38). On the other hand, nuclear ErbB-4 ICD contributed the breast cancer etiology by blocking the gene repression activity of ETO-2, a transcriptional factor with suggested tumor suppressor function (39).
Protein kinase
A common feature for the nuclear ErbB-1, ErbB-2, and ErbB-4, including their intracellular fragments, is the presence of a tyrosine kinase domain. The cyclin-dependent kinase p34Cdc2, a protein located primarily in the nucleus, has been reported to be a direct substrate of ErbB-2 (40). Phosphorylation of p34Cdc2 at tyrosine 15 by ErbB-2 inhibited the kinase activity of p34Cdc2 and delayed M phase entry, leading to taxol resistance in breast cancer. The ICD fragment of ErbB-4 is able to form homodimers in the nucleus and possesses constitutive kinase activity (41). ErbB-4 ICD phosphorylated and inhibited the nuclear protein HDM2, consequently enhanced p53 and p21 expression (42). The full-length EGFR in the nucleus is a bona fide tyrosine kinase to phosphorylate the chromatin-associated DNA replication processive factor PCNA (proliferative cell nuclear antigen) (43). Inhibition of the phosphorylation led to degradation of the chromatin-bound, but not the un-bound, form of PCNA through a proteasome-dependent manner and consequently suppressed its function in DNA synthesis and DNA damage repair.
CLINICAL-TRANSLATIONAL ADVANCES
Expression of EGFR in the nucleus, but not in the non-nuclear compartment, was shown significantly correlated with overall survival in a cohort of breast carcinoma patients (44). Nuclear expression of EGFR was also detected by immunofluorescence staining in oropharyngeal squamous cell carcinoma using an automated image acquisition and quantitation system (45, 46). The presence of EGFR in the nucleus was correlated with significant increase in local recurrence and decrease in disease-free survival after radiotherapy alone or adjuvant radiotherapy. Interestingly, in a study on a small cohort of esophageal cancer patients, only the nucleus-located phosphorylated EGFR but not the total EGFR was correlated with higher TNM stage, nodal metastasis, and poor patient outcome (47). Nuclear staining of EGFR was also found in epithelial ovarian cancer tumor tissues (48, 49), while it remains to determine whether nuclear EGFR is involved in tumor progression of ovarian cancer. Thus, nuclear expression of EGFR appears to be associated with cancer development. Nuclear expression of the commonly identified EGFRvIII variant of EGFR, a mutant with deletion of the cytoplasmic domain of the receptor rendering the receptor constitutively activated (50), has been described in prostate cancer, invasive breast cancer, and brain tumor (51–53). In hormone-refractory prostate cancer, nuclear expression of EGFRvIII was associated with decreased time to death from biochemical relapse and decreased overall survival (51). In glioblastoma, nuclear EGFRvIII was found forming a complex with STAT3 (53). The EGFRvIII-STAT3 oncogenic complex upregulated iNOS and further mediated the EGFRvIII-induced glial transformation (53).
There are multiple mechanisms underlying the EGFR-mediated tumor progression. In nasopharyngeal carcinoma (NPC) induced by Epstein-Barr virus (EBV) (54), the EBV-encoded latent membrane protein 1 (LMP1) oncoprotein was identified to enhance nuclear entry of EGFR, which subsequently transactivated the cyclin D1 and cyclin E gene promoters to foster cell proliferation. On the other hand, EGFR nuclear trafficking can be inhibited by the growth inhibitor vitamin D (1,25(OH)2D3), which subsequently repressed the cyclin D1 gene and suppressed tumor growth (55). A number of studies demonstrated that ionization irradiation activates EGFR kinase activity, which contributed to protecting cancer cells from the killing by radiotherapy (56–59). Contrarily, several studies reported that EGF enhanced radiosensitivity of EGFR-expressing cancer cells, in particular for cells in G1 phase of the cell cycle (60–62). These contradictory results might have been due to the cell proliferation stage or the different molecular makeup of these cells, for example, the nuclear EGFR status. A recent report showed that the gefitinib/erlotinib–sensitive EGFR mutants in a population of non–small cell lung cancers also conferred elevated sensitivity to ionizing irradiation. More importantly, these mutants were also defective in nuclear translocation (63). On the other hand, ionizing radiation enhanced nuclear translocation of wild-type EGFR which in turn contributed to EGFR-mediated radioprotection. Ionizing irradiation has been shown to cause nuclear transport of EGFR through caveolin- and protein kinase C-dependent mechanisms (64–66). It is therefore likely that nuclear sequestration of wild-type EGFR in cancer cells is a means to protect the cells from EGFR-targeting as well as other genotoxic stresses. In this regard, it has recently been shown that nuclear translocation of both EGFR and ErbB-2 is inhibited by the tyrosine kinase inhibitor lapatinib, which leads to the sensitization of cancer cells to fluoropyrimindine (67).
Radiation-induced nuclear transport of EGFR activated the DNA-dependent protein kinase (DNA-PK) which then mediated the repair of the double-strand DNA break induced by irradiation (64). Activation of DNA-PK function through interaction with EGFR has been documented previously (68, 69), while the molecular mechanism remains to be determined. Treatment with the anti-EGFR antibody C225 blocked nuclear transport of EGFR and consequently inhibited DNA-PK activity and resulted in increased radiosensitivity (70). It was reported recently that the cyclooxygenase-2 (COX-2) inhibitor celecoxib can also induce radiosensitization through blocking nuclear transport of EGFR, providing an example of COX-2-independent mechanism of celecoxib (71). Nuclear EGFR can also regulate cell sensitivity to genotoxic stress through PCNA (43). Inhibition of EGFR kinase activity rendered PCNA destabilized from the chromatin so that its DNA damage repair function was also impaired. Consistently, in primary breast tumor tissues, nuclear expression of EGFR and PCNA and phospho-PCNA was tightly correlated. More importantly, expression of the phospho-PCNA was a better tumor marker for overall survival in breast cancer patients. As the Y211-phosphorylated PCNA is the chromatin-bound, replicationally functional form of PCNA, this specific PCNA species is expected to be a better molecular marker to assess tumor malignancy. In addition to breast tumor tissues, this correlation was also found in oropharyngeal squamous cell carcinoma, in which PCNA expression was the only pathological factors tested being positively correlated with nuclear EGFR (46).
For ErbB-3, two recent studies showed that although nuclear expression of ErbB-3 was not frequently observed in normal or hyperplasia prostate tissues, its expression became much pronounced in prostate cancer, especially in the advanced hormone-refractory cases (72, 73). Tissue microarray analysis showed that positive nuclear staining of ErbB-3 was associated with higher Gleason grade and disease progression (72). In a prostate cancer xenograft model, although ErbB-3 predominantly located in the cytoplasm and plasma membrane in the tumors inoculated subcutaneously, significant nuclear expression of ErbB-3 was observed in the bone xenograft, suggesting a regulation by the microenvironment (73). Thus, nuclear expression of ErbB-3 can be a prognostic marker or a therapeutic target for advanced prostate cancer with bone metastasis and resistance to androgen ablation.
Nuclear transport of the ErbB proteins is increasingly recognized as a pathological feature in tumors. The clinical impact of this new field has been demonstrated in the correlation of nuclear ErbB proteins with disease progression in multiple types of cancer. From the perspective of cancer biology, it is possible that the nuclear localization of the ErbB proteins serve as a mechanism for sustained signaling and transactivation functions of these proteins to promote cancer progression. The nuclear localization may also contribute to the resistance to therapeutic agents designed for targeting these proteins in the plasma membrane. A major challenge is to gain substantial knowledge of this process that will permit tackling this out-side-in nuclear translocation of the ErbB receptors for cancer prognosis, targeting, and therapeutic sensitization.
Table 1.
Biological functions of nuclear ErbB receptors
| Cell proliferation | ErbB-1 (9, 22, 31, 43, 54) |
| DNA replication | ErbB-1 (43) |
| DNA damage repair | ErbB-1 (43, 64–66, 71) |
| Transcription | ErbB-1 (9, 30–32); ErbB-2 (10, 11); ErbB-4 (17, 37, 74) |
| Cancer progression | ErbB-1 (9, 30, 32, 42, 43, 45–47, 51, 63, 66); ErbB-2 (11, 18); ErbB-3 (72); ErbB-4 (75) |
| Development | ErbB-4 (74) |
Acknowledgments
We would like to apologize to the authors of the works we could not cite due to space limitations.
Grant Support: This work is supported in part by NIH RO1 CA109311, Breast Cancer SPORE CA116199, and the University of Texas M. D. Anderson Cancer Center/China Medical University Hospital Sister Institution Fund (to M.-C. H). S.-C.W. is supported by the tenure-track faculty starting fund from the University of Cincinnati College of Medicine Cancer Center and the Department of Surgery, and the UC Barrett Cancer Center Pilot Project Award.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–8. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planque N. Nuclear trafficking of secreted factors and cell-surface receptors: new pathways to regulate cell proliferation and differentiation, and involvement in cancers. Cell Commun Signal. 2006;4:7. doi: 10.1186/1478-811X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends in Cell Biology. 2006;16:649–56. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15:143–8. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 6.Wells A, Marti U. Signalling shortcuts: cell-surface receptors in the nucleus? Nat Rev Mol Cell Biol. 2002;3:697–702. doi: 10.1038/nrm905. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J, Lemmon MA. Nuclear Signaling by Receptor Tyrosine Kinases: The First Robin of Spring. Cell. 2006;127:45–8. doi: 10.1016/j.cell.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Marti U, Burwen SJ, Wells A, et al. Localization of epidermal growth factor receptor in hepatocyte nuclei. Hepatology. 1991;13:15–20. [PubMed] [Google Scholar]
- 9.Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 10.Xie YM, Hung MC. Nuclear Localization of p185neu Tyrosine Kinase and Its Association with Transcriptional Transactivation. Biochem Biophys Res Comm. 1994;203:1589–98. doi: 10.1006/bbrc.1994.2368. [DOI] [PubMed] [Google Scholar]
- 11.Wang S-C, Lien H-C, Xia W, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929–40. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueter W, Dammann O, Zscheppang K, Korenbaum E, Dammann CE. ErbB receptors in fetal endothelium--a potential linkage point for inflammation-associated neonatal disorders. Cytokine. 2006;36:267–75. doi: 10.1016/j.cyto.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zscheppang K, Korenbaum E, Bueter W, Ramadurai SM, Nielsen HC, Dammann CE. ErbB receptor dimerization, localization, and co-localization in mouse lung type II epithelial cells. Pediatric pulmonology. 2006;41:1205–12. doi: 10.1002/ppul.20518. [DOI] [PubMed] [Google Scholar]
- 15.Thompson M, Lauderdale S, Webster MJ, et al. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain research. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Raabe TD, Deadwyler G, Varga JW, Devries GH. Localization of neuregulin isoforms and erbB receptors in myelinating glial cells. Glia. 2004;45:197–207. doi: 10.1002/glia.10311. [DOI] [PubMed] [Google Scholar]
- 17.Ni C-Y, Murphy MP, Golde TE, Carpenter G. gamma -Secretase Cleavage and Nuclear Localization of ErbB-4 Receptor Tyrosine Kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 18.Anido J, Scaltriti M, Bech Serra JJ, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. The EMBO journal. 2006;25:3234–44. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 20.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 21.Giri DK, Ali-Seyed M, Li L-Y, et al. Endosomal Transport of ErbB-2: Mechanism for Nuclear Entry of the Cell Surface Receptor. Mol Cell Biol. 2005;25:11005–18. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao HJ, Carpenter G. Role of the sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Molecular biology of the cell. 2007;18:1064–72. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu S-C, Hung M-C. Characterization of a Novel Tripartite Nuclear Localization Sequence in the EGFR Family. J Biol Chem. 2007;282:10432–40. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 24.Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem. 2006;98:1570–83. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- 25.Wiertz EJ, Tortorella D, Bogyo M, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–8. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 26.King MC, Lusk C, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442:1003–7. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 27.Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–63. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi AD, Bunge RP, Lofgren JA, et al. The influence of heregulins on human Schwann cell proliferation. J Neurosci. 1995;15:1329–40. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–18. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo H-W, Hsu S-C, Ali-Seyed M, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–89. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Hung L-Y, Tseng JT, Lee Y-C, et al. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucl Acids Res. 2008;36:4337–51. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanada N, Lo HW, Day CP, Pan Y, Nakajima Y, Hung MC. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol Carcinog. 2006;45:10–7. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- 33.Flower RJ. The development of COX-2 inhibitors. Nature Reviews Drug Discovery Nat Rev Drug Discov. 2003;2:179–91. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 34.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. The Journal of biological chemistry. 2002;277:18649–57. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 36.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 37.Williams CC, Allison JG, Vidal GA, et al. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naresh A, Thor AD, Edgerton SM, Torkko KC, Kumar R, Jones FE. The HER4/4ICD estrogen receptor coactivator and BH3-only protein is an effector of tamoxifen-induced apoptosis. Cancer Res. 2008;68:6387–95. doi: 10.1158/0008-5472.CAN-08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linggi B, Carpenter G. ErbB-4 s80 Intracellular Domain Abrogates ETO2-dependent Transcriptional Repression. J Biol Chem. 2006;281:25373–80. doi: 10.1074/jbc.M603998200. [DOI] [PubMed] [Google Scholar]
- 40.Tan M, Jing T, Lan K-H, et al. Phosphorylation on Tyrosine-15 of p34Cdc2 by ErbB2 Inhibits p34Cdc2 Activation and Is Involved in Resistance to Taxol-Induced Apoptosis. Molecular Cell. 2002;9:993–1004. doi: 10.1016/s1097-2765(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 41.Linggi B, Cheng QC, Rao AR, Carpenter G. The ErbB-4 s80 intracellular domain is a constitutively active tyrosine kinase. Oncogene. 2005;25:160–3. doi: 10.1038/sj.onc.1209003. [DOI] [PubMed] [Google Scholar]
- 42.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. The Journal of biological chemistry. 2005;280:30783–7. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 43.Wang S-C, Nakajima Y, Yu Y-L, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8:1359–68. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 44.Lo H-W, Xia W, Wei Y, Ali-Seyed M, Huang S-F, Hung M-C. Novel Prognostic Value of Nuclear Epidermal Growth Factor Receptor in Breast Cancer. Cancer Res. 2005;65:338–48. [PubMed] [Google Scholar]
- 45.Psyrri A, Yu Z, Weinberger PM, et al. Quantitative Determination of Nuclear and Cytoplasmic Epidermal Growth Factor Receptor Expression in Oropharyngeal Squamous Cell Cancer by Using Automated Quantitative Analysis. Clin Cancer Res. 2005;11:5856–62. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 46.Psyrri A, Egleston B, Pectasides E, et al. Correlates and determinants of nuclear epidermal growth factor receptor content in an oropharyngeal cancer tissue microarray. Cancer Epidemiol Biomarkers Prev. 2008;17:1486–92. doi: 10.1158/1055-9965.EPI-07-2684. [DOI] [PubMed] [Google Scholar]
- 47.Hoshino M, Fukui H, Ono Y, et al. Nuclear expression of phosphorylated EGFR is associated with poor prognosis of patients with esophageal squamous cell carcinoma. Pathobiology. 2007;74:15–21. doi: 10.1159/000101047. [DOI] [PubMed] [Google Scholar]
- 48.Psyrri A, Kassar M, Yu Z, et al. Effect of Epidermal Growth Factor Receptor Expression Level on Survival in Patients with Epithelial Ovarian Cancer. Clin Cancer Res. 2005;11:8637–43. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 49.Xia W, Wei Y, Du Y, et al. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009 doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 51.Edwards J, Traynor P, Munro AF, Pirret CF, Dunne B, Bartlett JMS. The Role of HER1-HER4 and EGFRvIII in Hormone-Refractory Prostate Cancer. Clin Cancer Res. 2006;12:123–30. doi: 10.1158/1078-0432.CCR-05-1445. [DOI] [PubMed] [Google Scholar]
- 52.Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. International journal of cancer. 2002;98:357–61. doi: 10.1002/ijc.10224. [DOI] [PubMed] [Google Scholar]
- 53.de la Iglesia N, Konopka G, Puram SV, et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes & development. 2008;22:449–62. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao Y, Song X, Deng X, et al. Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein-Barr-encoded oncoprotein latent membrane protein 1. Experimental cell research. 2005;303:240–51. doi: 10.1016/j.yexcr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Cordero JB, Cozzolino M, Lu Y, et al. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. The Journal of biological chemistry. 2002;277:38965–71. doi: 10.1074/jbc.M203736200. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–7. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 57.Goldkorn T, Balaban N, Shannon M, Matsukuma K. EGF receptor phosphorylation is affected by ionizing radiation. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1997;1358:289–99. doi: 10.1016/s0167-4889(97)00063-3. [DOI] [PubMed] [Google Scholar]
- 58.Herbst RS. Review of epidermal growth factor receptor biology. International journal of radiation oncology, biology, physics. 2004;59:21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 59.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. International Journal of Radiation Biology. 2007;83:781– 91. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 60.Kwok TT, Sutherland RM. Differences in EGF related radiosensitisation of human squamous carcinoma cells with high and low numbers of EGF receptors. Br J Cancer. 1991;64:251–4. doi: 10.1038/bjc.1991.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwok TT, Sutherland RM. Cell cycle dependence of epidermal growth factor induced radiosensitization. International journal of radiation oncology, biology, physics. 1992;22:525–7. doi: 10.1016/0360-3016(92)90867-h. [DOI] [PubMed] [Google Scholar]
- 62.Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. International journal of radiation oncology, biology, physics. 1994;29:243–7. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 63.Das AK, Chen BP, Story MD, et al. Somatic Mutations in the Tyrosine Kinase Domain of Epidermal Growth Factor Receptor (EGFR) Abrogate EGFR-Mediated Radioprotection in Non-Small Cell Lung Carcinoma. Cancer Res. 2007;67:5267–74. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- 64.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. The Journal of biological chemistry. 2005;280:31182–9. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 65.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Molecular cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wanner G, Mayer C, Kehlbach R, Rodemann HP, Dittmann K. Activation of protein kinase C-epsilon stimulates DNA-repair via epidermal growth factor receptor nuclear accumulation. Radiother Oncol. 2008;86:383–90. doi: 10.1016/j.radonc.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 67.Kim HP, Yoon YK, Kim JW, et al. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS ONE. 2009;4:e5933. doi: 10.1371/journal.pone.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical Interaction between Epidermal Growth Factor Receptor and DNA-dependent Protein Kinase in Mammalian Cells. J Biol Chem. 1998;273:1568–73. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 69.Huang S-M, Harari PM. Modulation of Radiation Response after Epidermal Growth Factor Receptor Blockade in Squamous Cell Carcinomas: Inhibition of Damage Repair, Cell Cycle Kinetics, and Tumor Angiogenesis. Clin Cancer Res. 2000;6:2166–74. [PubMed] [Google Scholar]
- 70.Dittmann K, Mayer C, Rodemann H-P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiotherapy and Oncology. 2005;76:157–61. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Dittmann KH, Mayer C, Ohneseit PA, et al. Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: a COX-2 independent mechanism. International journal of radiation oncology, biology, physics. 2008;70:203–12. doi: 10.1016/j.ijrobp.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 72.Koumakpayi IH, Diallo JS, Le Page C, et al. Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res. 2006;12:2730–7. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]
- 73.Cheng C-J, Ye X-c, Vakar-Lopez F, et al. Bone Microenvironment and Androgen Status Modulate Subcellular Localization of ErbB3 in Prostate Cancer Cells. Mol Cancer Res. 2007;5:675–84. doi: 10.1158/1541-7786.MCR-06-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-Dependent ErbB4 Nuclear Signaling Regulates the Timing of Astrogenesis in the Developing Brain. Cell. 2006;127:185–97. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan R, Gillett CE, Barnes DM, Gullick WJ. Nuclear Expression of the c-erbB-4/HER-4 Growth Factor Receptor in Invasive Breast Cancers. Cancer Res. 2000;60:1483–7. [PubMed] [Google Scholar]