Abstract
Objective: Cervical spinal cord injury (tetraplegia) is known to interrupt sympathetic vasculature control, thereby preventing shunting of blood from the periphery to central organs when exposed to cold temperatures. As a result, persons with tetraplegia are at risk to develop hypothermia. However, information regarding the discomfort experienced during the cooler months (late fall, winter, early spring) is overwhelmingly anecdotal. It is not known, with any certainty, how those with tetraplegia perceive cold and if discomfort in colder environments restricts them from performing activities that they routinely would perform.
Design: Prospective, two-group, self-report surveys.
Setting: VA Medical Center and Kessler Institute for Rehabilitation.
Participants: Forty-four subjects with tetraplegia; 41 matched non-SCI controls.
Outcome Measures: Tetraplegic and control groups responded “yes” or “no” when asked whether cold seasonal temperatures allowed comfort or negatively affected participation in routine activities.
Results: Percentage of responses of tetraplegia compared to controls was different as to whether they felt cold when others in the same room were comfortable (82 vs. 24%; χ2 = 28.2, P < 0.0001), felt comfortable outdoors (17 vs. 43%; χ2 = 6.8, P = 0.009), or whether cold negatively affected bathing routines (55 vs. 15%; χ2 = 14.8, P = 0.0001), keeping physician appointments (46 vs. 12%; χ2 = 11.3, P = 0.0008), thinking clearly (41 vs. 7%; χ2 = 12.9, P = 0.0003), and completing usual work duties (46 vs. 10%; χ2 = 13.3, P = 0.0003).
Conclusion: Cold seasonal temperatures have a reported greater negative impact on personal comfort and ability to perform vital activities in persons with tetraplegia than that of non-SCI controls. These findings highlight the need to address thermoregulatory impairment in persons with tetraplegia.
Keywords: Activities of daily living, Hypothermia, Quadriplegia, Quality of life, Self report, Spinal cord injuries
Introduction
Injury to the spinal cord at the cervical level (tetraplegia) is known to interrupt motor, sensory, and sympathetic pathways below the lesion level.1–7 However, the impairment of hypothalamic regulation of cutaneous vasoconstriction and shivering during cold exposure and of cutaneous vasodilation and sweating during heat exposure after spinal cord injury (SCI) is not as well recognized, leaving persons with SCI vulnerable to both hypothermia and hyperthermia, respectively. This study addresses the impact of dysfunctional thermogenic mechanisms on comfort and quality of life (QoL) during the cooler seasons.
In able-bodied persons exposed to cold environments, sensory input from cutaneous and more central thermal receptors stimulate neurons in the preoptic-anterior hypothalamus, which mobilize neurons in the posterior hypothalamus and paraventricular nucleus to effect a sympathetic-mediated response.8 Within minutes of cold exposure, plasma levels of circulating norepinephrine, epinephrine, and cortisol have been shown to increase due to sympatho-adrenal stimulation.9 The resultant peripheral vasoconstriction shifts blood volume from the superficial to the deeper central compartment, rapidly increasing thermal insulation to preserve core temperature at the expense of less vital, more superficial tissues.10,11 With continued exposure to cold, the posterior hypothalamus will induce involuntary shivering to promote the production of heat above that of the basal metabolic rate (BMR).12
Persons with tetraplegia are predisposed to subnormal core temperatures (Tcore) and hypothermia secondary to multiple factors. The interruption of sensory pathways to the cortex and hypothalamus impairs behavioral and involuntary responses to cold ambient temperatures, respectively. The inability to appropriately augment norepinephrine levels, due to either partial or complete interruption of supraspinal modulation of the sympathetic nervous system,13 limits sympathetic vasculature control, thereby preventing shunting of blood from the periphery to central organs when exposed to cold temperatures. Shivering is typically both delayed and limited,3 thereby attenuating any increases in metabolic rate,6 and combined with impaired vasoconstriction and lean tissue atrophy,14 leads to steadily decreasing Tcore following cold exposure (18.0–24.0°C) in persons with tetraplegia.1,3,6,15,16 The combined effects of these impairments after a high-level SCI (lesion level above T6)17 results in an increased likelihood of subnormal Tcore (i.e. 35.5–36.5°C) and increased vulnerability to hypothermia (Tcore < 35.0°C), both of which have been reported in veteran in-patients with tetraplegia.18
Survey studies have been used to assess the impact of SCI and its secondary consequences on specific QoL factors.19–25 Westgren et al. used the Swedish version of the Short Form 36 (SF36) Health Survey to assess QoL and found that persons with SCI scored significantly lower in all subscales compared to the non-SCI population.25 Budh et al. assessed life satisfaction in persons with SCI and found that pain negatively affected QoL, while anxiety and depression were predictive for decreased life satisfaction.19 Clayton et al. used the Life Situation Survey to determine perceived QoL.20 Martin Ginis et al. found a positive relationship between level of physical activity and subjective well-being in persons with SCI.23 Djikers found that on average, persons with SCI reported fewer feelings of well-being than non-SCI persons, scored lower in areas of physical, mental, and social health, and scored lower in domains that most people consider important to QoL.21 However, none of these surveys have addressed the impact of thermal dysregulation after SCI on QoL.
More recently, Tulsky et al. developed the SCI-QOL as a comprehensive tool to categorize the various domains commonly affected by SCI in order to assess the impact of SCI on overall QoL.26,27 The SCI-QOL includes medical (bowel and bladder issues, pressure ulcers), emotional (depression, anxiety, resilience, positive affect and well-being, grief and loss, self-esteem, stigma, and psychological trauma), and social (participation, satisfaction, and independence) domains, along with the physical functioning items from the Spinal Cord Injury-Functional Index (SCI-FI),28 and the sleep disturbance from the Patient-Reported Outcomes Measurement Information System (PROMIS).29,30 However, although the SCI-QOL addresses the impact of many of the consequences of SCI on QoL, it too does not address the impact of thermal dysregulation on QoL.
Despite recent increased recognition of thermoregulatory dysfunction after SCI,31,32 along with the availability of a limited objective thermoregulatory dysfunction assessment instrument,33 instruments to assess the impact of thermal dysregulation on QoL are lacking. Information regarding the effect of dysfunctional thermoregulatory mechanisms on personal comfort, activities of daily living (ADL), instrumental ADL (IADL), and QoL issues experienced by persons with tetraplegia during the cooler seasons is overwhelmingly anecdotal. It is not known, with any certainty, how those with tetraplegia perceive cold and if discomfort in colder environments restricts them from activities that they routinely would perform.
The objective of this study was to determine if the cooler seasons affect personal comfort or performance of ADL and IADL, variables which contribute to QoL, differently in persons with tetraplegia than that of non-SCI controls. Based on our experience with persons with SCI during the winter months, we hypothesized that persons with tetraplegia would report greater discomfort and greater limitations in ADL and IADL during the cooler seasons compared to non-SCI controls.
Methods
The experimental design was a two-group causal comparative study. Forty-four persons with tetraplegia (male [n = 42], female [n = 2], C2–7, American Spinal Injury Association [ASIA] Impairment Scale [AIS] A-C) and 41 matched, non-SCI controls (male [n = 29], female [n = 12]) from the New York and New Jersey area volunteered for study participation by signing an informed consent document approved by either the James J. Peters Veterans Administration Medical Center (VAMC) or the Kessler Foundation Research Center Institutional Review Boards (Tables 1, 2).
Table 1.
Characteristics of the tetraplegic and control groups
| Characteristic | Tetraplegia (n = 44) Mean ± SD |
Controls (n = 41) Mean ± SD |
P-value |
|---|---|---|---|
| Age (yrs) | 48.7 ± 13.8 | 45.9 ± 16.1 | 0.38 |
| Height (m) | 1.77 ± 0.1 | 1.75 ± 0.1 | 0.52 |
| Weight (kg) | 77.05 ± 14.7 | 80.6 ± 14.7 | 0.27 |
| BMI (kg/m2) | 24.6 ± 4.3 | 26.1 ± 3.5 | 0.09 |
| DOI (yrs) | 17.8 ± 12.1 | NA | NA |
| Male/Female | 42/2 | 29/12 | 0.001 |
Group averages (± standard deviation) for age (years), height (meters), weight (kilograms), BMI, body mass index (kilograms/meter2), DOI, duration of injury (years), and number of male and female subjects.
Table 2.
AIS classification scores of subjects with tetraplegia
| LOI | AIS A (n = 16) | AIS B (n = 19) | AIS C (n = 9) |
|---|---|---|---|
| C2 | 1 | 1 | |
| C3 | 1 | 1 | |
| C4 | 3 | 6 | 1 |
| C5 | 7 | 7 | 6 |
| C6 | 4 | 4 | |
| C7 | 1 | 1 |
Level of Injury (LOI) and American Spinal Injury Association (ASIA) Impairment Scale (AIS) for each subject with tetraplegia.
Subjects were administered two surveys (Thermal Comfort, Thermal Activity). Although carefully constructed and based on the most frequent complaints and feedback of our subjects during the colder months, the survey questions were not validated through focus groups, formal interviews, etc., as has been performed with several of the other surveys mentioned, e.g. the SCI-QoL.27
The Thermal Comfort Survey was designed to determine how comfortable the subject felt indoors and outdoors during the colder months of the year (late fall, winter, early spring). Subjects were asked how many articles of clothing they needed to wear in order to be comfortable indoors and outdoors and to compare that amount with people without an SCI who were in the same immediate environment. Subjects were also asked to rate their typical thermal discomfort, indoors and outdoors, using the 7-point American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) Thermal Sensation scale (–3 = very cold, –2 = cold, –1 = cool, 0 = neutral/comfortable, +1 = warm, +2 = hot, +3 = very hot).34,35 Subjects were asked what percent of the time (0%, 25%, 50%, 75%, 100%) they felt each of the 7 ASHRAE thermal sensations, in indoor and outdoor conditions. We have converted subjects’ responses for the 7 ASHRAE sensations into dichotomous variables, “yes” or “no”, for feeling comfortable. Subjects who reported feeling “0” (neutral/comfortable) for only 0% or 25% of the time were rated as “no”, feeling uncomfortable in that environment. Subjects who reported feeling “0” (neutral/comfortable) for 50% to 100% of the time were rated as “yes”, feeling comfortable in that environment. The Thermal Activity Survey was designed to determine if the colder ambient temperatures during late fall, winter, and early spring negatively affected the subject's ability to complete their routine daily activities, work duties, and IADL, maintain their usual social schedule, and attend scheduled medical appointments. Tetraplegic and control groups responded “yes” or “no” when asked whether temperature-induced discomfort negatively affected their participation in these activities during colder months.
The surveys were administered at either the James J. Peters VAMC in the Veterans Affairs Rehabilitation Research and Development (VA RR&D) National Center for the Medical Consequences of SCI or at the Kessler Institute for Rehabilitation. The administration of the surveys was in the presence of a member of the investigators’ team or if travel was difficult for a subject, by phone. Subjects were asked to frame their responses based on their experience during the cooler months of the year, specifically during the late fall, winter, and early spring seasons.
Statistical analysis
To determine if the number of responses (either yes or no) differed between the two groups (tetra, non-SCI), a χ2 test for homogeneity was employed. Statistical significance was set at P < 0.05. Data were analyzed using IBM SPSS software (version 22.0, IBM Corp., Armonk, NY, USA).
Results
The tetraplegic and control groups were not significantly different for age, height, weight, or body mass index (Table 1). For the questions that assessed personal comfort in the Thermal Comfort Survey, the percent of subjects with tetraplegia who responded “yes” was significantly different compared to controls, when asked whether cold seasonal temperatures 1) required them to wear a hat, gloves, and/or a coat indoors in order to stay comfortable (80 vs. 12%; χ2 = 38.6, P < 0.0001), 2) required them to wear more articles of clothing than other persons in the same immediate environment (both indoors and outdoors) to stay comfortable (75 vs. 17%; χ2 = 28.6, P < 0.0001), 3) caused them to feel cold indoors while others in the same room were comfortable (82 vs. 24%; χ2 = 28.2, P < 0.0001), and 4) allowed them to feel comfortable indoors (28 vs. 67%; χ2 = 13, P = 0.0003) and outdoors (17 vs. 43%; χ2 = 6.8, P = 0.009) (Fig. 1).
Figure 1.
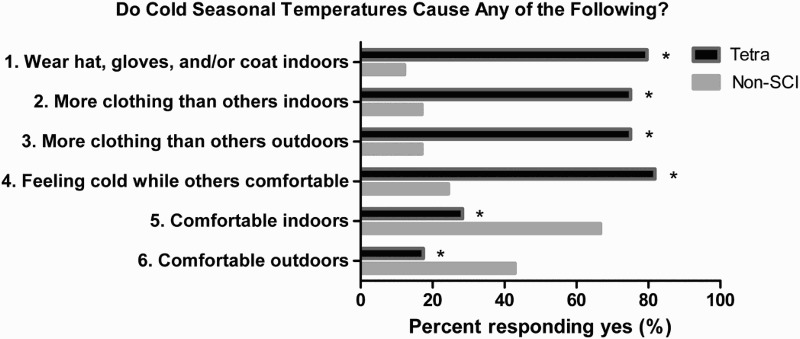
Percent (%) of subjects in each group who responded “yes” to each of the six questions of the Thermal Comfort Survey. The percent of subjects with tetraplegia who responded “yes” was significantly different compared to non-SCI controls. Asterisk (*) indicates χ2 P-value < 0.05.
For the eight questions that assessed performance of activities, a significantly greater percent of subjects with tetraplegia, compared to controls, responded “yes” that cold seasonal temperatures negatively affected their ability to 1) perform their bathing routine (55 vs. 15%; χ2 = 14.8, P = 0.0001), 2) maintain their social schedule (68 vs. 32%; χ2 = 11.3, P = 0.0008), 3) perform IADL (77 vs. 46%; χ2 = 8.6, P = 0.003), 4) keep scheduled medical appointments (46 vs. 12%; χ2 = 11.3, P = 0.0008), 5) sleep (55 vs. 24%; χ2 = 8, P = 0.005), 6) think clearly (41 vs. 7%; χ2 = 12.9, P = 0.0003), 7) complete their usual work duties (50 vs. 10%; χ2 = 16.2, P = 0.0001), and 8) perform usual work duties (46 vs. 10%; χ2 = 13.3, P = 0.0003) (Fig. 2).
Figure 2.
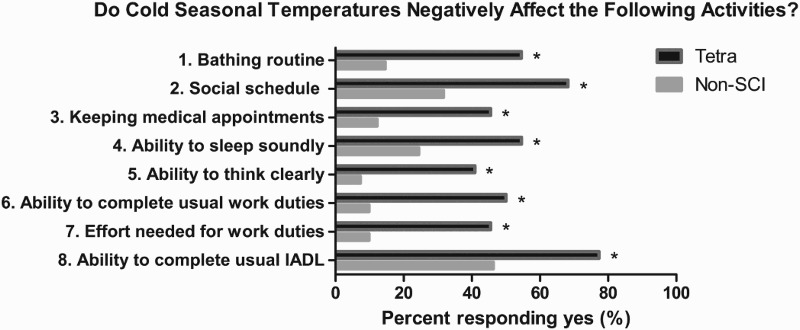
Percent (%) of subjects in each group who responded “yes” to each of the eight questions of the Thermal Activity Survey. The percent of subjects with tetraplegia who responded “yes” that cold seasonal temperatures negatively affected the performance of these activities was significantly different compared to controls. Asterisk (*) indicates χ2 P-value < 0.05.
Discussion
The subjective experience of the late fall, winter, and early spring months is different in persons with tetraplegia than non-SCI controls. The responses to questions 1 through 3 on the Thermal Comfort Survey demonstrate that persons with tetraplegia need significantly more clothing to provide insulation than is customary for indoor conditions (i.e. hat, gloves, and/or coat) and more insulation than non-SCI persons in the same environment, whether indoor or outdoor (Fig. 1).
We can attribute this need for greater external insulation to the interruption of brainstem control of sympathetic efferent pathways after high-level SCI.7,32,36 These pathways are normally responsive to hypothalamic modulation for vasoconstriction and shivering during cold exposure to conserve heat and increase thermogenesis, respectively.10,12 Peripheral vasoconstriction shifts blood volume from the superficial to the deeper central compartment, rapidly increasing thermal insulation to preserve core temperature at the expense of less vital, more superficial tissues.10,11 With continued exposure to cold, the posterior hypothalamus induces involuntary shivering to promote the production of heat above that of the basal metabolic rate.12
In persons with neurologically complete tetraplegia (AIS-A), the skin below the level of lesion is insentient, so information of cool ambient temperatures is not transmitted to the hypothalamus.2,3,37,38 This interruption of sensory feedback, along with the decentralization of descending sympathetic pathways, impairs the ability of the hypothalamus to regulate peripheral vasoconstriction and increase insulation, causing peripheral heat loss to be greater in persons with tetraplegia than in non-SCI persons. Additionally, the impaired sensory feedback of skin temperature also delays the onset of shivering, which, when combined with skeletal muscle paralysis below the level of lesion and the loss of lean tissue mass, severely limits thermogenesis. Indeed, the ability to increase BMR in persons with tetraplegia has been reported to be ∼50% compared to increases of 200–500% in non-SCI controls.6 Thus, the interruption of sympathetic integrity, after cervical SCI, impairs both peripheral vasoconstriction and thermogenesis, increasing the vulnerability of persons with tetraplegia to hypothermia.
The responses to questions 4 through 6 of the Thermal Comfort Survey demonstrate that, despite compensating with additional clothing, almost all persons with SCI complained of feeling cold even though able-bodied persons in the same environment were comfortable and, whether indoors or outdoors, persons with tetraplegia were much less likely to report feeling comfortable during the colder months than non-SCI controls (Fig. 1).
The subjective findings of the Thermal Comfort Survey are supported by the objective findings of Kahn et al. who demonstrated that, in veterans with tetraplegia, subnormal core body temperatures (35–35.5°C) occurred twice as frequently as in non-SCI individuals, causing those with higher SCI to be particularly vulnerable to hypothermia, despite exposure to relatively mild environmental temperatures.18 In the current study, the Tcores of persons with tetraplegia were not measured at the time of survey completion. However, in one of our recently published studies, similar levels of subjective discomfort were reported in persons with tetraplegia and controls, even though the average Tcore of those with tetraplegia approached mild hypothermia while the average Tcore of the control group was euthermic.16 Therefore, we speculate that the Tcore of those subjects with tetraplegia who reported discomfort on the survey may have been approaching mild hypothermia as well.
We speculate that this experience of greater discomfort during colder seasons than non-SCI counterparts may predispose persons with SCI to pain, anxiety, and depression. Life satisfaction was shown to be lower in persons with SCI who had pain compared to those that did not, while higher levels of depression and anxiety were predictive of decreased life satisfaction.19
The responses to questions 1, 2, 4, and 6 through 8 of the Thermal Activity Survey indicate that persons with tetraplegia responded “yes” much more frequently that cold seasonal temperatures negatively affected these routine activities compared to non-SCI controls. Discomfort due to the cold may have been the factor causing this negative impact on their bathing routine, social schedule, and ability to sleep (Fig. 2).
Of particular interest were the results from questions 3 and 5 (Fig. 2). For question 3, 46% of the group with tetraplegia reported that their adherence to medical appointments was negatively affected by cold seasonal temperatures. This negative effect may also be secondary to discomfort due to feeling cold, as discussed above. Persons with tetraplegia have increased medical needs, as noted by the greater prevalence urological conditions, pulmonary infections, diabetes, cardiovascular disease, constipation, osteoporosis, and skin breakdown.14,39–44 The impact of missing medical appointments, in persons who already have greater medical needs than typical able-bodied controls, may jeopardize optimal clinical care.
Question 5 of the Thermal Activity Survey indicates that 41% of the group with tetraplegia responded that cold seasonal temperatures negatively affected their ability to think clearly as opposed to only 7% of controls. Not thinking clearly in persons who require increased vigilance for attending to their medical needs and adhering to complex medical routines may also jeopardize attainment of optimal health and contribute to increased hospital re-admissions. The responses to question 5 are supported by the results of a previous study that exposed 7 persons with tetraplegia and 7 matched able-bodied controls to cool ambient temperature (18°C) for up to 2 hours.16 The study demonstrated a decline in Tcore, after cool challenge in the group with tetraplegia only. The decline in Tcore was associated with a decline in cognitive performance, in the areas of working memory and executive function.16 There was no such decline in Tcore or cognitive performance in the control group.
Limitations
The relatively small sample size of approximately 40 subjects in each group limits extrapolation of our results to the general population with SCI, as well as determining the influence of gender on our results. The ratio of male/female subjects is greater in the group with tetraplegia than controls. However, this gender distribution is more representative of the typical population with SCI. The small sample size limits our ability to discern differences among persons who have different AIS classifications. However, it may be anticipated that exposure to cold temperatures would be associated with greater discomfort and impairment on activities in those with higher and/or more complete (i.e. AIS A and B compared to AIS C) neurological injuries.
Although cold exposure had a negative impact on comfort and the performance of routine activities, there are other responses to cold exposure besides discomfort which could also negatively affect these activities, e.g. increased spasticity, muscle stiffness, arthralgia, etc.45–48 The presence of these additional factors and their impact on the performance of routine activities were not addressed in this survey.
Implications
There are no reports, other than anecdotal, that provide findings that the experience of the cooler seasons and its effects on routine activities are different in persons with tetraplegia compared to controls. The findings of this study help identify the existence of a problem (affecting personal comfort, ADL, and QoL) and identify the need for exploring safe and efficacious interventions (exercise, diet, medical, clothing, specific guidelines for cold exposure) to enhance maintenance of Tcore during exposure to cold ambient temperatures in persons with SCI. The results of research in this area of study may translate into improved clinical care, less hospitalizations, and an increased QoL for persons with SCI.
Conclusion
The cold seasonal temperatures of late fall, winter, and early spring have a greater negative impact on the personal comfort and ability to perform vital routine activities of persons with tetraplegia than that of non-SCI controls. These limitations have not been reported to date and appear to present a limiting factor to attaining optimal QoL in persons with tetraplegia.
The findings of this study warrant continued investigation on the effects of impaired thermoregulation in persons with SCI. Future research should explore safe, effective interventions that may enhance maintenance of Tcore in cool weather and mitigate the deleterious effects of lower environmental temperatures on comfort and ADL in persons with tetraplegia.
Acknowledgments
Veteran Affairs Rehabilitation Research and Development Service (#B4162-C) and the James J. Peters VA Medical Center. The authors would like to acknowledge Shou-An Liu, DPT, OCS, Megan Krajewski, DPT, Zhen Ni Guan, DPT, John Nulty, DPT, MS, and Chris Cirnigliaro, MS, CES, CBDT for their assistance in aspects of data collection for this study.
Disclosure
There are no commercial interests of the authors relevant to the subject of the manuscript and no commercial or financial conflicts of interest exist. All authors attest to the validity and legitimacy of data and the accuracy of its interpretation and presentation.
Clinical Trial Registration Number: NA
Disclaimer statements
Contributors: None.
Funding: None.
Conflicts of interest: None.
Ethics approval: None.
References
- 1.Claus-Walker J, Halstead LS, Carter RE, Campos RJ, Spencer WA, Canzoneri J 3rd. Physiological responses to cold stress in healthy subjects and in subjects with cervical cord injuries. Arch Phys Med Rehabil 1974;55(11):485–90. [PubMed] [Google Scholar]
- 2.Downey JA, Chiodi HP, Darling RC.. Central temperature regulation in the spinal man. J Appl Physiol 1967;22(1):91–4. [DOI] [PubMed] [Google Scholar]
- 3.Downey JA, Darling RC, Chiodi HP.. The response of tetraplegia patients to cold. Arch Phys Med Rehabil 1967;48(12):645–9. [PubMed] [Google Scholar]
- 4.Downey JA, Huckaba CE, Myers SJ, Darling RC.. Thermoregulation in the spinal man. J Appl Physiol 1973;34(6):790–4. [DOI] [PubMed] [Google Scholar]
- 5.Guttmann L. Spinal cord injuries: comprehensive management and research. 2nd ed. Oxford, UK: Blackwell Science Ltd; 1976. [Google Scholar]
- 6.Guttmann L, Silver J, Wyndham CH.. Thermoregulation in spinal man. J Physiol 1958;142(3):406–19. doi: 10.1113/jphysiol.1958.sp006026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathias CJ, Frankel HL.. Clinical manifestations of malfunctioning sympathetic mechanisms in tetraplegia. J Auton Nerv Syst 1983;7(3–4):303–12. doi: 10.1016/0165-1838(83)90083-8 [DOI] [PubMed] [Google Scholar]
- 8.Frank SM, Higgins MS, Fleisher LA, Sitzmann JV, Raff H, Breslow MJ.. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol 1997;272(2 Pt 2):R557–62. [DOI] [PubMed] [Google Scholar]
- 9.Wilkerson JE, Raven PB, Bolduan NW, Horvath SM.. Adaptations in man's adrenal function in response to acute cold stress. J Appl Physiol 1974;36(2):183–9. [DOI] [PubMed] [Google Scholar]
- 10.Castellani JW, Young AJ, Ducharme MB, Giesbrecht GG, Glickman E, Sallis RE.. American College of Sports Medicine position stand: prevention of cold injuries during exercise. Med Sci Sports Exerc 2006;38(11):2012–29. [DOI] [PubMed] [Google Scholar]
- 11.Young AJ, Muza SR, Sawka MN, Pandolf KB.. Human vascular fluid responses to cold stress are not altered by cold acclimation. Undersea Biomed Res 1987;14(3):215–28. [PubMed] [Google Scholar]
- 12.Guyton A, Hall J.. Textbook of Medical Physiology 12th Edition Philadelphia, PA: Elsevier Science; 2010. p. 1607–27. [Google Scholar]
- 13.Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Spalding JM.. Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Circ Res 1976;39(2):204–8. doi: 10.1161/01.RES.39.2.204 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Spungen AM.. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 2000;11(1):109–40. [PubMed] [Google Scholar]
- 15.Attia M, Engel P.. Thermoregulatory set point in patients with spinal cord injuries (spinal man). Paraplegia 1983;21(4):233–48. doi: 10.1038/sc.1983.37 [DOI] [PubMed] [Google Scholar]
- 16.Handrakis JP, Liu SA, Rosado-Rivera D, Krajewski M, Spungen AM, Bang C, et al. Effect of Mild Cold Exposure on Cognition in Persons with Tetraplegia. J Neurotrauma 2015;32(15):1168–75. doi: 10.1089/neu.2014.3719 [DOI] [PubMed] [Google Scholar]
- 17.Phillips AA, Krassioukov AV, Ainslie PN, Warburton DE.. Perturbed and spontaneous regional cerebral blood flow responses to changes in blood pressure after high-level spinal cord injury: the effect of midodrine. J Appl Physiol 2014;116(6):645–53. doi: 10.1152/japplphysiol.01090.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S, Plummer M, Martinez-Arizala A, Banovac K.. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med 2007;30(1):27–30. doi: 10.1080/10790268.2007.11753910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budh CN, Osteraker AL.. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil 2007;21(1):89–96. doi: 10.1177/0269215506070313 [DOI] [PubMed] [Google Scholar]
- 20.Clayton KS, Chubon RA.. Factors associated with the quality of life of long-term spinal cord injured persons. Arch Phys Med Rehabil 1994;75(6):633–8. doi: 10.1016/0003-9993(94)90184-8 [DOI] [PubMed] [Google Scholar]
- 21.Dijkers MP. Quality of life of individuals with spinal cord injury: a review of conceptualization, measurement, and research findings. J Rehabil Res Dev 2005;42(3 Suppl 1):87–110. [DOI] [PubMed] [Google Scholar]
- 22.Hetz SP, Latimer AE, Arbour-Nicitopoulos KP, Martin Ginis KA.. Secondary complications and subjective well-being in individuals with chronic spinal cord injury: associations with self-reported adiposity. Spinal Cord 2011;49(2):266–72. doi: 10.1038/sc.2010.100 [DOI] [PubMed] [Google Scholar]
- 23.Martin Ginis KA, Jetha A, Mack DE, Hetz S.. Physical activity and subjective well-being among people with spinal cord injury: a meta-analysis. Spinal Cord 2010;48(1):65–72. doi: 10.1038/sc.2009.87 [DOI] [PubMed] [Google Scholar]
- 24.Migliorini CE, New PW, Tonge BJ.. Quality of life in adults with spinal cord injury living in the community. Spinal Cord 2011;49(3):365–70. doi: 10.1038/sc.2010.102 [DOI] [PubMed] [Google Scholar]
- 25.Westgren N, Levi R.. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil 1998;79(11):1433–9. doi: 10.1016/S0003-9993(98)90240-4 [DOI] [PubMed] [Google Scholar]
- 26.Bertisch H, Kalpakjian CZ, Kisala PA, Tulsky DS.. Measuring positive affect and well-being after spinal cord injury: development and psychometric characteristics of the SCI-QOL positive affect and well-being bank and short form. J Spinal Cord Med 2015;38(3):356–65. doi: 10.1179/2045772315Y.0000000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulsky DS, Kisala PA.. The Spinal Cord Injury—Quality of Life (SCI-QOL) measurement system: development, psychometrics, and item bank calibration. J Spinal Cord Med 2015;38(3):251–6. doi: 10.1179/2045772315Y.0000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tulsky DS, Jette AM, Kisala PA, Kalpakjian C, Dijkers MP, Whiteneck G, et al. Spinal cord injury-functional index: item banks to measure physical functioning in individuals with spinal cord injury. Arch Phys Med Rehabil 2012;93(10):1722–32. doi: 10.1016/j.apmr.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33(6):781–92. doi: 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson AK, Krassioukov A, Alexander MS, Donovan W, Biering-Sørensen F.. International spinal cord injury skin and thermoregulation function basic data set. Spinal Cord 2012;50(7):512–6. doi: 10.1038/sc.2011.167 [DOI] [PubMed] [Google Scholar]
- 32.Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ.. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J Rehabil Res Dev 2007;44(1):103–12. doi: 10.1682/JRRD.2005.10.0159 [DOI] [PubMed] [Google Scholar]
- 33.Karlsson AK, Krassioukov A, Sipski M, Donovan W, Mathias CJ, Biering-Sørensen F.. International Spinal Cord Injury Skin and Thermoregulation Basic Data Set (Version 1.0)—2011.05.28 2011.
- 34.Yau YH, Chew BT.. Thermal comfort study of hospital workers in Malaysia. Indoor Air 2009;19(6):500–10. doi: 10.1111/j.1600-0668.2009.00617.x [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhao R.. Overall thermal sensation, acceptability and comfort. Building and Environment 2008;43:44–50. doi: 10.1016/j.buildenv.2006.11.036 [DOI] [Google Scholar]
- 36.Krassioukov A. Which pathways must be spared in the injured human spinal cord to retain cardiovascular control? Prog Brain Res 2006;152:39–47. doi: 10.1016/S0079-6123(05)52003-X [DOI] [PubMed] [Google Scholar]
- 37.Downey JA, Miller JM, Darling RC.. Thermoregulatory responses to deep and superficial cooling in spinal man. J Appl Physiol 1969;27(2):209–12. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt KD, Chan CW.. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clin Proc 1992;67(5):469–75. doi: 10.1016/S0025-6196(12)60394-2 [DOI] [PubMed] [Google Scholar]
- 39.Bauman WA, Spungen AM.. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77. doi: 10.1080/10790268.2001.11753584 [DOI] [PubMed] [Google Scholar]
- 40.Bauman WA, Spungen AM, Wang J, Pierson RN Jr., Schwartz E.. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int 1999;10(2):123–7. doi: 10.1007/s001980050206 [DOI] [PubMed] [Google Scholar]
- 41.Williams RE 3rd, Bauman WA, Spungen AM, Vinnakota RR, Farid RZ, Galea M, et al. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord 2011;50(1):81–4. doi: 10.1038/sc.2011.92 [DOI] [PubMed] [Google Scholar]
- 42.Deitrick G, Charalel J, Bauman W, Tuckman J.. Reduced arterial circulation to the legs in spinal cord injury as a cause of skin breakdown lesions. Angiology 2007;58(2):175–84. doi: 10.1177/0003319707300353 [DOI] [PubMed] [Google Scholar]
- 43.Javadi M, Hafezi-Nejad N, Vaccaro AR, Rahimi-Movaghar V.. Medical complications and patient outcomes in Iranian veterans with spinal cord injury. Adv Clin Exp Med 2014;23(2):269–75. doi: 10.17219/acem/37075 [DOI] [PubMed] [Google Scholar]
- 44.Krassioukov AV, Furlan JC, Fehlings MG.. Medical co-morbidities, secondary complications, and mortality in elderly with acute spinal cord injury. J Neurotrauma 2003;20(4):391–9. doi: 10.1089/089771503765172345 [DOI] [PubMed] [Google Scholar]
- 45.Hunter DJ, Riordan EA.. The impact of arthritis on pain and quality of life: an Australian survey. Int J Rheum Dis 2014;17(2):149–55. doi: 10.1111/1756-185X.12232 [DOI] [PubMed] [Google Scholar]
- 46.Pienimaki T, Karppinen J, Rintamaki H, Borodulin K, Laatikainen T, Jousilahti P, et al. Prevalence of cold-related musculoskeletal pain according to self-reported threshold temperature among the Finnish adult population. Eur J Pain 2014;18(2):288–98. doi: 10.1002/j.1532-2149.2013.00368.x [DOI] [PubMed] [Google Scholar]
- 47.Shirado O, Shundo M, Kaneda K, Strax TE.. Outdoor winter activities of spinal cord-injured patients. With special reference to outdoor mobility. Am J Phys Med Rehabil 1995;74(6):408–14. [DOI] [PubMed] [Google Scholar]
- 48.Phadke CP, Balasubramanian CK, Ismail F, Boulias C.. Revisiting physiologic and psychologic triggers that increase spasticity. Am J Phys Med Rehabil 2013;92(4):357–69. doi: 10.1097/PHM.0b013e31827d68a4 [DOI] [PubMed] [Google Scholar]


