Abstract
Context: Acquired copper deficiency represents a rare cause of progressive myelopathy presenting with sensory ataxia and spastic gait. The time interval from neurological symptoms onset to diagnosis of myelopathy ranges from 2 months to several years in almost all cases, mimicking the clinical course of subacute combined degeneration due to vitamin B12 deficiency.
Findings: A 60-year-old man, without any gastrointestinal symptoms, developed over the course of one week rapidly progressive gait imbalance, tingling and numbness in his feet and ascending lower limb weakness. Spine magnetic resonance imaging revealed hyperintensity involving cervical and dorsal posterior columns of spinal cord. Blood analysis revealed undetectable serum copper levels, low serum ceruloplasmin and positive serum Immunoglobulin A anti-tissue transglutaminase. Upper gastrointestinal endoscopy was performed revealing duodenal villous atrophy consistent with a malabsorption pattern. A gluten-free diet in association with intravenous then oral copper supplementation prompted sustained normalization of serum copper levels and progressive clinical improvement.
Conclusion/Clinical Relevance: We report a rare case of myelopathy induced by copper deficiency secondary to undiagnosed celiac disease, peculiarly presenting with a subacute onset. This case expands the neurological presentation and clinical course of myelopathy due to acquired copper deficiency. We suggest investigation of copper deficiency in patients presenting with subacute or even acute sensory ataxia and spastic gait. Detection of hypocupremia in patients without a previous history of gastric surgery should lead to diagnostic testing for celiac disease even in the absence of any obvious gastrointestinal symptoms.
Keywords: Copper, Spinal cord diseases, Celiac disease, Neurophysiology
Introduction
Acquired copper deficiency represents a rare treatable cause of progressive non-compressive myelopathy presenting with sensory ataxia and spastic gait. Despite being frequently associated with a history of gastric surgery, malabsorption syndromes and excessive zinc consumption, the cause of copper deficiency remains unclear in 20% of cases.1, 2 The time interval from the onset of neurological symptoms to diagnosis of myelopathy ranges from two months to several years in almost all cases, mimicking the clinical course of subacute combined degeneration due to vitamin B12 deficiency.1–3 Whole-spine magnetic resonance imaging (MRI) shows increased signal on T2-weighted images involving the dorsal column in the cervico-thoracic cord in only about half of the patients.3 Treatment of copper deficiency myelopathy requires copper supplementation and management of underlying risk factors. We report a case of patient who developed subacute myelopathy associated with copper deficiency due to undiagnosed and asymptomatic celiac disease.
Case report
A 60-year-old man without any gastrointestinal symptoms or reported weight loss was admitted to our hospital because of a one-week history of progressive gait imbalance, tingling and numbness in his feet and ascending lower limbs weakness. Neurological examination showed diffuse sensory ataxia, with an inability to stand upright and absent vibration and position sense, as well as distal lower limb weakness but increased tendon reflexes and bilateral Babinski's sign. Spinal MRI revealed a longitudinal hyperintensity involving the cervical and dorsal posterior columns on T2-weighted and Short Time Inversion Recovery (STIR) sequences (Fig. 1A). Brain MRI was normal without brain atrophy or signs of paramagnetic substances accumulation. Nerve conduction study was also unremarkable. Transcranial magnetic stimulation showed motor evoked potentials (MEP) in the right tibialis anterior (Tib. Ant.) reduced in amplitude and central conduction times mildly increased bilaterally (Fig. 1B). Somatosensory evoked potentials (SSEP) revealed impaired central sensory conduction in lower limbs. Normocytic anaemia (haemoglobin: 9 g/dL, range 12–16) was found associated with high red cell distribution width (RDW) (19%, range 12.6–15.8), very mild vitamin B12 deficiency (185 pg/ml, range 200–960), low blood iron levels (30 ug/dl, range 50–150), normal serum ferritin (74 ng/ml, range 25–400) and normal serum transferrin (262 mg/dl, range 200–360). Furthermore, undetectable serum copper level, low serum ceruloplasmin (5 mg/dl, range 20–60), normal serum zinc (1016 μg/L, range 600–1080), normal 24-hour urinary copper excretion (22.40 µg/24 h, range 5–60) and mildly elevated 24-hour urinary zinc excretion (845.00 µg/24 h, range 250–650) were also detected. Extensive cerebrospinal fluid examination (cell count, chemical tests, viral PCR, isoelectric focusing, microscopic examination, onconeural antibodies) did not reveal any abnormalities. Ophthalmological evaluation with the use of slit-lamp did not reveal Kaiser-Fleischer ring or retinal degeneration. The screening test for celiac disease (CD) detected high-titre circulating serum Immunoglobulin A (IgA) anti-tissue transglutaminase (45.0 U/ml, range 4.0–10.0), with normal anti-gliadin and anti-endomysial antibodies. Upper gastrointestinal endoscopy was performed revealing duodenal villous atrophy, supporting the clinical hypothesis of a malabsorption syndrome. The histological features of duodenal biopsy specimens were consistent with CD showing increased intraepithelial CD3-lymphocytes, (80 lymphocytes / 100 enterocytes), crypt hypertrophy, and villous atrophy (Marsh type IIIa lesions based on the Marsh grading system of CD).4 A gluten-free diet in association with a one-week intravenous copper repletion (1.5 mg/day of elemental copper) led to an almost complete normalization of serum copper level (474 μg/L, range 500–1250) within two weeks, and concurrent progressive clinical improvement. Prolonged oral copper supplementation over the next two months (3 mg/day of elemental copper) was associated with further improvement in lower limbs power, although mild gait instability and distal paraesthesia persisted.
Fig. 1.
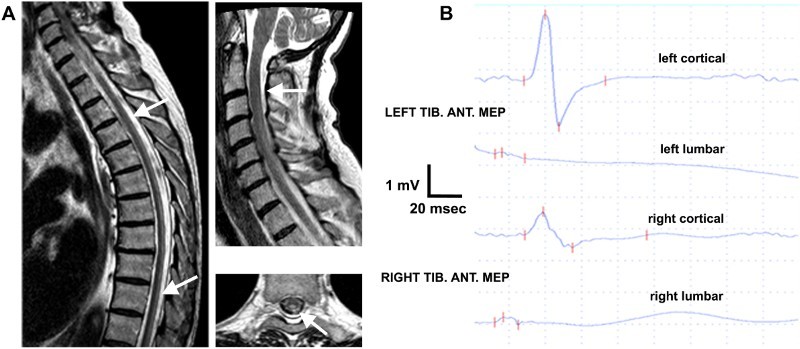
A: Sagittal and axial spine T2-weighted Magnetic Resonance Imaging revealed longitudinally hyperintensity involving cervical and dorsal posterior columns of spinal cord (white arrows). B: Transcranial magnetic stimulation shows amplitude reduction of cortical motor evoked potentials (MEP) on right tibialis anterior (Tib. Ant.) with mild bilateral increment of central conduction time
Discussion
Clinical, neurophysiological and neuroradiological findings in copper deficiency myelopathy may overlap with those of subacute combined degeneration due to vitamin B12 deficiency.1 Dysfunction of the methylation cycle, which is thought to be impaired in copper deficiency disorders, could represent a potential pathophysiological substrate explaining the phenotypic similarity between copper deficiency myelopathy and subacute combined degeneration.2 In our patient, the mild B12 deficiency was considered disproportionate to neurological impairment and neuroradiological features, prompting us to search for alternative causes. Wilson's disease was considered unlikely, because of normal brain MRI, absence of Kaiser-Fleischer ring and normal 24-hour urinary copper excretion.5 Aceruloplasminemia was ruled out on the basis of normal serum ferritin levels together with the absence of supportive brain MRI findings (iron accumulation in basal ganglia, brain atrophy) and typical clinical manifestations (retinal degeneration, extrapyramidal features, diabetes mellitus). In our patient the presence of low serum ceruloplasmin could be explained with impaired copper absorption, as in Menkes’ disease or occipital horn syndrome.5 Normocytic anaemia could be considered multifactorial, including deficiencies in vitamin B12, iron and copper serum. Regarding the elevated zinc urine excretion with normal serum level, we suppose an external zinc intake, even if we did not identify in our patient. Therefore, it is improbable that zinc overload is responsible for copper deficiency. The prevalence of copper deficiency in CD is unknown, but it can represent an uncommon complication of this pathological condition.6, 7 Indeed, we report a very rare case of myelopathy induced by copper deficiency secondary to undiagnosed celiac disease, peculiarly presenting with subacute onset.
Conclusion
In conclusion, this case expands the neurological presentation and clinical course of myelopathy due to acquired copper deficiency. We suggest to investigate for copper deficiency in patients presenting with subacute or acute sensory ataxia and spastic gait. Detection of hypocupremia in patients without a previous history of gastric surgery should lead to diagnostic testing for celiac disease even in the absence of any obvious gastrointestinal symptoms.
Author contributions
Dr. F. Cavallieri: acquisition of data, analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. N. Fini: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. S. Contardi: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, revising the manuscript for important intellectual content, final approval of the version to be submitted.
Dr. M. Fiorini: analysis and interpretation of data, drafting the article, final approval of the version to be submitted.
Dr. E. Corradini: acquisition of data, analysis and interpretation of data, revising the manuscript for important intellectual content, final approval of the version to be submitted.
Dr. F. Valzania: acquisition of data, analysis and interpretation of data, drafting the article, revising the manuscript for important intellectual content, final approval of the version to be submitted.
All authors have approved the final article.
Declarations: The authors report no conflicts of interest or financial disclosures.
Disclaimer statements
Contributors None.
Funding None.
Conflict of interest None.
Ethics approval None.
ORCiD
Massimo Fiorini http://orcid.org/0000-0002-9165-539X
Elena Corradini http://orcid.org/0000-0001-9477-2164
References
- 1.Plantone D, Primiano G, Renna R, Restuccia D, Iorio R, Patanella KA, et al. . Copper deficiency myelopathy: a report of two cases. J Spinal Cord Med 2015;38(4):559–62. doi: 10.1179/2045772314Y.0000000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiser SR, Winston GP.. Copper deficiency myelopathy. J Neurol 2010;257(6):869–81. doi: 10.1007/s00415-010-5511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar N, Ahlskog JE, Klein CJ, Port JD.. Imaging features of copper deficiency myelopathy: a study of 25 cases. Neuroradiology 2006;48(2):78–83. doi: 10.1007/s00234-005-0016-5 [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Rostami K, Marsh MN.. Diagnosis of coeliac disease. Best Pract Res Clin Gastroenterol 2005;19(3):389–400. doi: 10.1016/j.bpg.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Bandmann O, Weiss KH, Kaler SG.. Wilson's disease and other neurological copper disorders. Lancet Neurol 2015;14(1):103–13. doi: 10.1016/S1474-4422(14)70190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman BP, Mistry DH, Pasha SF, Bosch PE.. Copper deficiency myeloneuropathy due to occult celiac disease. Neurologist 2009;15(6):355–6. doi: 10.1097/NRL.0b013e31819428a8 [DOI] [PubMed] [Google Scholar]
- 7.Halfdanarson TR, Kumar N, Hogan WJ, Murray JA.. Copper deficiency in celiac disease. J Clin Gastroenterol 2009;43(2):162–4. doi: 10.1097/MCG.0b013e3181354294 [DOI] [PubMed] [Google Scholar]


