Abstract
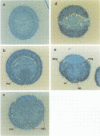
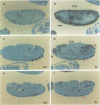
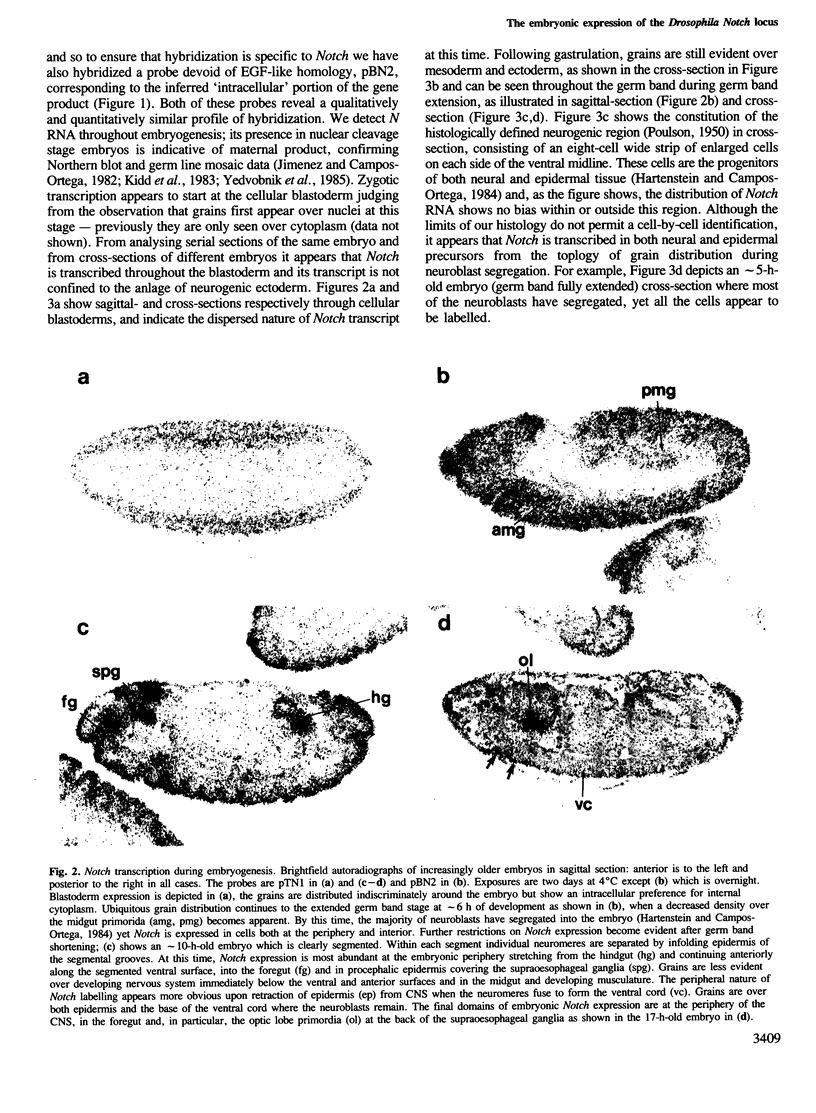
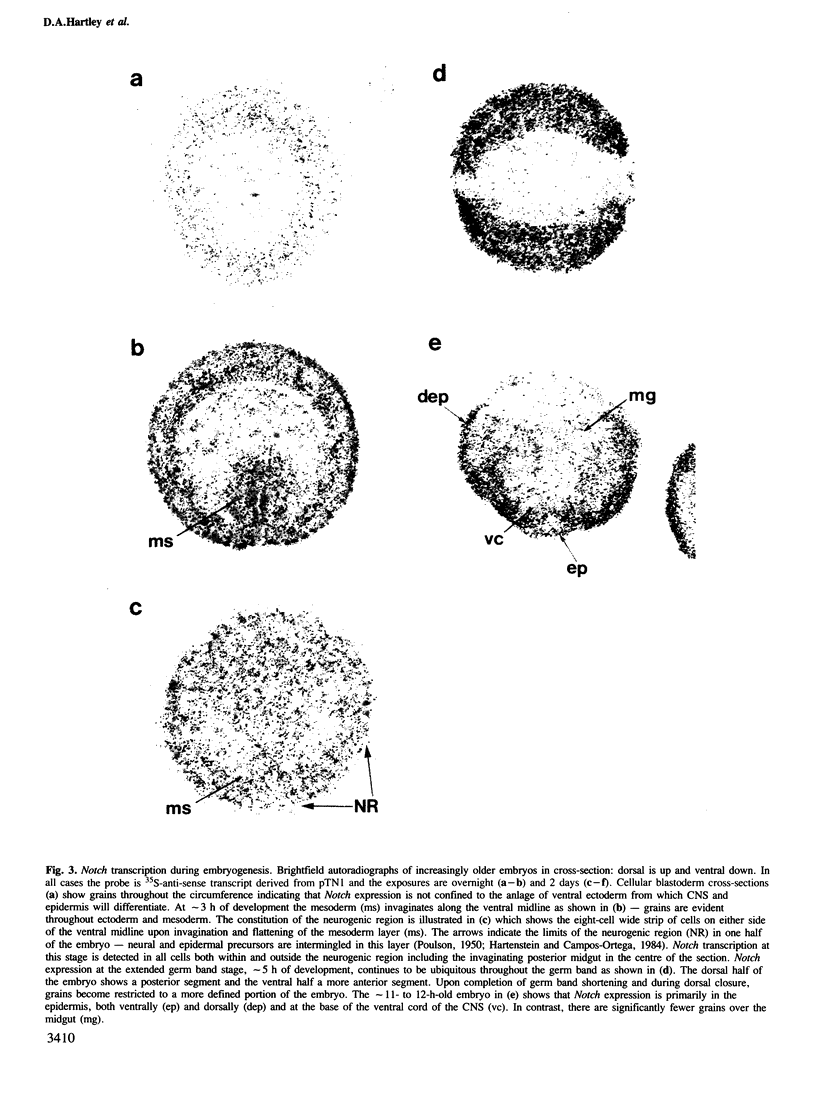
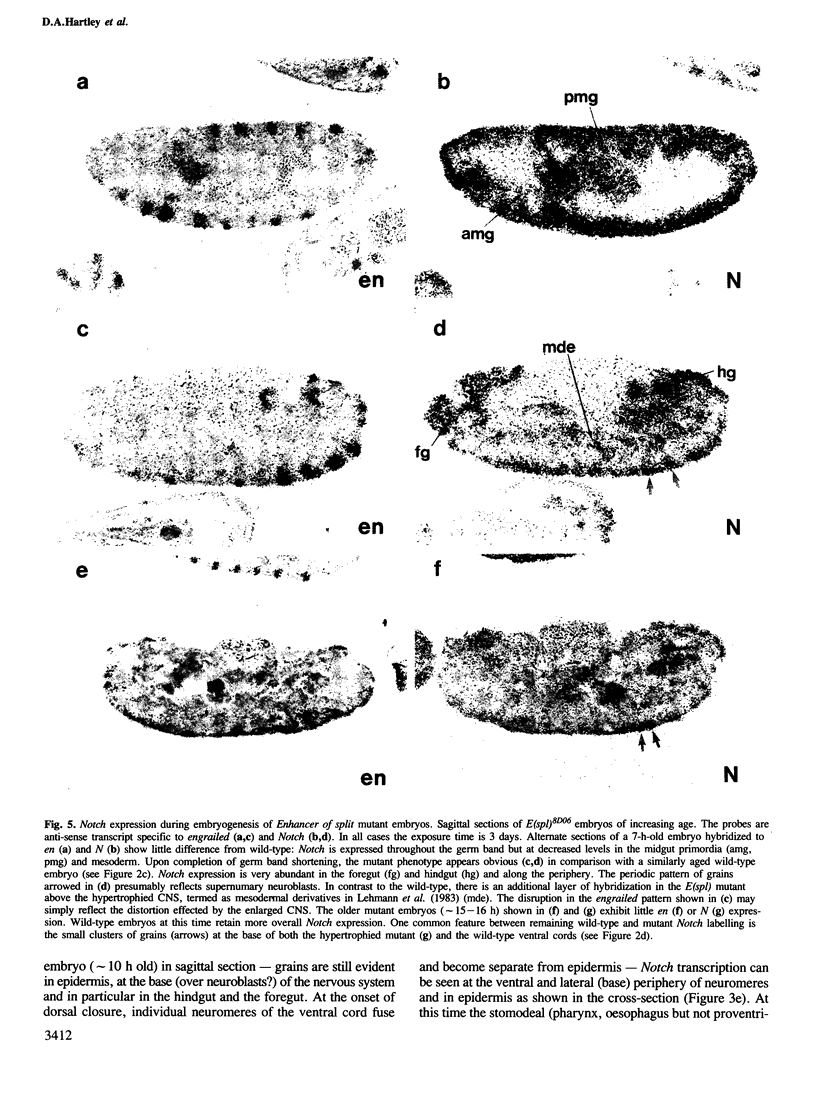
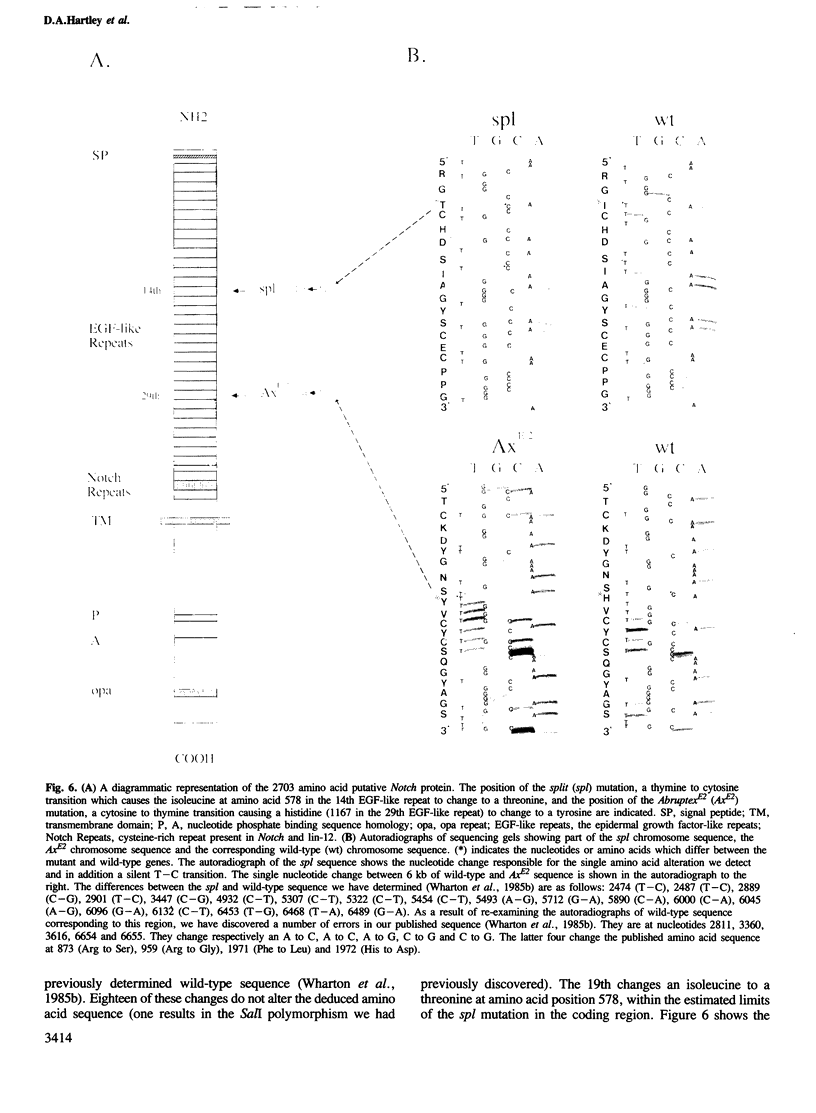
The Notch locus of Drosophila melanogaster is one of a small number of zygotically acting 'neurogenic' genes necessary for the correct segregation of neural from epidermal lineages during embryogenesis. The predicted gene product is implicated in a cell interaction mechanism required to achieve this ectodermal differentiation. We have examined wild-type Notch expression by in situ hybridization and find it to be expressed in more cells than we would have predicted given a sole function in regulating neurogenesis. We conclude from these data that Notch plays a more general role in development. In order to assess the dependence of Notch expression on other neurogenic gene function we have hybridized Notch probes to Enhancer of split mutants which are known to interfere with expression of Notch phenotypes. We intimate that the nature of interaction between these genes is not at the level of transcription. Instead, the DNA sequence of split, which is a missense mutation in the EGF-like extracellular domain of the Notch protein, suggests a direct biochemical interaction between Notch and E(spl) proteins. The similar site of a second point mutation, AxE2, implies that protein interactions also occur between Notch proteins. Finally we discuss the general implications of our findings with a view to the models and mechanisms of Notch action in regulating individual cellular interactions during development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artavanis-Tsakonas S., Muskavitch M. A., Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1977–1981. doi: 10.1073/pnas.80.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich U., Campos-Ortega J. A. The expression of neurogenic loci in imaginal epidermal cells of Drosophila melanogaster. J Neurogenet. 1984 Dec;1(4):315–332. doi: 10.3109/01677068409107094. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Goodman C. S. Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol. 1985 Sep;111(1):206–219. doi: 10.1016/0012-1606(85)90446-4. [DOI] [PubMed] [Google Scholar]
- Foster G. G. Negative complementation at the notch locus of Drosophila melanogaster. Genetics. 1975 Sep;81(1):99–120. doi: 10.1093/genetics/81.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Grimwade B. G., Muskavitch M. A., Welshons W. J., Yedvobnick B., Artavanis-Tsakonas S. The molecular genetics of the Notch locus in Drosophila melanogaster. Dev Biol. 1985 Feb;107(2):503–519. doi: 10.1016/0012-1606(85)90331-8. [DOI] [PubMed] [Google Scholar]
- Hoppe P. E., Greenspan R. J. Local function of the Notch gene for embryonic ectodermal pathway choice in Drosophila. Cell. 1986 Aug 29;46(5):773–783. doi: 10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Kidd S., Young M. W. Transposon-dependent mutant phenotypes at the Notch locus of Drosophila. Nature. 1986 Sep 4;323(6083):89–91. doi: 10.1038/323089a0. [DOI] [PubMed] [Google Scholar]
- Knust E., Dietrich U., Tepass U., Bremer K. A., Weigel D., Vässin H., Campos-Ortega J. A. EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J. 1987 Mar;6(3):761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Nakanishi M., Ali Z., Drees B., Gustavson E., Theis J., Kauvar L., Kornberg T., O'Farrell P. H. Molecular cloning of engrailed: a gene involved in the development of pattern in Drosophila melanogaster. Cell. 1985 Aug;42(1):309–316. doi: 10.1016/s0092-8674(85)80126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. Developmental genetics of the 2E-F region of the Drosophila X chromosome: a region rich in "developmentally important" genes. Genetics. 1984 Nov;108(3):559–572. doi: 10.1093/genetics/108.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin P. Allelic negative complementation at the Abruptex locus of Drosophila melanogaster. Genetics. 1975 Sep;81(1):121–133. doi: 10.1093/genetics/81.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson D. F. Chromosomal Deficiencies and the Embryonic Development of Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1937 Mar;23(3):133–137. doi: 10.1073/pnas.23.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M. P. The development of eggs produced by the female-sterile mutant almondex of Drosophila melanogaster. J Exp Zool. 1973 Mar;183(3):383–400. doi: 10.1002/jez.1401830312. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Technau G. M., Campos-Ortega J. A. Cell autonomy of expression of neurogenic genes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4500–4504. doi: 10.1073/pnas.84.13.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vässin H., Vielmetter J., Campos-Ortega J. A. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. 1985 Nov;2(5):291–308. doi: 10.3109/01677068509102325. [DOI] [PubMed] [Google Scholar]
- Welshons W. J. Analysis of a gene in drosophila. Science. 1965 Nov 26;150(3700):1122–1129. doi: 10.1126/science.150.3700.1122. [DOI] [PubMed] [Google Scholar]
- Welshons W. J. Genetic basis for two types of recessive lethality at the notch locus of Drosophila. Genetics. 1971 Jun;68(2):259–268. doi: 10.1093/genetics/68.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Wright T. R. The genetics of embryogenesis in Drosophila. Adv Genet. 1970;15:261–395. doi: 10.1016/s0065-2660(08)60075-9. [DOI] [PubMed] [Google Scholar]
- Yedvobnick B., Muskavitch M. A., Wharton K. A., Halpern M. E., Paul E., Grimwade B. G., Artavanis-Tsakonas S. Molecular genetics of Drosophila neurogenesis. Cold Spring Harb Symp Quant Biol. 1985;50:841–854. doi: 10.1101/sqb.1985.050.01.102. [DOI] [PubMed] [Google Scholar]