Abstract
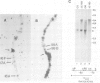
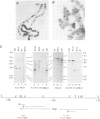
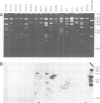
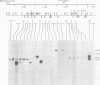
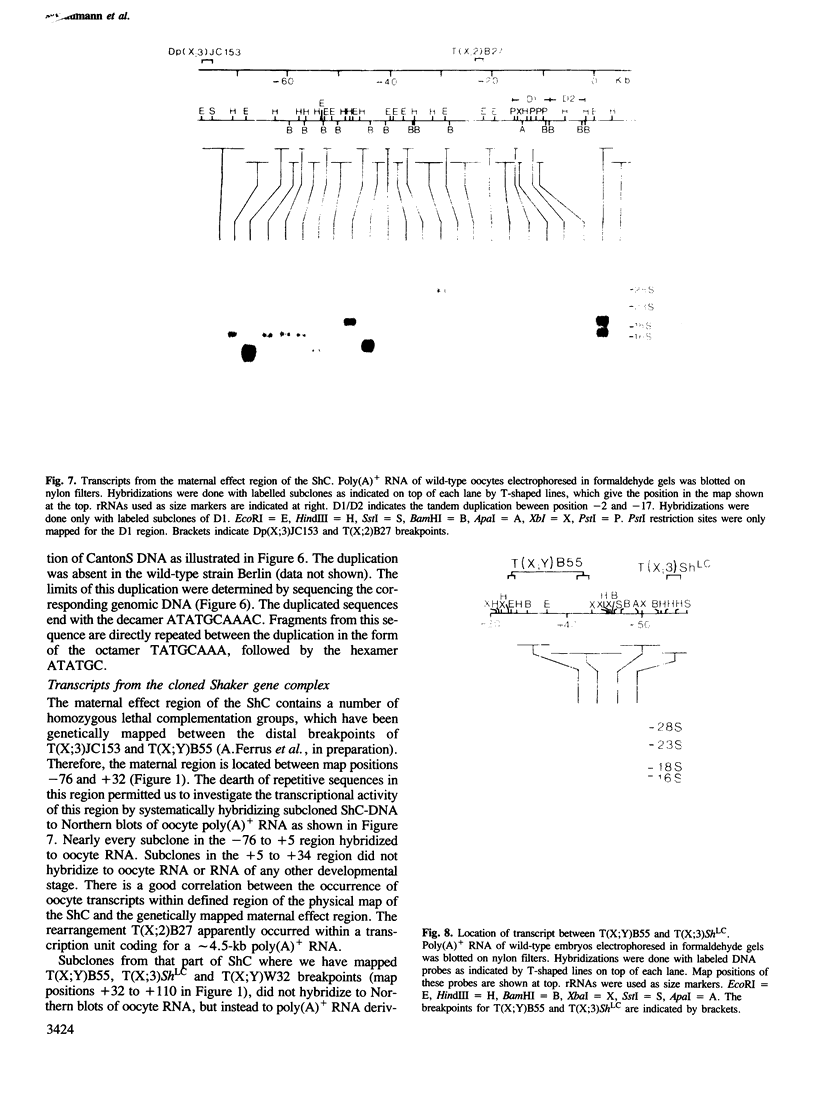
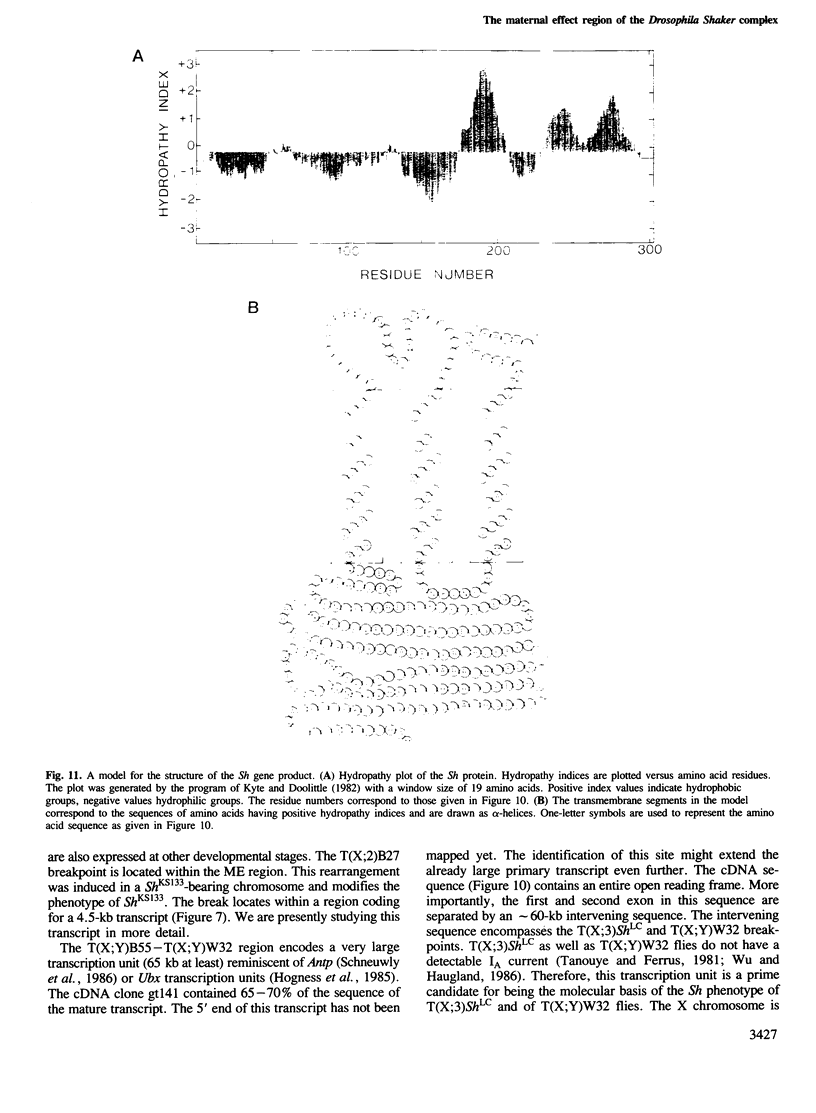
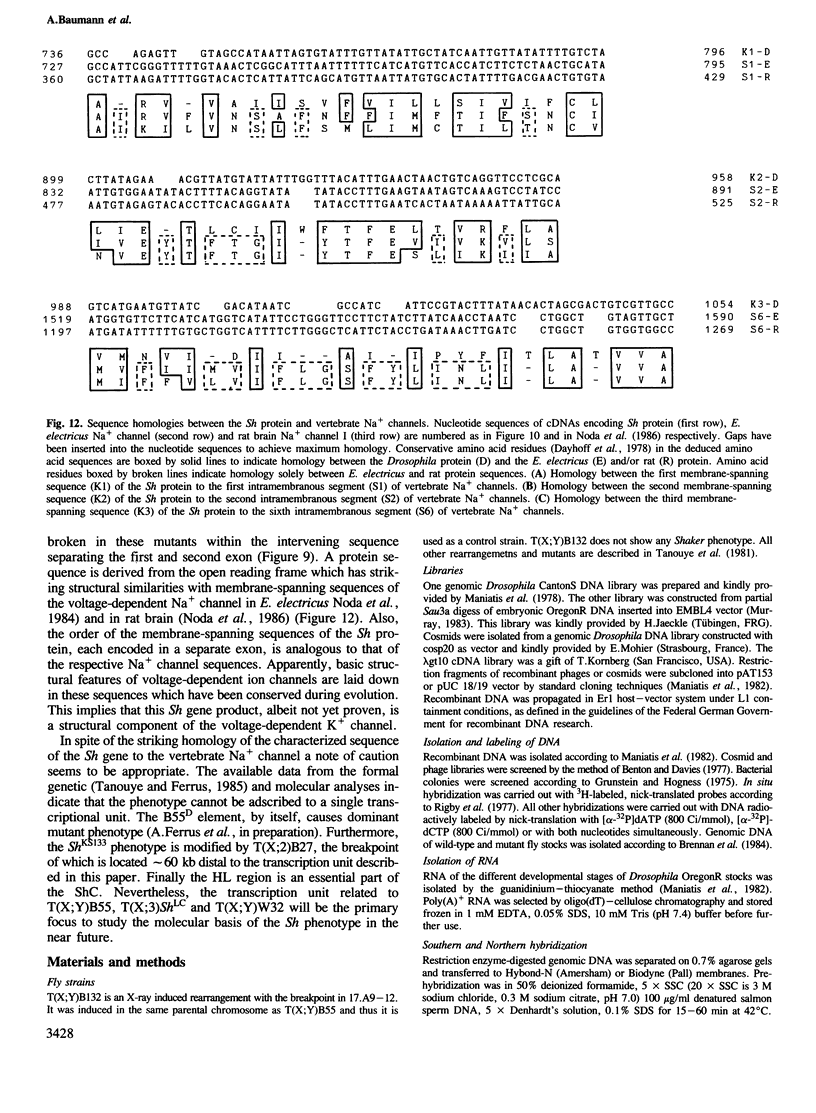
We have cloned 215-kb DNA containing the maternal effect region (ME) of the Shaker gene complex (shC) at 16F of the Drosophila X chromosome. Five translocation and deletion breakpoints have been mapped on the cloned DNA allowing a correlation of the genetic map to transcription units. The ME region spans ˜100 kb. The genetic behavior of this region correlates with the occurrence of maternal RNAs in this part of the ShC. Two transcripts have been identified in the vicinity of chromosomal rearrangements which cause a Sh phenotype. These are a 4.5-kb transcript interrupted by T(x;2)B27 and a 2-kb transcript interrupted by T(X;3)ShLC and T(X;Y)W32. The latter transcript is derived from a primary transcript which spans >65 kb genomic DNA. The cDNA-sequencing data show that this Shaker (IAchannel) gene can encode a protein of ˜35 kd with three α-helical membrane-spanning sequences near its carboxyl terminus. These have a striking homology with membrane-spanning sequences of the vertabrate Na+ channel.
Keywords: Drosophila, Shaker locus, K+ channel, Na+ channel
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Deber C. M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. D., Rowan R. G., Dickinson W. J. Introduction of a functional P element into the germ-line of Drosophila hawaiiensis. Cell. 1984 Aug;38(1):147–151. doi: 10.1016/0092-8674(84)90535-x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogness D. S., Lipshitz H. D., Beachy P. A., Peattie D. A., Saint R. B., Goldschmidt-Clermont M., Harte P. J., Gavis E. R., Helfand S. L. Regulation and products of the Ubx domain of the bithorax complex. Cold Spring Harb Symp Quant Biol. 1985;50:181–194. doi: 10.1101/sqb.1985.050.01.024. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Koto M., Tanouye M. A., Ferrus A., Thomas J. B., Wyman R. J. The morphology of the cervical giant fiber neuron of Drosophila. Brain Res. 1981 Sep 28;221(2):213–217. doi: 10.1016/0006-8993(81)90772-1. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G. H. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 1983;2(6):927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J. L., Changeux J. P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984 Oct;64(4):1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Brorein W. J., Jr, Dunsmuir P., Rubin G. M. Transposition of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):415–427. doi: 10.1016/0092-8674(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981 Sep 17;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985 Sep;2(4):253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye M. A., Kamb C. A., Iverson L. E., Salkoff L. Genetics and molecular biology of ionic channels in Drosophila. Annu Rev Neurosci. 1986;9:255–276. doi: 10.1146/annurev.ne.09.030186.001351. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. F., Ganetzky B., Haugland F. N., Liu A. X. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983 Jun 3;220(4601):1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Haugland F. N. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985 Oct;5(10):2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]