Abstract
Background
Composite pheochromocytoma/paragangliomas are very rare tumors composed of ordinary pheochromocytoma paragangliomas associated with neurogenic tumors. Several hereditary susceptibility disorders are known to be associated with pheochromocytoma/paragangliomas such as multiple endocrine neoplasia type 2 (2A or B). To the best of our knowledge, only four cases of composite pheochromocytoma/paragangliomas associated with multiple endocrine neoplasia type 2 have been reported.
Case presentation
A 40-year-old Arabic woman presented with headache, palpitations, paroxysmal hypertension, and weight loss, which she had had for the last 3 years. She had a familial history of diabetes and multiple endocrine neoplasia type 2. A radiological examination revealed thyroid lesions and bilateral adrenal medulla tumors. Our patient had undergone bilateral adrenalectomy, total thyroidectomy with cervical lymphadenectomy, and parathyroidectomy. A pathological examination confirmed the multiple endocrine neoplasia type 2A consisting of left medullary pheochromocytoma, right medullary composite pheochromocytoma-ganglioneuroma, medullary carcinoma of the thyroid with lymph node metastasis and parathyroid hyperplasia. A genetic analysis also revealed that our patient had a RET germline mutation.
Conclusion
Composite pheochromocytoma/paraganglioma associated with multiple endocrine neoplasia type 2 is a very rare occurrence, as the current literature provides only a few cases. Further reported cases are needed in order to understand the behavior and the pathogenesis of this uncommon entity.
Keywords: Composite pheochromocytoma/paraganglioma, MEN 2, Ganglioneuroma
Background
Composite pheochromocytoma/paraganglioma (CPC/PG) is a rare entity composed of classic PC/PG associated with other neurogenic tumors such as neuroblastoma, ganglioneuroblastoma, ganglioneuroma (GN), or malignant peripheral nerve sheath tumors (MPNST) [1]. In the literature, ganglioneuroma is the most reported component of composite tumors [2]. The adrenal medulla is the common site of these tumors, however, extramedullary locations (CPG) can be encountered. Cases of CPC/PG have been reported in association with neurofibromatosis (NF), gastrointestinal stromal tumors (GIST), multiple endocrine neoplasia (MEN), and von Hippel-Lindau syndrome (vHL) [2, 3]. The management and the prognosis of CPC/GN seem to be similar to their ordinary-type counterpart (PC/PG), but based on a few reported cases to date, little is known about their real behavior [2, 4, 5]. To the best of our knowledge, only four cases of CPC/PG associated with MEN 2 have been previously reported in the literature [6–9]. The first case was reported by Brady et al. in 1997 in a 34-year-old man with MEN 2A. In 1998, Matias-Guiu et al. reported another case of CP in a 49-year-old man with MEN 2A. The two reported cases were located in the left adrenal medulla and the neurogenic components were ganglioneuroma and ganglioneuroblastoma, respectively. Cases associated with MEN 2B have also been reported. Charfi et al. reported in 2008 the first case of CP associated with MEN type 2B. More recently, in 2016, Yamasaki et al. reported a case of a composite retroperitoneal paraganglioma in a 59-year-old man.
We report here an additional case of CPC in a 40-year-old woman with MEN 2A. We discuss the clinicopathological issues and perform a review of the literature in order to improve understanding of this very rare type of tumor.
Case presentation
A 40-year-old Arabic woman presented with headache, palpitations, paroxysmal hypertension and weight loss, which she had had for 3 years. She had a history of treated type 2 diabetes. She had also a familial history of type 2 diabetes and MEN 2A. Her sister had undergone thyroidectomy and adrenalectomy for medullary carcinoma of the thyroid and pheochromocytoma. A physical examination showed an enlarged and firm thyroid mass which moved with swallowing, without cervical adenopathy. Our patient had no hypertension on admission. A cervical ultrasound scan found nodular heterogeneous calcified and cystic lesions of the thyroid gland, the larger lesion was located at the left lobe and measured 1.8 × 1.3 cm. An abdominal computed tomography (CT) scan revealed a left adrenal mass and a well-encapsulated nodule of the right adrenal gland (Fig. 1). The laboratory tests showed high levels of serum parathyroid hormone and calcitonin, normal levels of serum calcium, phosphorus, and Vitamin D. A 24-hour urine collection found elevated levels of norepinephrine, epinephrine, and methyldopa (Table 1). Because of our patient’s familial history of MEN 2A, a genetic mutational analysis for the RET proto-oncogene was performed at our hospital’s medical genetics department. The test was carried out on our patient’s peripheral blood. Her genomic deoxyribonucleic acid (DNA) was amplified by using polymerase chain reaction (PCR) and oligonucleotide primers for exon 11. A direct sequencing analyses showed a RET (rearranged during transfection proto-oncogene) germline mutation at exon 11 (codon 634), with a substitution of tyrosine by a cysteine residue (C1900T→C (C634R).
Fig. 1.
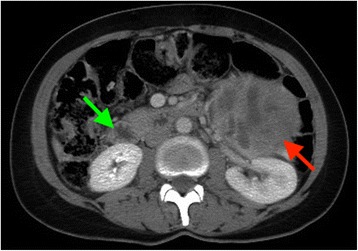
The abdominal computed tomography scan shows a left adrenal mass (red arrow) and a well-encapsulated nodule of the right adrenal gland (green arrow). The two lesions have central hypodense areas
Table 1.
Laboratory tests results of our patient
| Results | Normal range | |
|---|---|---|
| Parathyroid hormone (serum) | 222.2 pg/mL | 11–54pg/mL |
| Calcitonin (serum) | 3130 pg/mL | ˂ 6.4 pg/mL |
| Calcium (serum) | 10.2 mg/L | 8.7–10.3mg/dL |
| Phosphorus (serum) | 3.5 mg/L | 2.5–4.5 mg/dL |
| Vitamin D (serum) | 28 ng/mL | 24–40 ng/mL |
| Norepinephrine (urine) | 345,350 nmol/24 h | 90–500 nmol/24 h |
| Epinephrine (urine) | 217,152 nmol/24 h | ˂ 120 nmol/24 h |
| Methyldopa (urine) | 9722 nmol/24 h | 300–3000 nmol/24 h |
pg picogram, mL milliliter, L liter, nmol nanomole, h hour
Our patient had undergone bilateral adrenalectomy, total thyroidectomy with cervical lymphadenectomy, and parathyroidectomy. A pathological examination confirmed the MEN 2A consisting of left medullary pheochromocytoma, right medullary composite pheochromocytoma-ganglioneuroma, medullary carcinoma of the thyroid (MCT) with lymph node metastasis, and parathyroid hyperplasia. Our patient recovered well after surgery and had no signs of disease 3 years after treatment.
Pathological findings
Grossly, the resected right adrenal specimen measured 9 × 6.5 × 5 cm. The tumor was well-encapsulated, firm, and the cut surface was yellow-brown, with areas of hemorrhage (Fig. 2a).
Fig. 2.
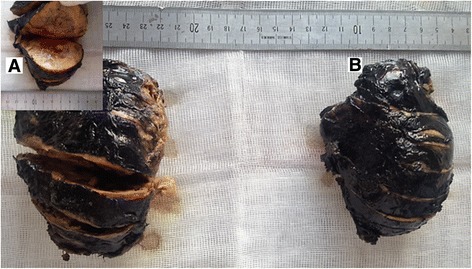
The macroscopic view of the resected adrenal glands. a The right adrenal gland shows a well-encapsulated tumor, the cut surface is yellow-brown, with areas of hemorrhage. b The left resected specimen has quite similar characteristics
The left adrenal specimen measured 13 × 8 cm. The cut surface of the tumor was light tan and yellowish, with hemorrhagic changes (Fig. 2b).
The left lobe of the resected thyroid gland measured 4.5 × 3.5 × 1.5 cm; the right lobe measured 5 × 3 × 3 cm and the isthmus measured 2 × 1.5 cm. A 1.7 × 1.2 cm nodule was found in the middle third of the left lobe; it was fleshy, grayish-white, and ill-defined. Cystic and fibrous degeneration were seen in the remaining parenchyma. The right lobe was enlarged with multinodular colloid structures with cystic degeneration.
The resected parathyroid gland measured 2.4 × 1 × 0.5 cm, with no macroscopic lesion identified.
Lymphadenectomy consisted of fibroadipocytic fragments measuring from 0.5 cm to 3 × 2.5 cm, containing several lymph nodes.
A histologic examination with routine hematoxylin-eosin staining (HES) of the right adrenal resected specimen showed tumor cells arranged in trabecular and anastomosing cords. The cells were polygonal, spindle or oval-shaped with a moderate amphophilic cytoplasm. The nuclei were round to spindle-shaped with inconspicuous nucleoli. Some bizarre nuclei were found with pseudoinclusion (Figs. 3 and 4). Beside this component, a second one was found consisting of mature ganglion cells with abundant eosinophilic cytoplasm and a prominent nucleolus, embedded in a thick fibrillary matrix background (Fig. 5). The two components were slightly separated by dilated blood vessels. Mitosis figures or necroses were not observed. The ganglioneuroma component represented approximately 20% of the tumor surface.
Fig. 3.
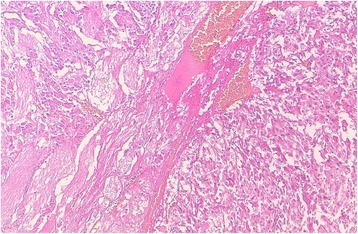
The two components of the tumors of the right adrenal gland: pheochromocytoma (the right part of the image) and ganglioneuroma (the left part), separated by dilated blood vessels (Hematoxylin-eosin-safran (HES) ×100)
Fig. 4.
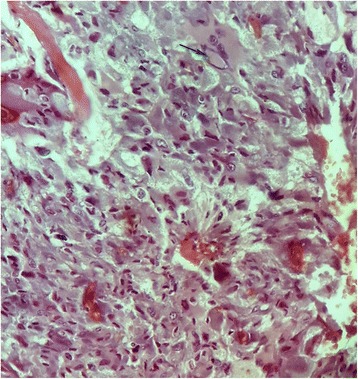
The pheochromocytoma component of the tumor shows polygonal, oval-shaped and spindle cells with amphophilic cytoplasm. The nuclei were round to spindle-shaped with inconspicuous nucleoli. A bizarre cell is seen with pseudoinclusion at the top part of the image (black arrow) (Hematoxylin-eosin-safran (HES) ×400)
Fig. 5.
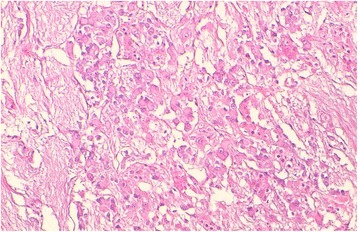
The ganglioneuroma component of the tumor shows multiple mature ganglion cells with abundant eosinophilic cytoplasm and round nuclei with conspicuous nucleoli, within a fibrillary background (Hematoxylin-eosin-safran (HES) ×200)
The microscopic aspect of the left adrenal resected specimen was similar to the pheochromocytoma component seen in the right adrenal specimen.
At immunohistochemistry, the cells of the pheochromocytoma component were strongly positive for synaptophysin and chromogranin, but negative for neurofilament (Fig. 6a). The ganglion cells of the ganglioneuroma components were negative for chromogranin and stained positive for neurofilament, as well as the Schwannian fibrillary matrix (Fig. 6b).
Fig. 6.
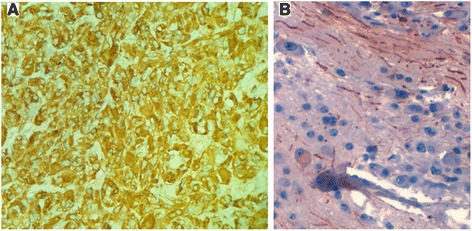
The cells of the pheochromocytoma component are strongly positive for chromogranin (a). The ganglion cells of the ganglioneuroma component are positive for neurofilament as well as the Schwannian fibrillary matrix (b) (×100)
Microscopically, the nodule of the left lobe of the resected thyroid gland showed sheets of polygonal cells with amphophilic cytoplasm and oval nuclei with granular chromatin and inconspicuous nucleoli. Amyloid deposits were seen as pink amorphous globules within the tumor sheets (Fig. 7a).
Fig. 7.
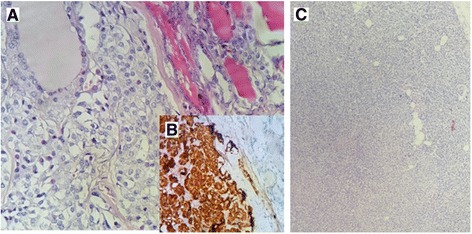
The medullary carcinoma of the thyroid shows sheets of polygonal cells with amphophilic cytoplasm and oval nuclei with granular chromatin and inconspicuous nucleoli (a) (HES ×200). These cells are strongly positive for chromogranin (b). The hyperplastic parathyroid shows a significant decrease in adipocytic lobules (c) (Hematoxylin-eosin-safran (HES) ×200)
Tumor cells stained positive for synaptophysin and chromogranin, but negative for thyroid transcription factor 1 (TTF1) (Fig. 7b).
The histologic examination of the parathyroid gland showed hyperplastic chief cells with reduced adipose tissue (Fig. 7c).
The lymphadenectomy consisted of 26 lymph nodes with two metastatic lymph nodes from medullary carcinoma of the thyroid. The tumor was classified as pT1bN1b (AJCC 2009).
Discussion
Composite pheochromocytoma/paragangliomas (CPC/PG) are uncommon tumors combining the histologic features of the ordinary PC/PG with those of ganglioneuroma, ganglioneuroblastoma, neuroblastoma or peripheral nerve sheath tumors [2]. Paragangliomas (PG) are tumors derived from paraganglia located along the supradiaphragmatic (parasympathetic) nerves, the pre- and paravertebral sympathetic chains or sympathetic nerve fibers of the pelvic and retroperitoneal organs. Pheochromocytoma is a paraganglioma of the adrenal gland. Cells of both adrenal medulla and paraganglia derived embryologically from neural crest cells that migrated to other organs [2, 3]. This embryologic common origin allows understanding the histogenesis of composite tumors. In fact, neural crest cells migrate to other sites and mature into melanocytes, chromaffin cells, Schwann cells or ganglion cells [2, 10]. Disorders of crest cells migration or maturation at various stages of the cellular development are thought to lead to CPC/PG [2, 5]. The possibility of chromaffin cells to undergo divergent differentiation into neuronal elements is another hypothesis to explain histogenesis of CPC/PG [11].
Macroscopically, CPC/PG have similar features of ordinary pheochromocytoma, presenting generally, as solid and firm tumors, the cut section appearing gray-white, light tan, or dusky red [10, 12]. The histologic diagnosis of composite pheochromocytoma/paraganglioma (CPC/PG) must be based both on architecture and on cell populations deriving from neural crest. Any tumor component that does not share common embryological origin with paraganglia or adrenal medulla cells should be considered as a collision tumor [2, 12]. In addition, the presence of degenerating chromaffin cells, lymphocytes, or scattered neuron-like cells, should not be misinterpreted as CPC/PG, as they are occasionally found in ordinary PC/PG [6, 12]. On microscopic examination, the two components of the tumor are admixed or separate, usually the pheochromocytoma is the predominant component [6, 7]; but the case reported by Brady et al. was composed of approximately equal parts of PC and PG [6]. The PC cells are arranged in syncytial-like growth pattern (Zellballen pattern), or arranged in an alveolar, diffuse, trabecular or solid pattern. Spindle cells can also be found in the tumor. Cellular pleomorphism is often present, with admixture of large and small cells. Some cells with bizarre appearance with nuclear pseudoinclusion may be observed, but this is not criteria for malignancy [12]. The stroma and the vasculature of the tumor may be prominent, causing unhabitual appearance, with diagnostic challenge [2]. In our case, the PC component showed trabecular and diffuse pattern with a rich vasculature; cells with pseudoinclusion and spindle cells have been observed. The ganglioneuroma component was slightly demarcated from the PC component by dilated blood vessels. Immunochemistry is very helpful, to rule out differential diagnosis or to distinguish easily the different components of the CPC/PG. The cells of the PC/PG component stain positive for neuroendocrine markers such as chromogranin and synaptophysin; functional tumors are positive for catecholamine-synthetizing enzymes such as thyrosine hydroxylase (TH) or phenylethanolamine N-methyltransferase (PNMT). Tumor cells have also capacity to produce a large variety of neuropeptides like vasoactive intestinal peptide (VIP), bombesin, substance P, and so on. [2, 6, 7]. The neurogenic cells are positive for neuron-specific enolase (NSE), neurofilament (NF), but show weak or focal staining for chromogranin. The sustentacular cells and Schwann cells are positive for S-100 protein [6, 12]. There is no reliable biological marker that reflects the malignant potential of CPC/PG. Comstock et al. reported the absence of N-myc amplification in four cases of CPC, and concluded that CPC does not have adverse prognostic significance conferred by the neuroblastic elements [13].
Usually, CPC/PG are functional tumors, clinical symptoms are related to the type of hormones produced by the component of the tumor. Symptoms range from palpitations, headache, hypertension, and excessive sweat. These signs are due to excessive production of epinephrine or norepinephrine. Cases presenting with watery diarrhea have been described attributable to increased production of vasoactive intestinal peptide (VIP) [12]. According to Shawa et al., CPC/PG are similar clinically and radiologically, and their management should be so [14].
PC/PG, ordinary or composite types, are usually associated with certain hereditary disorders: von Hippel-Lindau syndrome (vHL), multiple endocrine neoplasia syndrome type 2A and 2B (MEN 2A and 2B), NF1 and the recently described familial paraganglioma syndrome [2, 12]. Several genes are involved in the pathogenesis of PC, such as the RET proto-oncogene, von Hippel–Lindau disease tumor suppressor (VHL), neurofibromatosis type 1 tumor suppressor (NF1), genes encoding the succinate dehydrogenase (SDH) complex subunits, TMEM127, and MYC-associated factor X (MAX) [4].
To the best of our knowledge, only four cases of CPC/PG associated with MEN 2 have been reported [6–9] (Table 2). MEN type 2 is an autosomal-dominant disease with major components of MTC, PC, and hyperparathyroidism [7, 9]. There were two cases of MEN 2A and two cases of MEN 2B. The case reported by Matias-Guiu et al. was in fact a postmortem diagnosis on autopsy as the patient had died after an orthopedic surgery [7]. He had a RET germline mutation on the exon 11 at the codon 634, like our patient. In fact the vast majority of patients with MEN 2A carry a RET germline mutation on exon 10 or 11 [7, 9]. The MEN 2B represents less than 10% of the MEN 2 entity and has a more aggressive course. Patients with MEN 2B develop MTC, pheochromocytomas, developmental abnormalities, mucosal neuromas, and intestinal ganglioneuromas. They carry mostly a germline methionine-to-threonine mutation at codon 918 (M918T) in exon 16 of the RET proto-oncogene [9]. The case reported by Yamasaki et al. had a composite retroperitoneal paraganglioma with a M918T germline mutation of the RET proto-oncogene [9]. Patients’ age ranged from 27 years to 59 years, with a male predominance (three men for one woman). Our case was a woman aged 40 years. The adrenal medulla was the most common site of the tumors in the previously reported cases. However, the case reported by Yamasaki et al. had a retroperitoneal CPG. Our patient had bilateral pheochromocytomas with a CP at the right adrenal medulla. The neurogenic components of CPC/PG are, in decreasing frequency: ganglioneuroma, ganglioneuroblastoma, neuroblastoma, malignant peripheral nerve sheath tumors (MPNST), and neuroendocrine carcinoma [2].
Table 2.
Reported cases of composite pheochromocytoma/paragangliomas associated with MEN 2A/B
| Age(years)/sex | MEN type | Tumor size/weight | Organ | Neurogenic component | Outcome | Reference/country/year |
|---|---|---|---|---|---|---|
| 34/M | 2A | 1.6 cm/11,8g | Left adrenal medulla | Ganglioneuroma | Uneventful postoperative course | Brady et al. [6]/USA/1997 |
| 49/M | 2A | 600g | Left adrenal medulla | Ganglioneuroblastoma | Died after orthopedic surgery (postmortem incidental finding) | Matias-Guiu et al. [7]/Spain/1998 |
| 27/F | 2B | 10 cm/124g | Left adrenal medulla | Ganglioneuroma | Tumor free for 16 months after surgery | Charfi et al. [8]/Tunisia/2008 |
| 59/M | 2B | 3 cm | Retroperitoneum | Ganglioneuroma | Metastatic disease at diagnosis | Yamasaki et al. [9]/Japan/2016 |
| 40/F | 2A | 9 cm | Right adrenal medulla | Ganglioneuroma | Tumor free for 3 years | Our case/Morocco |
MEN2A/2B multiple endocrine neoplasia type 2A or 2B, M Male, F Female
The clinical course of CPC/PG is not clearly defined because of the rarity of these tumors. Patients usually present with clinical signs related to hormone hyperproduction, such as headache, palpitations or hypertension. It has been reported in the literature that the prognosis of composite tumors with neuroblastoma (NB), ganglioneuroblastoma (GNB) and MPNST is worse, and the clinical behavior is determined by these components [2]. The behavior of CPC/PG associated with MEN 2 seems to depend on the MCT. In fact, cases reported by Matias-Guiu et al. and Yamasaki et al., as well as our case, had metastasis from MCT [7, 9]. In general, the prognosis of patients with MEN 2 relies on the occurrence of the MCT, as the associated pheochromocytomas or other symptoms often have a benign course [15]. Recently, the American Thyroid Association (ATA) has updated its guidelines for the management of MTC [15]. In fact, the aggressiveness of MTC in MEN 2 depends on the type of RET proto-oncogene mutations, and a subsequent risk stratification has been suggested by the ATA. Patients with MEN 2A and C634 mutation on exon 11, like our patient, have a high risk of aggressive MTC. Patients with M918T RET mutation on exon 16, in patients with MEN 2B, have the highest risk. According to ATA’s guidelines, patients with a high-risk MEN 2 (ATA-H category), like our current patient, should be treated by surgery and their first-degree relatives should be offered genetic counseling and genetic testing for RET germline mutations [15]. In fact, such management has been proposed to our patient’s relatives but we do not know if they have had it done elsewhere, as they have not been screened at our center. The sister of our patient had also undergone surgery for MEN 2A years before; unfortunately, her sister (our current patient) had not been screened until she developed the complete disease syndrome with a locally advanced MTC.
Conclusions
CPC/PGs are very rare tumors and are supposed to have similar clinical behavior of ordinary pheochromocytoma/paraganglioma. The association with MEN 2 is uncommon. We have described here a case of a patient with CPC associated with MEN 2A, together with a review of the literature. Less than 100 cases of CPC/PG have been reported, emphasizing the need for further studies in order to know more about the epidemiological, diagnostic, and prognostic aspects of these rare tumors.
Acknowledgements
The authors gratefully thank Prof. S. Bennis and her team for carrying out additional immunohistochemical staining.
Funding
The authors received no specific funding for this study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ATA
American Thyroid Association
- CPC/PG
Composite pheochromocytoma-paraganglioma
- DNA
Deoxyribonucleic acid
- GN
Ganglioneuroma
- GNB
Ganglioneuroblastoma
- HES
Hematoxylin-eosin Saffron
- MCT
Medullary carcinoma of the thyroid
- MEN 2A/B
Multiple endocrine neoplasia syndrome type 2A/type 2B
- MPNST
Malignant peripheral nerve sheath tumors
- NF1
Neurofibromatosis type 1
- NSE
Neuron-specific enolase
- PC
Pheochromocytoma
- PCR
Polymerase chain reaction
- PG
Paraganglioma
- RET
Rearranged during transfection proto-oncogene
- vHL
von Hippel Lindau syndrome
- VIP
Vasoactive intestinal peptide
Authors’ contributions
BE wrote the report and made substantial contributions to conception and design of the manuscript. GAE, ST, KM, NH, and HEF critically revised the manuscript. LC was involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Boubacar Efared, Email: befared2013@gmail.com.
Gabrielle Atsame-Ebang, Email: diaraepidemio@gmail.com.
Soufiane Tahirou, Email: befared@yahoo.fr.
Khalid Mazaz, Email: yassinethoracique@gmail.com.
Nawal Hammas, Email: serrajpneumo@yahoo.fr.
Hinde El Fatemi, Email: fatemianapath@gmail.com.
Laila Chbani, Email: laylaanapath@gmail.com.
References
- 1.DeLellis R, Heitz P, Lloyd R, Eng C. WHO classification of tumours: pathology and genetics of tumours of endocrine organs. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Papathomas TG, de Krijger RR, Tischler AS. Paragangliomas: update on differential diagnostic considerations, composite tumors, and recent genetic developments. Semin Diagn Pathol. 2013;30(3):207–23. doi: 10.1053/j.semdp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Gorgel A, Cetinkaya DD, Salgur F, Demirpence M, Yilmaz H, Karaman EH, et al. Coexistence of gastrointestinal stromal tumors (GISTs) and pheochromocytoma in three cases of neurofibromatosis type 1 (NF1) with a review of the literature. Intern Med. 2014;53(16):1783–9. doi: 10.2169/internalmedicine.53.2012. [DOI] [PubMed] [Google Scholar]
- 4.Shida Y, Igawa T, Abe K, Hakariya T, Takehara K, et al. Composite pheochromocytoma of the adrenal gland: a case series. BMC Res Notes. 2015;8:257. doi: 10.1186/s13104-015-1233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namekawa T, Utsumi T, Imamoto T, Kawamura K, Oide T, Tanaka T, et al. Composite pheochromocytoma with a malignant peripheral nerve sheath tumor: case report and review of the literature. Asian J Surg. 2016;39(3):187–90. doi: 10.1016/j.asjsur.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Brady S, Lechan RM, Schwaitzberg SD, Dayal Y, Ziar J, Tischler AS. Composite pheochromocytoma/ganglioneuroma of the adrenal gland associated with multiple endocrine neoplasia 2A: case report with immunohistochemical analysis. Am J Surg Pathol. 1997;21(1):102–8. doi: 10.1097/00000478-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Matias-Guiu X, Garrastazu MT. Composite phaeochromocytoma-ganglioneuroblastoma in a patient with multiple endocrine neoplasia type IIA. Histopathology. 1998;32(3):281–2. doi: 10.1046/j.1365-2559.1998.0372g.x. [DOI] [PubMed] [Google Scholar]
- 8.Charfi S, Ayadi L, Ellouze S, Ghorbel R, Khabir A, Gouiaa N, et al. Composite pheochromocytoma associated with multiple endocrine neoplasia type 2B. Ann Pathol. 2008;28(3):225–8. doi: 10.1016/j.annpat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki M, Sato Y, Nomura T, Sato F, Uchino S, Mimata H. Composite paraganglioma-ganglioneuroma concomitant with adrenal metastasis of medullary thyroid carcinoma in a patient with multiple endocrine neoplasia type 2B: a case report. Asian J Endosc Surg. 2017;10(1):66–9. doi: 10.1111/ases.12332. [DOI] [PubMed] [Google Scholar]
- 10.Chang H, Jeong H, Kim Y, Park SH, Kim A. Composite pheochromocytoma or paraganglioma of adrenal gland: a case report with immunohistochemical studies and electron microscopic examination. Korean J Pathol. 2011;45(3):306–10. doi: 10.4132/KoreanJPathol.2011.45.3.306. [DOI] [Google Scholar]
- 11.Tischler AS. Divergent differentiation in neuroendocrine tumors of the adrenal gland. Semin Diagn Pathol. 2000;17(2):120–6. [PubMed] [Google Scholar]
- 12.Lack EE, Wieneke J. Tumors of the adrenal gland. In: Fletcher CDM, editor. Diagnostic histopathology of tumors. Philadelphia: Elsevier Saunders; 2013. pp. 1307–14. [Google Scholar]
- 13.Comstock JM, Willmore-Payne C, Holden JA, Coffin CM. Composite pheochromocytoma: a clinicopathologic and molecular comparison with ordinary pheochromocytoma and neuroblastoma. Am J Clin Pathol. 2009;132(1):69–73. doi: 10.1309/AJCPN76VTIGWPOAG. [DOI] [PubMed] [Google Scholar]
- 14.Shawa H, Elsayes KM, Javadi S, Sircar K, Jimenez C, Habra MA. Clinical and radiologic features of pheochromocytoma/ganglioneuroma composite tumors: a case series with comparative analysis. Endocr Pract. 2014;20(9):864–9. doi: 10.4158/EP14010.OR. [DOI] [PubMed] [Google Scholar]
- 15.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


