Abstract
Previous research has demonstrated that before puberty, parents are able to buffer, and often completely block, cortisol responses to social evaluative stressors (e.g., Trier Social Stress Test; TSST). However, after puberty, parents no longer provide a powerful buffer of the HPA axis from a social-evaluative stressor. The current study investigates whether friends can buffer the HPA axis in both children and adolescents compared to parents and whether similar stress-ameliorating patterns can also be observed in oxytocin activity. A total of 109 participants (54 children ages 9–10 and 55 adolescents ages 15–16; half of each sex) completed the TSST and were randomly assigned to prepare for their speech with their parent or friend for 5 minutes beforehand. Salivary cortisol and urinary oxytocin were measured before and after the TSST. For children, cortisol responses were comparable regardless of who helped the child prepare the speech. For adolescents, however, friends actually amplified the cortisol response compared to parents. In addition, adolescents produced less oxytocin than children, as did males compared to females. Notably, for boys, oxytocin levels decreased across the session if participants prepared with a friend rather than their parent. The mean change was in the same direction but not significant for girls. These results indicate that friends do not take over the social buffering role by age 15–16, which may inform interventions in at-risk children and adolescents.
Keywords: social buffering, HPA axis, cortisol, oxytocin, adolescence
Social stress buffering has been defined as the reduction of physiological stress responses to an acute stressful event due to the presence or assistance of another individual, often a person with a close relationship to the person undergoing the stressor (Hennessy et al., 2009). For many mammalian species, social buffering by parents (i.e., parental stress buffering) may serve to protect the young from the deleterious effects of stress by decreasing activation of stress-mediating systems and reducing allostatic load impacts during development even when a child lives in an otherwise adverse environment (Gunnar & Donzella, 2002). This phenomenon may be a primary reason why social support has positive effects on mental and physical health. As a result, understanding social buffering during development may enhance our ability to improve outcomes for children.
The ability of an attachment figure to buffer the infant’s physiological response to stress is well documented. Numerous studies in non-human primates have shown that the presence of the parent buffers elevations in cortisol for monkey infants (see Hennessy, Kaiser, & Sachser, 2009). Similarly, in human infants and young children, the presence of the attachment figure in secure relationships reduces or blocks elevations in cortisol to physical (i.e., inoculations) and psychosocial stressors (Gunnar et al., 1996; Nachmias et al., 1996; Spangler & Schieche, 1998; Ahnert et al., 2004). Social buffering effects also have been demonstrated in adults, but with romantic partners and close friends instead of parents. These close relationship figures have been shown to reduce both HPA and autonomic responses to stressors (Fontana, Diegnan, Villeneuve & Lepore, 1999; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Uno, Uchino, & Smith, 2002).
The animal literature is also replete with evidence that familiar conspecifics reduce reactivity of the HPA axis to stressors (Hennessy et al., 2009). For example, in adolescent rhesus monkeys, both mothers and peers can buffer cortisol responses to stress, but in an inconsistent manner (Gunnar, Gonzales, & Levine, 1980; Rilling et al., 2001; Winslow et al., 2003). Likewise, squirrel monkeys that have been peer housed show little to no increase in cortisol secretion in response to novelty (Hennessy, 1984; Hennessy, Mendoza, & Kaplan, 1982). In guinea pigs, adolescents are buffered from stress by maternal care weeks beyond the weaning period (Hennessy, Nigh, Sims, & Long, 1995). Taken together, these studies of post-weaning to pre-adulthood social buffering in animal models suggest significant but inconsistent alterations in the effectiveness of social buffering by parents and peers that may be moderated by a number of factors.
Current evidence suggests that stress buffering by the parent may continue through childhood but become less potent during adolescence. Seltzer and colleagues (2010) demonstrated that among 7- to 12-year old females, recovering from a social stressor task with their mother reduced cortisol to baseline faster than doing so without any maternal contact, and even talking to their mother on the phone provided some benefit. Our research group has demonstrated that parents remain an effective buffer of the HPA axis among 9- and 10-year old children but not for adolescents aged 15- and 16-years-old (Hostinar et al., 2015). Both children and adolescents exhibited a significant cortisol response to the TSST if they prepared with a stranger; however, only adolescents and not children showed a cortisol stress response if they prepared with their parent. A recent study in 11–14 year olds demonstrated that this switch away from parental effectiveness as a buffer of HPA axis reactivity was associated with changes in pubertal status rather than increasing age (Doom, Hostinar, VanZomeren-Dohm, & Gunnar, 2015), which suggests that developmental mechanisms related to puberty may underlie changes in social buffering. Using neuroimaging methods, researchers have shown that with adolescence, the mother’s presence no longer buffers amygdala responses to threat stimuli, allowing fear conditioning to occur even when the mother is present and not indicating fear of the conditioned stimulus (Gee et al., 2014).
If parents are not as effective in stress buffering post-puberty, is it the case that social buffering shifts to others with whom the child is intimate, notably close friends? Certainly it is the case that during adolescence youth report that they become more emotionally distant from parents and closer to friends (Hunter and Youniss, 1982; Cauce, 1986; Harris, 1995; Hartup, 1996). Thus, it is theoretically plausible that friends might buffer HPA responses to threatening situations. On the other hand, researchers have documented an overall increase in cortisol reactivity to social stressors between childhood and adolescence associated with puberty (van den Bos, de Rooij, Miers, Bokhorst, & Westenberg, 2013). Adolescents also report increased self-consciousness, social anxiety, embarrassment, and social fear compared to other types of fear (Forbes & Dahl, 2010; Weems & Costa, 2005; Westenberg, Drewes, Goedhart, Siebelink, & Treffers, 2004; Westenberg, Gullone, Bokhorst, Heyne, & King, 2007). Thus, it is also possible that the sense of social evaluation might be increased by a friend’s presence and therefore friends might not be capable of taking over the social stress buffering role from parents during adolescence for social evaluative stressors. Of course, many of the stressors of adolescence involve being evaluated by others. Furthermore, the incidence of stress-related psychological disorders increases in adolescence (Nelson, Leibenluft, McClure, & Pine, 2005). Therefore, questions about the capacity of youth to benefit from social buffering and who in their lives can provide such support are particularly noteworthy.
Currently, there are no experimental studies assessing the effectiveness of friends to serve as social stress buffers among either children or adolescents. A study by Adams, Santo, and Bukowski (2011) involved 10–11 year-olds collecting their own saliva at several points throughout the day and recording in a diary what they were doing, who they were with, and how they were feeling in the 20 minutes before the sample. Results indicated that, across participants, children demonstrated lower cortisol levels after a negative event when they reported being with their best friend relative to negative events without their best friend present (Adams, Santo, & Bukowski, 2011). There is also evidence that when allowed to debrief with a friend, 12- to 16-year-olds who had good quality relationships with the friend who debriefed them returned to baseline faster following a social evaluative stressor than did same-aged youth who had poor relationships with the friend who debriefed them (Calhoun et al., 2014). Of course, in both of these cases it could be that being the type of individual who can develop and maintain high quality friendships might be associated with characteristics that make one more stress-resilient. The present study provided an experimental test of whether parents versus same-sex friends provide equivalent or different social stress buffering effects in children (aged 9 and 10 years) as compared to adolescents (aged 15 and 16 years).
While the study of social stress buffering has focused on the reduction or prevention of heightened reactivity in stress-mediating systems, part of the positive effects of close relationships may also come from the stimulation of hormones and other neurochemicals that have restorative or anti-stress effects. One such hormone is oxytocin. It is well documented that the contact with close relationship partners increases oxytocin production (Carter, 1998; Seltzer, Ziegler, & Pollak, 2010; Uvnas-Moberg, 1998). Although most of the work has focused on the impact of oxytocin in the brain and its role in the formation of social bonds (Argiolas & Gessa, 1991; Liu & Wang, 2003; Nelson & Panksepp, 1996; Smith & Wang, 2013; Witt et al., 1992; Young, Lim, Gringrich, & Insel, 2001), oxytocin is also released into the periphery of the body where it has stress-reducing or reversing impacts on numerous systems, including the cardiovascular system (Gouin et al., 2010; Karelina & DeVries, 2011; Uvnas-Moberg, 1998). It is also the case that in adults, nasal oxytocin administration lowers the cortisol response to stress after the TSST with an effect size similar to the buffering effect of friend support (Heinrichs et al., 2003), and thus may be one of the mechanisms through which social stress buffering is produced. Research in the animal literature has demonstrated that at least in female prairie voles, oxytocin in the paraventricular nucleus of the hypothalamus (PVN) mediates social buffering effects during a 1-hour immobilization stressor, which may point to a similar mechanism operating in humans (Smith & Wang, 2013). In the Smith and Wang study, voles that recovered alone after immobilization showed more anxiety-like behaviors and increased corticosterone levels, which were not observed in voles that recovered with their male partner (Smith & Wang, 2013). Voles who recovered with their partner, and were therefore biologically and behaviorally buffered from the stressor, demonstrated a rise in oxytocin in the PVN. Furthermore, injections of oxytocin in the PVN reduced behavioral and corticosterone responses to stress while administration of an oxytocin receptor antagonist blocked social buffering effects provided by partner support (Smith & Wang, 2013). Similarly, in female squirrel monkeys, chronic intranasal oxytocin administration has been shown to lower ACTH levels after an acute social isolation stressor, and this HPA attenuation appears to operate at the level of the pituitary rather than the adrenal gland (Parker, Buckmaster, Schatzberg, & Lyons, 2005).
The production of both oxytocin and corticotropin-releasing hormone (CRH), the hormone involved in initiating the production of cortisol, in neurons of the PVN suggests that this location may be prime for interactions between the HPA and oxytocin systems. One study in which oxytocin was administered in the PVN resulted in reduced HPA responses in rats (Nishioka et al., 1998). In addition, oxytocin receptors have been localized in specific areas of the human brain (Boccia, Petrusz, Suzuki, Marson, & Pedersen, 2013), suggesting that oxytocin has both central and peripheral actions on emotions, behavior, and health. Developmental changes in the oxytocin system reported in animal models may also be present in humans. For example, in the pig hypothalamus, oxytocin-containing neurons nearly tripled across puberty (van Eerdenburg et al., 1990). This finding could indicate greater involvement of oxytocin in stress regulation and social behavior starting in adolescence if replicated in humans. In addition, neuronal oxytocin mRNA has been shown to be upregulated during puberty and this upregulation is dependent on gonadal steroids (Chibbar, Toma, Mitchell, & Miller, 1990). Due to evidence of direct interactions between the HPA and oxytocin systems as well as evidence of developmental changes in the central oxytocin system, at least in animal models, oxytocin was examined in this study to investigate whether changes in the oxytocin system paralleled changes in the HPA axis between childhood and adolescence.
In the present study, we measured oxytocin in urine, which reflects oxytocin in the periphery as well as in the brain. As most of the oxytocin in the body is produced in the hypothalamus (Carter, 2005), the bladder is a reservoir for oxytocin that is non-invasive and thus ideal for use in children. Although more research is needed to understand the association between central and peripheral oxytocin in humans, animal models suggest that central and peripheral oxytocin release is coordinated under certain conditions (Kendrick, Keverne, Baldwin, & Sharman; Landgraf & Neumann; 2004; Wotjak et al., 1998). We asked two questions about oxytocin production in this study: 1) would the pattern of oxytocin production mirror the pattern of social stress buffering observed for parents versus friends at the two ages in our assessment? 2) If so, when entered as a covariate, would it reduce the association between condition and age and the cortisol response to social evaluative stress? Because we are measuring urinary oxytocin, we cannot assume oxytocin is functioning the same way in the brain. Nonetheless, we can conservatively conclude that its levels provide at least one pathway through which social buffering may help protect the individual from deleterious impacts of social evaluative stress. We predicted that if friends become a potent source of stress buffering in adolescence then we would also expect to see them increasing the production of oxytocin by their presence.
Thus, to summarize, the aims of the current study are to test the effectiveness of parents versus friends in buffering the HPA response to a social evaluative stressor in childhood and adolescence. In addition, the oxytocin system will be tested as a potential correlate of developmental changes in social buffering. It is hypothesized that for children ages 9–10, preparing with a parent for a social evaluative stressor (TSST) will be associated with lower cortisol reactivity and faster recovery than preparing with a friend. Conversely, 15–16 year olds are expected to have lower cortisol reactivity and faster recovery when preparing with a friend than with a parent due to adolescents reporting becoming more emotionally close to friends than parents. However, because part of the stress stimulus in the TSST is the expectation that a group of peers will be evaluating their recorded speech, it is possible that by bringing a friend to the session, it increases sensitivity to the peer-evaluation component of the task, and thus increases the stressfulness of the task. Due to this possibility and research on heightened sensitivity to social evaluation in adolescence, friends might actually increase the response to threat in participants compared to parents. Finally, we predict that changes in the potency of parents and friends as social buffers of the HPA axis will be accompanied by changes in these individuals’ capacity to enhance the production of oxytocin during this social evaluative stressor. It is expected that changes in oxytocin will parallel changes in cortisol levels, suggesting that oxytocin may be involved in developmental changes in social buffering and HPA regulation. To examine whether oxytocin might be a potential mechanism of stress buffering, we will examine its role as a covariate of the responses observed, recognizing that we are only examining statistical mediation, and not a true causal relationship.
Methods
Participants
A total of 54 children ages 9–10 and 55 adolescents ages 15–16 were recruited from a department-maintained participant pool and were enrolled in the study. These age ranges were selected as the children were either pre-pubertal or in the very early stages of puberty, and adolescents at this age were either post-pubertal or in the late stages of puberty (pubertal screening described below). A total of 4 pubertal 9–10 year olds were excluded and all numbers and demographics in this study are reported without these participants. Exclusion criteria included the use of steroid medications, and diagnosis of Autism Spectrum Disorder, Fetal Alcohol Spectrum Disorder, or any other developmental disorder. These 54 typically developing children (M age = 9.9 years, SD = 0.5, range = 9–10.8 years; 26 females) and 55 adolescents (M age = 15.8 years, SD = 0.5, range = 15.1–16.9 years; 30 females) were included in all analyses. Participants reported the following racial/ethnic backgrounds: white/Caucasian (87.2%), African American (2.8%), Hispanic (1.8%), Asian (0.9%), other (0.9%), and more than one race (6.4%).
Annual household income ranged from $15,000–25,000 to over $200,000. The percent of families that had incomes greater than $150,000 was 21.1%, 56.8% had incomes from $75,001–150,000, 15.6% had incomes from $35,001–75,000, and 4.6% had incomes less than $35,000. There were 2 individuals who refused to report income. The distribution for parental educational level (of the parent who attended the session) was 7.3% high school or GED graduate, 15.6% 2-year college or associate’s degree, 39.4% bachelor’s or 4-year college degree, and 36.7% postgraduate degree. One individual did not report education level. Neither parent education nor family income differed as a function of sex, age group (child/adolescent), or parent/friend condition. The University of Minnesota Institutional Review Board approved all study procedures. Parents were recruited by phone, and those who did not meet exclusion criteria and agreed to have their child participate were scheduled. Parents of the friend who attended the session either filled out a consent form online before the session or the friend brought a consent form signed by their parent to the session.
Procedures
Pubertal status
Once at the laboratory, pubertal status was assessed using adolescent self-report. This was done to ensure that all 9–10 year olds were pre-pubertal and all 15–16 year olds were past the halfway point in puberty. The Pubertal Development Scale (Petersen et al., 1988; Carskadon and Acebo, 1993) assessed the extent of participants’ sex-specific bodily changes associated with puberty onset: growth in height, body hair, skin changes, deepening of voice, and facial hair for males; growth in height, body hair, skin changes, breast development, and menstruation for females. Responses were 1 = not yet started, 2 = barely started, 3 = definitely started, and 4 = seems complete (Carskadon & Acebo, 1993). Menstruation was coded as 1 if it had not begun and 4 if it had begun. This measure yields a mean score from 1 (puberty has not begun) to 4 (puberty is complete) in order to exclude pre-pubertal adolescents and pubertal children. Consistent with previous studies of pubertal timing (Doom et al., 2015), any 9–10-year-old who reported above a 2.5 on the scale was excluded from analyses for being pubertal, and any 15–16-year-old below a 2.5 was likewise excluded for being pre-pubertal.
Session timeline
Participants were scheduled for one session in which all data were collected. All participants were accompanied by a parent and arrived to the laboratory between 14:30 and 16:30 in order to account for diurnal variation in cortisol. Parents were told over the phone that the primary caregiver was preferred to accompany the participant to the session. Mothers (88.1%) were the parent in most of the sessions with no difference in sex of parent across age group, χ2(1, N = 109) = 2.08, p = 0.15, sex, χ2(1, N = 109) = 2.51, p = 0.11, or condition, χ2(1, N = 109) = 0.99, p = 0.32. All participants also arrived for testing with a friend. Friends were the same sex as the participant within 2 years of their age and could not be a sibling.
Once at the laboratory, each participant was randomly assigned to prepare for the stress task with their parent or with their friend (see Table 1 for summary of age/sex/condition). Sessions adhered to the following timeline (see Figure 1): (1) Parent, friend, and participant worked independently on questionnaires in the laboratory after the consent process (25 min), (2) urine sample #1 collection (5 min), (3) saliva sample #1 collection, then participant was told who they were going to prepare the speech with (parent or friend), moved to a separate room with that individual, and received TSST-M instructions for the speech preparation period (5 min), (4) speech preparation with parent or friend (5 min), (5) sample #2; participant moved to speech room and completed TSST alone regardless of condition (10 min), (6) samples #3–7; participant relaxed with parent and friend regardless of condition while working on questionnaires (samples collected every 10 min), (7) urine sample #2 was collected within 10 minutes of the final saliva sample, (8) debriefing of participants, parents, and friends was conducted. The 5-minute speech preparation period with the parent or friend was the only part that differed by condition. All participants were with their parent and friend before speech preparation and after the TSST-M.
Table 1.
Number of male and female participants by each age group and study condition (parent vs. friend).
| Group | Male | Female | Total |
|---|---|---|---|
| Younger/Parent Condition | 15 | 11 | 26 |
| Younger/Friend Condition | 13 | 15 | 28 |
| Older/Parent Condition | 15 | 12 | 27 |
| Older/Friend Condition | 10 | 18 | 28 |
| Total | 53 | 56 | 109 |
Note. Total N = 109.
Figure 1.
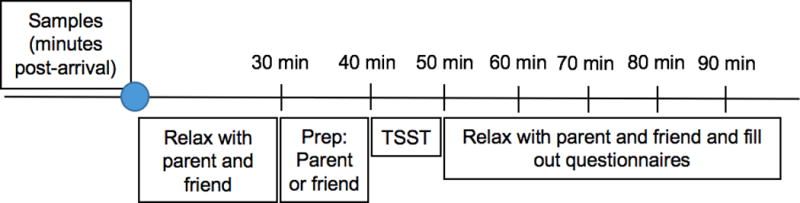
A timeline of the TSST-M protocol by minutes since the beginning of the session (marked by the blue circle). The first saliva sample used to compute reactivity was collected at 30 minutes after the start of the session, and the following 6 samples were collected every 10 minutes after the first. Note that the only difference in parent vs friend condition is during speech preparation.
Stress paradigm
A modified Trier Social Stress Test (TSST-M; Yim, et al., 2010) was used in which participants are asked to imagine they are introducing themselves to a classroom of students and that they should tell the class about their personality and some good and bad characteristics about themselves. The speech was followed by the standard TSST-C mental arithmetic (Buske-Kirschbaum et al., 1997). Rather than two live judges, the experimenter told the participant that there were two teachers behind a one-way mirror who would judge their speech and math performance and that a classroom of students would later evaluate their recorded speech. The teachers introduced themselves through a pre-recorded audiotape. Participants in the parent condition received support for 5 minutes before the TSST from their parent, who was instructed to assist their child in any way thought useful. In the friend condition, the participant’s parent remained in the waiting room while the friend assisted the participant in speech preparation for 5 minutes in any way they deemed useful. In both conditions, parents and friends were given the same instructions about assisting the participant, and the participants were alone in the room during the TSST-M.
Cortisol
Seven saliva samples were collected throughout the session to provide pretest, response, and recovery cortisol levels. Participants used the passive drool method to collect saliva through a straw into 1.5mL Eppendorf (Hamburg, Germany) tubes. Participants were asked not to consume large, protein-filled meals, milk, caffeine, or energy drinks for two hours before the session. After sample collection, saliva was stored in a −80°C laboratory freezer until being shipped to the University of Trier, Germany for assay. A time-resolved fluorescence immunoassay (dissociation-enhanced lanthanide fluorescent immunoassay [DELFIA]) detected cortisol levels. Intra-assay CVs ranged from 4.0% to 6.7%, and inter-assay CVs ranged from 5.1% to 7.2%. All seven samples from each participant were assayed in duplicate and in the same batch to prevent between-batch variation. An average of duplicate samples was used for the final analyses; these values were log-transformed. A total of 6 cortisol values were considered outliers (> 3 SD from the mean) and were winsorized.
Oxytocin
Two urine samples were collected to measure pretest oxytocin and oxytocin levels in response to the TSST-M. Urine was collected approximately 25 minutes after arrival for pretest levels and again within approximately 10 minutes of the final saliva sample. At least 5 mL of urine was snap frozen on dry ice in 15mL vials immediately after urination, and these samples were stored in a −80°C freezer until shipment on dry ice to the University of Wisconsin-Madison National Primate Research Center for assay of oxytocin and creatinine to adjust for sample volume. Urine samples were subjected to controlled thawing and then to solid-phase extraction with 1 ml SepPak C18 cartridges (Waters, no. WAT023590). Pretreatment of each column was done with 1 mL methanol, 1 mL water, and then 1 mL urine, and this was followed by a 10% acetonitrile (ACN) plus 1% trifluroacetic acid (TFA) wash (1 mL). The elutant was then collected via application of 1 mL of 80/20 percent ACN solution with 1% TFA. Samples were dried in a water bath with air stream and were then reconstituted in the buffer supplied in the 96-well enzyme linked immunosorbent assay (ELISA) kit used (Assay Designs. no. 901-153). Intra- and intercoefficients of variation were determined using oxytocin standards (intra-assay/inter-assay coefficient of variation = 6.0%/10.6%). A Molecular Devices Spectramax 340PC 384 at 405 nm was used to read each plate. Weighted least-squares regression was used for data analysis and log-logit transformation was used for reduction for peptide concentrations. Variation in water content was corrected by dividing the simple creatinine value by peptide concentration (Ziegler et al., 1995). Coefficients of variation for the creatinine assay were 1.7% for intra-assay and 5.2% for inter-assay. The final adjusted value was pg oxytocin/mg creatinine. Both pretest oxytocin and oxytocin at the end of the session were log-transformed for analysis. One sample was considered an outlier and was winsorized.
Daily diary
The parent and participant both completed a diary to report information about the participant relevant to cortisol collection. This information included time of wake, medication usage, physical activity, caffeine consumption, distressing events that day (e.g. arguments), and number of hours of sleep the previous night. Participant reports were the primary source of information. However, for type of medication used by the adolescent, the parent’s report on this variable was used when the child’s information was incomplete. After excluding participants taking medications with substantial effects on cortisol, a medication count variable was created using the method by Granger and colleagues (2009; M = .39, SD = 0.77, range: 0–2). Time since wake was calculated by subtracting the participant’s reported time of wake from the time of first saliva collection.
Self-reported stress
Participants completed a questionnaire about their stress level at 5 points in the assessment: arrival, speech preparation, speech delivery, math assessment, and the end of the session (e.g., “How stressful was giving the speech?”). Responses included: 1 = calm, 2 = low stress, 3 = medium stress, 4 = somewhat high stress, 5 = very high stress. Participants generally reported large increases in perceived stress levels during the speech and math portions of the TSST-M: arrival (M = 1.8, SD = 1.0), speech preparation (M = 2.7, SD = 1.2), speech (M = 3.8, SD = 1.2), math (M = 4.2, SD = 1.1), and end of session (M = 1.5, SD = 0.9).
Data Analytic Plan
A piecewise latent growth curve model was conducted to analyze cortisol around a theoretically meaningful time point (TSST onset). For a fine-grained analysis, cortisol was divided into reactivity and recovery (Juster et al., 2012). Reactivity and recovery slopes were extracted using Mplus in order to examine reactivity and recovery with linear regression models. Although most individuals’ cortisol levels were highest 10 minutes post-TSST-M, some individuals peaked 10–20 minutes later. As a result, landmark registration was used so that each person’s peak cortisol response was the point of highest reactivity and the beginning of recovery. Thus, reactivity was the cortisol slope between speech preparation onset and their peak cortisol level. Recovery was measured from the peak to the final sample. Two linear regressions were conducted for cortisol (one for reactivity and one for recovery) with age group (−1 = 9–10 year olds, 1 = 15–16 year olds), sex (−1 = female, 1 = male), and condition (−1 = parent, 1 = friend) as effect-coded independent variables in Step 1 of the regressions. Step 2 had all the 2-way interactions between age, sex, and condition, and Step 3 had the 3-way interaction. Medication count and time-since-wake were included as covariates. For cortisol reactivity, the log-transformed cortisol value after the 30-minute rest period (pretest) was used as a covariate to control for initial cortisol levels. For cortisol recovery, both the pretest cortisol level and the reactivity slope were included as covariates. To examine predictors of pre- and post-test oxytocin corrected for fluid volume (pg/mg creatinine), a repeated measures ANCOVA was conducted with Time 1 and Time 2 oxytocin as dependent variables to assess within- and between-subjects differences by sex, condition, and age group, and all 2-way and 3-way (e.g., time × sex × condition) interactions. The 4-way interaction was not tested due to power concerns. If the age × condition interaction significantly predicted both cortisol reactivity and change in oxytocin across the session, as hypothesized, change in oxytocin would be examined as a mediator between the age × condition interaction and cortisol reactivity. Finally, regression analyses were conducted with self-reported stress reactivity (difference between stress level at arrival and during speech/math) and recovery (speech/math to end of session) as dependent variables and age group, sex, and condition (and their interactions) to determine whether stress buffering acts by decreasing subjective feelings of stress.
Results
Cortisol
Tables 2 and 3 show the cortisol regression results. Higher pretest cortisol predicted lower reactivity, and more time since morning awakening also predicted lower reactivity. Sex and medication count did not predict reactivity. Likewise, neither age group nor condition yielded main effects. In step 2 of the regression, there was a significant age group by condition effect (see Figure 2). Follow-up analyses indicated that the effect of condition was significant in the 15–16 year olds, such that individuals in the friend condition showed greater reactivity than individuals in the parent condition, β = 0.33, t(54) = 2.63, p = 0.01. There was no effect of condition on cortisol reactivity in the 9–10 year olds, t(53) = −0.65, p = 0.52. The reactivity slope for each age × condition group was significantly greater than zero, ps < 0.01, indicating that each group showed significant cortisol reactivity to the TSST-M.
Table 2.
| Variable | B | SE(B) | β | t-value | p-value |
|---|---|---|---|---|---|
| Step 1 | |||||
| Pretest Cortisol | −0.10 | 0.03 | −0.37 | −3.85 | 0.00 |
| Med Count | −0.00 | 0.02 | −0.01 | −0.14 | 0.89 |
| Male | 0.00 | 0.02 | 0.02 | 0.22 | 0.83 |
| Time Since Wake | −0.00 | 0.00 | −0.31 | −3.26 | 0.00 |
| Age Group | 0.02 | 0.02 | 0.11 | 1.06 | 0.29 |
| Condition | 0.03 | 0.02 | 0.15 | 1.66 | 0.099 |
| Step 2 | |||||
| Pretest Cortisol | −0.10 | 0.03 | −0.40 | −4.17 | 0.00 |
| Med Count | 0.00 | 0.02 | 0.02 | 0.23 | 0.82 |
| Male | 0.00 | 0.02 | 0.03 | 0.32 | 0.75 |
| Time Since Wake | −0.00 | 0.00 | −0.33 | −3.53 | 0.00 |
| Age Group | 0.02 | 0.02 | 0.11 | 1.12 | 0.27 |
| Condition | 0.02 | 0.02 | 0.14 | 1.64 | 0.10 |
| Age × Condition | 0.03 | 0.02 | 0.21 | 2.33 | 0.02 |
Note. N = 109. Hierarchical linear regression results with cortisol reactivity (Mplus-generated) as the dependent variable. Age group was coded as −1 = 9–10 year olds, 1 = 15–16 year olds. Condition was coded as −1 = parent, 1 = friend.
Table 3.
| Variable | B | SE(B) | β | t-value | p-value |
|---|---|---|---|---|---|
| Step 1 | |||||
| Pretest Cortisol | 0.02 | 0.02 | 0.12 | 1.02 | 0.31 |
| Cortisol Reactivity | 0.10 | 0.06 | 0.17 | 1.57 | 0.12 |
| Time Since Wake | 0.00 | 0.00 | −0.11 | −1.05 | 0.30 |
| Med Count | 0.02 | 0.01 | 0.18 | 1.81 | 0.07 |
| Male | −0.01 | 0.01 | −0.15 | −1.53 | 0.13 |
| Age Group | −0.01 | 0.01 | −0.15 | −1.41 | 0.16 |
| Condition | −0.00 | 0.01 | −0.01 | −0.11 | 0.91 |
| Step 2 | |||||
| Pretest Cortisol | 0.02 | 0.02 | 0.12 | 1.07 | 0.29 |
| Cortisol Reactivity | 0.10 | 0.06 | 0.18 | 1.61 | 0.11 |
| Time Since Wake | −0.00 | 0.00 | −0.11 | −0.98 | 0.33 |
| Med Count | 0.02 | 0.01 | 0.17 | 1.72 | 0.09 |
| Male | −0.01 | 0.01 | −0.15 | −1.54 | 0.13 |
| Age Group | −0.01 | 0.01 | −0.15 | −1.41 | 0.16 |
| Condition | −0.00 | 0.01 | −0.01 | −0.12 | 0.91 |
| Age × Condition | −0.00 | 0.01 | −0.04 | −0.36 | 0.72 |
Note. N = 109. Hierarchical linear regression results with cortisol recovery (Mplus-generated) as the dependent variable. Age group was coded as −1 = 9–10 year olds, 1 = 15–16 year olds. Condition was coded as −1 = parent, 1 = friend.
Figure 2.
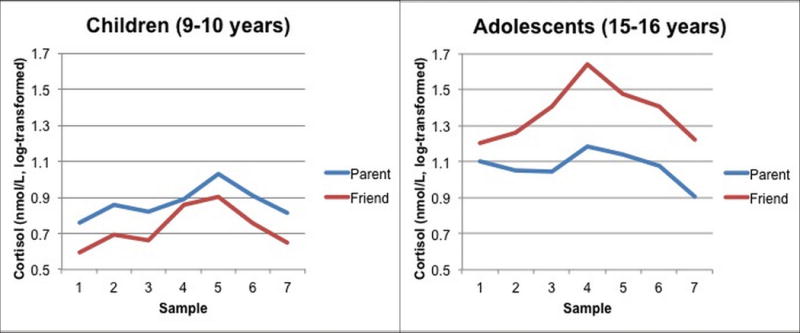
N = 109. Cortisol levels in log-transformed units by age group (9–10 years vs. 15–16 years) and speech preparation condition (parent vs. friend). Sample 1 was collected at the beginning of the speech preparation period, and samples 2–7 were collected every 10 minutes thereafter. Samples 4–6 represent the peak of cortisol production post-TSST that occurred 20–40 minutes after the onset of the TSST. Means for each group were calculated controlling for the effect of sex and time since wake. The effect of condition is significant in the adolescents age 15–16, p < 0.05, but not the children age 9–10, p > .10.
In the regression with cortisol recovery as the dependent variable (Table 3), there were no significant effects of medication count, age group, condition, sex, reactivity, pretest cortisol, and time since awakening. In addition, none of the interactions significantly predicted cortisol recovery.
Oxytocin
A repeated-measures ANCOVA was conducted with oxytocin at pretest (Time 1) and posttest (Time 2) corrected for fluid volume (pg/mg creatinine) to examine within- and between-subjects differences by sex, condition, and age group, and all 2-way and 3-way interactions. Overall, there was a main effect of age with adolescents producing less oxytocin than children (see Table 4, Figure 3). There was also a significant main effect of trials with oxytocin decreasing from pre- to post-test. However, this trials effect was qualified by significant trials by condition and trials by sex by condition effects (Table 4 and Figure 4). As the age × condition interaction did not significantly predict change in oxytocin, it was not examined as a potential mediator between the age × condition interaction and cortisol reactivity.
Table 4.
| Variable | Mean Square | F-value | p-value |
|---|---|---|---|
| Within-Subjects | |||
| Time | 1.98 | 5.57 | 0.02 |
| Time × Condition | 2.60 | 7.29 | 0.01 |
| Time × Male | 0.88 | 2.46 | 0.12 |
| Time × Age Group | 0.08 | 0.22 | 0.64 |
| Time × Condition × Male | 1.55 | 4.36 | 0.04 |
| Time × Condition × Age Group | 0.00 | 0.00 | 0.98 |
| Time × Male × Age Group | 0.01 | 0.02 | 0.89 |
| Between-Subjects | |||
| Male | 7.95 | 7.15 | 0.01 |
| Age Group | 11.90 | 10.71 | 0.00 |
| Condition | 2.83 | 2.54 | 0.11 |
| Male × Condition | 0.00 | 0.00 | 0.98 |
| Male × Age Group | 1.36 | 1.22 | 0.27 |
| Condition × Age Group | 0.22 | 0.20 | 0.66 |
Note. N = 95. Within- and between-subjects results of the repeated measures ANCOVA results with log-transformed oxytocin at pre- and posttest as the repeated measures.
Figure 3.
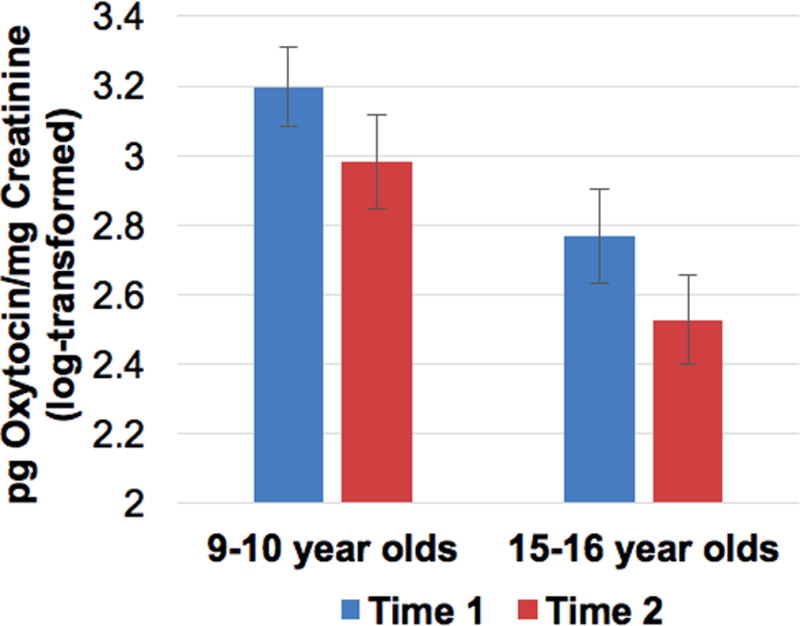
Oxytocin adjusted for urine volume (pg oxytocin/mg creatinine, log-transformed) 30 minutes after arrival (Time 1) and at the end of the session (Time 2) by age group.
Figure 4.
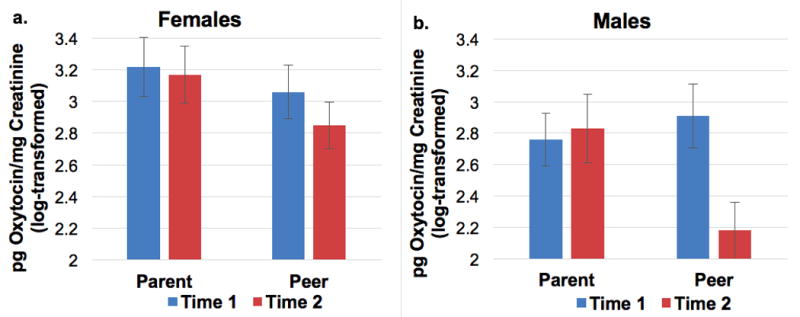
Oxytocin adjusted for urine volume (pg oxytocin/mg creatinine, log-transformed) 30 minutes after arrival (Time 1) and at the end of the session (Time 2) by condition for females (panel a) and males (panel b).
Self-Reported Stress
With regard to self-reported stress reactivity between arrival and speech/math, neither sex, age group, condition, nor their interactions predicted differences in reported stress that might account for the cortisol reactivity findings, ps > .10. However, there was a significant increase in self-reported stress overall with participants on average reporting a 2.65-point increase (SD = 1.08) from arrival to speech/math. Similarly, self-reported stress recovery from speech/math to the end of the session was not predicted by sex, age group, condition, or the interactions, ps > .10. Only self-reported stress reactivity predicted the amount of self-reported recovery from the stressor, with higher reactivity linked to greater recovery, β = −0.39, t(108) = −4.39, p < .001. [These analyses are available by request.]
Discussion
The results of the current study demonstrate that parents and friends have similar ability to buffer the HPA axis from a psychosocial stressor at 9–10 years of age, but by 15–16 years of age, the effects of parents and friends diverge. Specifically, having a same-sex friend help adolescents prepare for a social-evaluation stressor increased cortisol reactivity to the stressor compared to having a parent help them prepare. In addition, preparing for the speech with a parent resulted in higher levels of urinary oxytocin than preparing with a friend. This was true at both ages, although with age pretest oxytocin levels were lower. These effects were not due to different self-reported experiences of the stressfulness of the task as a function of preparation with a parent versus a friend for children and adolescents. Thus, consistent with much of the social stress buffering literature (Hostinar, Sullivan, & Gunnar, 2013), social partners modify physiological response to the psychological experience of stress.
Although we hypothesized that friends could potentially buffer the HPA axis, particularly for adolescents, we found the opposite. Preparing for this social evaluation task with a friend markedly amplified the HPA response to stress for adolescents and decreased the overall amount of oxytocin produced, regardless of age. There could be a number of possible explanations for these findings. First, researchers have observed a general increase in stress sensitivity, including cortisol reactivity, in adolescence that is closely related to pubertal development (Sumter, Bokhorst, Miers, Van Pelt, & Westenberg, 2010). Peers may further increase this sensitivity post-puberty as their salience increases across adolescence (Nelson et al., 2005). Thus, planning a self-focused speech with a close friend during adolescence appears to make the social evaluative nature of the stressor more potent and the stress system more sensitive to this threat, contrary to the hypothesis that buffering from stress may switch from parents to friends across puberty. Interestingly, studies in adults have demonstrated that friends and romantic partners are able to buffer the autonomic nervous system and HPA axis responses to stress (Fontana, et al., 1999; Kirschbaum, et al., 1995; Uno et al., 2002). Thus, adolescence might be a time between childhood and adulthood when friends do not buffer the response to a social evaluative stressor, but actually amplify it. This result is similar to studies of social buffering in adult females in the presence of their male romantic partners, who, unlike men with female romantic partners, show increased cortisol in response to the TSST (Kirschbaum et al., 1995). It may be that similar stress sensitization may be operating in adolescents in the presence of friends, potentially due to increased salience of friends and their friends’ opinions.
The increased cognitive ability of adolescents compared to children may allow for greater reflection and rumination about the meaning of a social evaluative task, which may increase anxiety about the task and potentially increase cortisol reactivity (Adam, 2006; Nelson et al., 2005; Westenberg et al., 2004, 2007). This may be amplified in the presence of a salient social figure. In addition to increased cognitive ability, heightened emotional reactivity that has been consistently reported in adolescents may further intensify negative emotions surrounding social evaluation (Dahl & Gunnar, 2009). Increased emotional reactivity, greater cognitive ability to reflect on social evaluation, and an amplified HPA response to social stress in the presence of peers may be related to the increased vulnerability of adolescents to emotional disorders and substance abuse (Paus et al., 2008; Spear, 2009). The dramatic shift in the social context of adolescence towards independence and greater interaction with peers may place an enhanced meaning on social interactions and increase the amount of time that adolescents spend in these peer environments with high levels of perceived social evaluation (Nelson et al., 2005; Pine et al., 1998; Steinberg & Morris, 2001).
Interestingly, preparing with friends before the TSST did not stimulate oxytocin production. In fact, oxytocin production decreased across the task when the participants prepared with a friend. When they prepared with the parent oxytocin levels did not decline. Thus, it was not that preparing with the parent increased oxytocin production as much as preparing with the friend inhibited the production of this anti-stress hormone. Our measure of oxytocin in urine does not allow us to make direct comparisons to oxytocin present in the central nervous system, although oxytocin is excreted into the urine between 30 minutes to one hour after release. Indeed, it has been noted that nasal oxytocin is capable of markedly reducing cortisol increases to the TSST among adults. The oxytocin system has been shown to be protective against acute and chronic stress, often by promoting antioxidant and anti-inflammatory processes (Gutkowska & Jankowski, 2012; Szeto et al., 2008). We found no evidence that urinary levels of oxytocin statistically mediated age, sex or condition effects on cortisol responses in our participants. However, as cortisol and oxytocin have different time courses for response to acute stress, and the development of the HPA and oxytocin systems may proceed at different rates over time, this conclusion should be viewed cautiously.
It is important to note that the paradigm used in the current study is different from the one used in our earlier studies (Doom et al., 2015; Hostinar et al., 2015), as in the earlier paradigm, participants came to the lab with only their primary caregiving parent and only directly interacted with their parent and the experimenter throughout the session. In this paradigm, children and adolescents came with their friend and their parent, and they interacted with their friend and parent both before and after the TSST. This may help explain why our parent findings differed from previous studies. Specifically, unlike previously, we obtained a statistically significant elevation in cortisol among the 9- and 10-year-olds, although the response was fairly small. It may be that having a friend come to the session, even if they did not help you prepare, made the whole session more arousing for the children and adolescents. Indeed, it is critical for interpretation to recognize that in this paradigm, parent and friends are with the participants from arrival until speech preparation and speech/math delivery and then they are present with the participant once the task is over and throughout recovery. The only time that differs is the five minutes of speech preparation when the participant is either with the parent or the friend and the time during the speech and math when the participant is alone with the assessors. Interestingly, social buffering effects have been observed when the supportive figure is present before, during, and after the stressor (e.g., Calhoun et al., 2014; Coan, Schaefer, & Davidson, 2006; Hostinar et al., 2015), but the time course of these effects (reactivity vs. recovery) likely differs depending on the timing of supportive figure’s presence.
In addition, the results of the present study should temper our view of whether parents can provide a stress-buffering role in adolescence. In this study parents of adolescents were certainly more effective than friends in reducing, or at least not amplifying, the cortisol response. Parents were also associated with higher urinary oxytocin levels than were friends at both ages. Again, this difference in oxytocin was due to the few minutes of speech preparation when the paradigm differed for the two conditions. Thus, this study may be only the tip of the iceberg regarding differences in oxytocin production during stress when children and adolescents have access to attachment figures. These results strongly suggest that parents continue to be capable of providing stress-protecting effects, at least in the oxytocin system, well into the adolescent period.
The sex differences in oxytocin production in both childhood and adolescence are intriguing considering a vast literature on sex differences in mental and physical health problems across the lifespan. Finding that even in childhood, social challenge is associated with lower oxytocin production in males than females might suggest that oxytocin should be targeted as a potential contributor to sex differences in mental and physical health outcomes in response to stress. Oxytocin has been associated with more social, passive coping strategies in the face of stress, which some theorize is directly related to sex differences in coping strategies between men and women (e.g., tend and befriend vs. fight or flight; Carter, 2007; Taylor et al., 2000). Our finding that females produce more oxytocin than males in response to social evaluation in childhood and adolescence further supports the idea that there are significant sex differences in oxytocin responses to stress at multiple points in development.
The study did have limitations that must be considered. First, we had half of participants of each sex within each age by condition group. Due to low power, we should be cautious about the lack of any interaction effects of sex in the cortisol results. Future research must examine whether there are sex differences in peer and parental social buffering before and after puberty. Second, it must also be noted that we asked for the primary caregiver to attend the session with the child. Most of the time, the mother accompanied the participant, but it was the father in 13 cases. Sex of the parent did not differ across sex, age group, or condition, so our results cannot be attributed to having the mother versus father attend, but it would be interesting to examine how mothers and fathers may differentially impact social buffering. Third, regardless of condition, children and adolescents were with their parent and friend for 30 minutes before speech preparation and again after the TSST-M until the end of the session. This time spent with their parent and friend may have influenced cortisol levels throughout the session. However, it is interesting to note the effects on cortisol and oxytocin observed when the only difference between the parent and friend conditions was the 5-minute speech preparation period. Finally, there are different TSST protocols that have been used in the literature, and these might lead to differences in cortisol reactivity and recovery. In this study, the participants were asked to give a speech about their good and bad qualities (self-referential speech), while in other speech-stems the participant does not talk about themselves. It might be especially threatening to have a friend help you prepare a self-referential speech because doing so, by definition, involves the friend evaluating the participant. In the present version of the TSST there were no judges in the room with the participant, and although each group showed significant cortisol reactivity, cortisol levels could be different in a protocol where judges are in the room. Future research should explore this possibility. Finally, it is possible that these findings are particular to social evaluative stressors. Having a friend or your parent present during other types of stressors, such as facing a painful medical procedure or entering a new situation, might have different social stress buffering effects. This needs to be explored.
As higher early adolescent friendship quality has been shown to predict better physical health in adulthood (Allen, Uchino, & Hafen, 2015), the association between adolescent peer relationships and stress reactivity must be examined longitudinally to understand whether stress reactivity may mediate the association between child/adolescent friendship and later physical and mental health. In addition, longitudinal studies across childhood, adolescence, and adulthood should investigate when friends become effective social buffers. In adults, there is a great deal of evidence that friends and romantic partners can buffer the HPA response to social evaluation. These findings indicate that at ages 15–16, adolescents have not yet transitioned to using friends as a social buffer, and studies that target at what point friends become effective buffers are greatly needed. Increased HPA reactivity to social evaluative threat, especially in the presence of peers, may contribute to heightened risk for stress-related psychopathology in adolescence (Costello, Copeland, & Angold, 2011), so understanding when friends can block HPA activation is crucial. It is also unknown what mechanisms may underlie the shift from enhanced to attenuated reactivity in the presence of friends. Although the sex of the child/adolescent and the context appear to affect oxytocin production, the lack of change between childhood and adolescence for oxytocin production in response to stress signals that other neurobiological systems may be associated with the shift in social buffering of the HPA axis over time. There is strong evidence in the animal literature that oxytocin in the PVN mediates the social buffering effect (Smith & Wang, 2013), and this has not been examined in humans, so it could be that peripheral oxytocin does not accurately reflect oxytocin and CRH interactions in the PVN and that there could be a role for oxytocin in developmental shifts in social buffering. Future studies should examine social buffering in contexts other than social evaluation to understand whether parents and friends may be helpful or stress provoking in the face of other challenges. Research on both acute and long-term effects of social relationships on the HPA and oxytocin systems may inform interventions that improve mental and physical health, especially in the face of chronic or severe life stress.
Acknowledgments
The authors were supported by the following grants: an Interdisciplinary Doctoral Fellowship and a Doctoral Dissertation Fellowship from the University of Minnesota to JR Doom, and a University of Minnesota Clinical and Translational Science Institute (CTSI) Advanced Research Fellowship grant to CM Doyle. This research was supported by funds from the Canadian Institute for Advanced Research Experience-based Brain and Biological Development Program, NICHD HD075349, and NSF BCS-1439258 to MR Gunnar. In addition, this research was supported by an APA Dissertation Research grant, APA Division 7 Dissertation Research grant, University of Minnesota Women’s Philanthropic Leadership Circle grant, and a University of Minnesota Institute of Child Development small grant to JR Doom. The conference that occasioned this special issue was supported by NSF grant BCS-1439258. The authors would like to thank Bonny Donzella, MA, Zamzam Ahmed, Sydney Pauling, Kaylee Broussard, Clare Tollefson, Brittany Smith, and Jenna Allard for their assistance with the study. We would also like to thank Dante Cicchetti, PhD, Michael Georgieff, MD, and Jonathan Gewirtz, PhD, for their input on the study design.
The authors were supported by the following grants: an Interdisciplinary Doctoral Fellowship and a Doctoral Dissertation Fellowship from the University of Minnesota to JR Doom. This research was supported by funds from the Canadian Institute for Advanced Research Experience-based Brain and Biological Development Program, NICHD HD075349, and NSF BCS-1439258 to MR Gunnar. In addition, this research was supported by an APA Dissertation Research grant, APA Division 7 Dissertation Research grant, University of Minnesota Women’s Philanthropic Leadership Circle grant, and a University of Minnesota Institute of Child Development small grant to JR Doom. The conference that occasioned this special issue was supported by NSF grant BCS-1439258.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adams RE, Santo JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2012;47:1786–1791. doi: 10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Development. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Allen JP, Uchino BN, Hafen CA. Running with the pack: Teen peer-relationship qualities as predictors of adult physical health. Psychological Science. 2015;26:1574–83. doi: 10.1177/0956797615594118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Gessa GL. Central function of oxytocin. Neuroscience and Biobehavior. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauth W, Hellhammer DH. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, Prinstein MJ. Relational victimization, friendship, and adolescents’ hypothalamic–pituitary–adrenal axis responses to an in vivo social stressor. Development and Psychopathology. 2014;26:605–618. doi: 10.1017/S0954579414000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo CA. Self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. The chemistry of child neglect: Do oxytocin and vasopressin mediate the effects of early experience? Proceedings of the National Academy of the Sciences. 2005;102:18247–18248. doi: 10.1073/pnas.0509376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Cauce AM. Social networks and social competence: Exploring the effects of early adolescent friendships. American Journal of Community Psychology. 1986;14:607–628. doi: 10.1007/BF00931339. [DOI] [PubMed] [Google Scholar]
- Chibbar R, Toma J, Mitchell B, Miller F. Regulation of neural oxytocin gene expression by gonadal steroids in pubertal rats. Molecular Endocrinology. 1990;4:2030–2038. doi: 10.1210/mend-4-12-2030. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Copeland W, Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? Journal of Child Psychology and Psychiatry. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana AM, Diegnan T, Villeneuve A, Lepore S. Nonevaluative social support reduces cardiovascular reactivity in young women during acutely stressful performance situations. Journal of Behavioral Medicine. 1999;22:75–91. doi: 10.1023/a:1018751702934. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science. 2014;25:2067–78. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35(7):1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss KA, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. Oxytocin re-visited: its role in cardiovascular regulation. Journal of Neuroendocrinology. 2011;24:599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- Harris JR. Where is the child’s environment? A group socialization theory of development. Psychological Review. 1995;102:458–489. [Google Scholar]
- Hartup WW. The company they keep: Friendships and their developmental significance. Child Development. 1996;67:1–13. [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–82. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science. 2015;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychological Bulletin. 2013;140(1):256–82. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter FT, Youniss J. Changing functions of three relationships during adolescence. Developmental Psychology. 1982;18:806–811. [Google Scholar]
- Juster RP, Perna A, Marin MF, Sindi S, Lupien SJ. Timing is everything: Anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress. 2012;15:569–577. doi: 10.3109/10253890.2012.661494. [DOI] [PubMed] [Google Scholar]
- Karelina K, DeVries AC. Modeling social influences on human health. Psychosomatic Medicine. 2011;73:67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levels of acetylcholinesterase, monoamines and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behavioral Neuroscience. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Research. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: Reliability and validity of a self-report measure. Journal of Youth and Adolescence. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biological Psychiatry. 2013;76(3):281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: the differential function of emotional expression. International Journal of Behavioral Development. 1998;22:681–706. [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, Westenberg PM. Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35:1510–1516. doi: 10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. American Journal of Physiology: Endocrinology and Metababolism. 2008;295(6):E1495–501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Uno D, Uchino BN, Smith TW. Relationship quality moderates the effect of social support given by close friends on cardiovascular reactivity in women. International Journal of Behavioral Medicine. 2002;9:243–262. doi: 10.1207/s15327558ijbm0903_06. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Van den Bos E, de Rooij M, Miers AC, Bokhorst CL, Westenberg PM. Adolescents’ increasing stress response to social evaluation: pubertal effects on cortisol and alpha-amylase during public speaking. Child Development. 2013;85(1):220–236. doi: 10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- van Eerdenburg FJ, Poot P, Molenaar GJ, van Leeuwen FW, Swaab DF. A vasopressin and oxytocin containing nucleus in the pig hypothalamus that shows neuronal changes during puberty. Journal of Comparative Neurology. 1990;301:138–146. doi: 10.1002/cne.903010113. [DOI] [PubMed] [Google Scholar]
- Weems CF, Costa NM. Developmental differences in the expression of childhood anxiety symptoms and fears. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:656–663. doi: 10.1097/01.chi.0000162583.25829.4b. [DOI] [PubMed] [Google Scholar]
- Westenberg PM, Drewes MJ, Siebelink BM, Treffers PDA. A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? Journal of Child Psychology & Psychiatry. 2004;45:481–496. doi: 10.1111/j.1469-7610.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Westenberg PM, Gullone E, Bokhorst CL, Heyne DA, King NJ. Social evaluation fear in childhood and adolescence: Normative developmental course and continuity of individual differences. British Journal of Developmental Psychology. 2007;25:471–483. [Google Scholar]
- Witt DM, Winslow JT, Insel T. Enhanced social interaction in rats following chronic, centrally infused oxytocin. Pharmology and Biochemical Behavior. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayawaka CM. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 2010;35:241–248. doi: 10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gringrich BG, Insel TR. Cellular mechanisms of social attachment. Hormones and Behavior. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oepdipus. Hormones and Behavior. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]


