Abstract
Cocaine use is prevalent among HIV-infected individuals. While cross-sectional studies suggest that cocaine users may be at increased risk for depression, long-term effects of cocaine on depressive symptoms remain unclear. This is a longitudinal study of 341 HIV-infected and uninfected men (135 cocaine users and 206 controls) ages 30 to 60 enrolled in the Multicenter AIDS Cohort Study during 1996–2009. The median baseline age was 41; 73% were African-American. In mixed-effects models over a median of 4.8 years of observation, cocaine use was associated with higher depressive symptoms independent of age, education level, and smoking (n=288; p= 0.02); HIV infection modified this association (p= 0.03). Latent class mixed models were used to empirically identify distinct depressive trajectories (n=160). In adjusted models, cocaine use was associated with 3-fold increased odds of membership in the class with persistent high depressive symptoms (95% confidence interval (CI) 1.38–6.69) and 8-fold increased odds (95% CI (2.73–25.83) when tested among HIV-infected subjects only. Cocaine use is a risk factor for chronic depressive symptoms, particularly among HIV-infected men, highlighting the importance of integrating mental health and substance use treatments to address barriers to well-being and successful HIV-care.
Keywords: HIV, cocaine, crack cocaine, depression, cognitive function
Background
Cocaine use is prevalent among HIV-infected populations and has been associated with lower rates of viral suppression, faster decline of CD4 cell counts, and increased AIDS-related morbidity (1–8). Among 3000 HIV-infected individuals receiving care in United States (U.S.) cities, 8.5% reported using crack cocaine and recent use was associated with detectable viral loads and nonadherence to antiretroviral therapy (ART) (4). While white Americans are more likely to report lifetime cocaine use (powder or crack cocaine) than black or Hispanic Americans, black Americans report more crack cocaine use than white or Hispanic Americans (9). The high reporting of crack cocaine use is particularly evident among impoverished black communities in the U.S. South (10, 11), an area that comprises a disproportionate share of new HIV infections and deaths among persons living with HIV (11–14). Moreover, crack compared to powder cocaine is specifically associated with an immediate euphoric effect, greater risk for dependence, compulsive use, and faster HIV disease progression (2, 15); controversial laws mandating unequal prison sentences for crack cocaine relative to powder cocaine use remain in place, and continue to impact black and economically disadvantaged Americans disproportionately (9).
Cocaine users, including individuals using crack cocaine, are more likely to engage in high-risk behaviors linked to increased HIV transmission rates including unsafe sexual and needle-sharing behaviors and ART non-adherence (4, 8, 16–21). Among HIV-infected cohorts, living alone or with someone other than a spouse or partner, was predictive of screening positive for drug dependence, which may result in part from factors that include difficulty sustaining relationships or successfully living with others (1); among crack users, homelessness was associated with reduced pharmacy refills for ART despite access to free medication (19). Cocaine use may influence behavior by increasing impulsivity and reducing inhibitory control, reasoning, planning, and emotional regulation (22–26), suggesting that understanding the effects of cocaine use, particularly crack cocaine, on mood and cognitive function in HIV-infected individuals is important for the development of policies and HIV-care programs that enhance access to appropriate care providers, improve prevention and disease management, and increase individual well-being (27).
Major depressive disorders and cognitive impairment are common co-morbidities in people living with HIV infection that have been associated with poor health outcomes (1, 4, 27–30). In a U.S.-based national survey, people living with HIV infection were five times more likely to report a diagnosis of major depression compared to similar survey responses in the general population; increased likelihood of screening positive for a psychiatric disorder was greater among persons who were unemployed, disabled, and living alone (1). Additionally, mental health, including depression, has a major influence on sexual risk behavior and HIV infection across several studies (31, 32), and is of particular concern among men who have sex with men (MSM), especially in racial/ethnic minority communities including black men (32, 33).
Chronic cocaine use has been associated with greater depressive symptoms (27, 34) and cognitive impairments in domains that include attention, executive function, and verbal learning/memory (23, 28, 35, 36). In the general population, cocaine users who report high depressive symptoms are also more likely to exhibit cognitive impairment and impulsivity (37), suggesting a multifaceted relationship between depression and cognitive function in the context of cocaine use. Chronic cocaine exposure has neurotoxic effects that include alterations in glutamate levels in brain regions such as prefrontal cortex, and mesolimbic structures such as ventral tegmentum and nucleus accumbens (38). Decreases in functional connectivity between prefrontal cortex and underlying subcortical limbic structures among daily or weekly cocaine users suggests that neuroadaptive changes lead to large-scale alterations in brain circuits engaged in emotional regulation (39). While cross-sectional studies suggest that HIV-infected cocaine users may be at greater risk for depression and cognitive impairment, longitudinal studies using matched controls are limited (11, 28, 40–43).
The relative contributions of all factors including psychosocial and social contextual (including stigma), behavioral (including drug use), and HIV-related host factors in the pathogenesis of depression in HIV-positive persons require deeper understanding (44–46). Given the integrated prefrontal and limbic circuitry involved in both depression and neurocognitive impairment influenced by drug use and HIV infection, the purpose of the current study was to understand the influence of daily or weekly cocaine use and HIV infection on baseline and longitudinal reporting of depressive symptoms and cognitive trajectories. In a study of 341 HIV-infected and HIV-uninfected predominantly black men participating in the Multicenter AIDS Cohort Study (MACS) from 1996–2009, we used mixed-effects models to determine whether cocaine use (primarily crack cocaine) and HIV infection have independent effects on depressive symptoms based on the Center for Epidemiological Studies Depression Scale (CES-D) and cognitive scores over time. We then used latent class mixed models (LCMM) to empirically identify individuals with similar trajectories of depressive symptoms, and examined relationships between cocaine use, HIV infection, and persistently high depressive symptoms.
Methods
Participants and Data Collection
The MACS is an ongoing prospective study of the natural and treated histories of HIV infection in men who have sex with men. The study recruited men from four urban centers (Los Angeles, Chicago, Pittsburgh, and Baltimore/Washington D.C.) in three enrollment waves: 1984–1985, 1987–1991, and 2001–2003 with a focus on racial and ethnic minority recruitment during recruitment waves two and three; details of study design and enrollment patterns have been described elsewhere (47). The Institutional Review Board at each clinical site approved the study protocols, and informed consent was obtained from all participants. MACS participants return every six months for detailed interviews, physical examinations, and biological specimen collection. Interviews include questions about medical conditions and treatments, cigarette and alcohol consumption, and cocaine, marijuana, amphetamines/methamphetamine, heroin, 3,4-methylenedioxy-methamphetamine (MDMA)/3,4-Methylenedioxyamphetamine (MDA), and nitrite inhalant (poppers) use. Questions about sexual and drug use history are administered by audio computer assisted self-interview (ACASI). In 1988, the MACS began a neuropsychological substudy which includes neurocognitive tests evaluating multiple cognitive domains related to HIV-associated neurocognitive disorders; biannual testing for all parcipants began in 2005 (47). All data were obtained from the MACS public dataset (p23 release; http://www.ntis.gov/search/index.aspx) and translated to create a local SQL database.
Study Design
This longitudinal nested study was restricted to MACS participants ages 30 to 60, an age window with consistent data coverage for testing of depressive symptoms and cognitive performance, enrolled in the neuropsychological substudy between 1996 and 2009. Exclusion criteria included a diagnosis of central nervous system (CNS) toxoplasmosis or lymphoma, progressive multifocal leukoencephalopathy, or cryptococcal meningitis; participants who seroconverted during the study period were excluded. To evaluate cocaine-related effects on depression and cognitive performance, participants using daily marijuana, or daily or weekly amphetamine/methamphetamine, MDMA/MDA, or heroin were also excluded (n=38; 22% of heavy cocaine users). HIV-infected and HIV-uninfected cocaine users were matched to respective control groups (minimal or/no cocaine use) for baseline age, race, education level and hepatitis C virus (HCV)-antibody serostatus in a 1:2 ratio using the MatchIt package in R (Version 3.1); controls lacking cognitive test scores were excluded. The final cohort consisted of 341 men ages 30 to 60 years (n=135 cocaine users, n=206 controls).
Demographic and Clinical Covariates
Cocaine use was the primary exposure of interest. Subjects were classified based on self-reported frequency of cocaine use as heavy users (daily or weekly use for at least one year in the study interval) and compared to controls (monthly or no use at all visits). Eighty-four percent of heavy cocaine users reported crack cocaine use. Crack and powder cocaine were combined into a single cocaine exposure variable given insufficient statistical power to test their respective effects. Race and ethnicity was categorized as African-American/Black, White, and Other (Hispanic, Asian/Pacific Islander, American Indian/Alaskan Native). Education was categorized as ≤12 or >12 years of education; HCV status was categorized as HCV-positive (detectable antibody and/or RNA) or HCV-negative. Self-reported alcohol consumption of more than 14 drinks/week or 5 drinks/day at two visits in the study interval was classified as heavy drinkers and compared to moderate/nondrinkers. Self-reported tobacco cigarette use of ½ pack/day or more on two visits during the study interval was classified as heavy smokers and compared to light/nonsmokers (0 to < ½ pack/day).
HIV-Related Characteristics
At each six-month visit, HIV-uninfected participants were tested for HIV seroconversion to validate continued enrollment in the uninfected group. HIV-infected participants were tested for T-cell subsets using standardized flow cytometry, and plasma HIV RNA was quantified using the Roche Amplicor RNA kit (detection limit <400 copies/mL) or the Roche Ultrasensitive RNA PCR assay (detection limit <50 copies/mL) (34). Self-reported ART adherence was measured at baseline and categorized at ≥95% of visits in study.
Depression and Neurocognitive Scores
The CES-D scale includes 20 items measuring frequency of depressive symptoms ranging on a four-point rating scale with maximum score of 60; higher scores indicate more frequent depressive symptoms. CES-D scores can be separated into four subscales assessing: (1) depressed affect, (2) vegetative depression, (3) loss of wellbeing and (4) interpersonal relationships (48).
The neurocognitive battery included the Grooved Pegboard Test, Rey-Auditory Verbal Learning Test, Stroop Task, Trailmaking Test, Symbol-Digit Modalities Test, and California Computerized Assessment Package (49). Raw scores on neurocognitive tests were normalized by z-scoring, using the mean and standard deviation from education-matched HIV-uninfected controls aged 30 to 39 years from the MACS as the reference group. Reference norms from the MACS, as opposed to national reference norms, were used to account for biases typical for study recruitment procedures in HIV-seropositive cohorts (i.e. incentives may attract participants who are unemployed, have prior or current substance use, high prevalence of Hepatitis C or other coinfections) (50). The signs for some timed tests were transformed so that higher z-scores always denote better performance. Z-scores allow for neurocognitive tests to be on a common scale with approximately equal weighting for creation of composite domains and is a preferred method for examining longitudinal effects (51). The summary cognitive score (NPZ16) used to capture heterogeneity was derived from a battery of 16 individual cognitive tests testing verbal learning and memory, executive function, speed of processing and attention, and motor skills (Supplementary Table I).
Statistical Analyses
Simple univariate/bivariate tests were conducted using Wilcoxon Rank-Sum Test, paired t-test, Pearson χ2, or analysis of variance (ANOVA) for cohort characteristic comparisons (Table I) and baseline depression and cognitive analyses (Supplementary Table II). Longitudinal analyses of depressive trajectories and cognitive decline were conducted in subjects with ≥2 CES-D evaluations (n=288, average 9 time points/participant [range 2–14]) or neurocognitive scores (n=242, average 3 time points/participant [range 2–9]), respectively. The association between heavy cocaine use, HIV-infection, and change in depression scores was examined using mixed-effects models with interaction terms for HIV-infection with time, cocaine use with time, and their joint interaction with time. Baseline age at study entry was treated as a continuous variable; education, HIV infection, cocaine, and smoking were analyzed as binary covariates. Baseline age was calculated based on year of birth and study visit at entry, providing only an approximate but reasonably accurate assessment of age. While using baseline age and year after study entry provides an indirect measurement of a subject’s age (i.e. study increments occur in tandem with age within a subject), the age of each subject was not directly modeled. A backward elimination procedure (cutoff p< 0.05) was used on a large initial pool of fixed predictors and variances/covariances of random terms, as described (52). The association between covariates and the summary cognitive z-score was analyzed in a separate mixed-effects model. Random predictors were correlated intercepts and linear slopes of time. Analyses were performed using SAS PROC MIXED version 9.3 (SAS Institute, Cary, NC).
Table I.
Cohort characteristics by HIV Serostatus and Cocaine use
| HIV− (n =147) | HIV+ (r)=194) | p valuec | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls (n=94) | Cocainea (n=53) | Controls (n=112) | Cocainea (n=82) | HIV− controls vs. HIV− cocaine | HIV+ controls vs. HIV+ cocaine | |
|
| ||||||
| Baseline Age (years; mean, SD) | 41.8 (7.0) | 39.8 (6.3) | 39.0 (5.4) | 39.1 (4.8) | ns | ns |
|
| ||||||
| Follow-up (years; mean, SD) | 5.9 (3.1) | 6.1 (2.5) | 6.6 (3.7) | 6.5 (3.3) | ns | ns |
|
| ||||||
| Demographic and Clinical Characteristics (%) | ||||||
|
| ||||||
| Race | ns | ns | ||||
| Black | 79.8 | 67.9 | 72.3 | 70.7 | ||
| White, non-Hispanic | 18.1 | 20.8 | 21.4 | 22.0 | ||
| Other | 2.1 | 11.3 | 6.3 | 7.3 | ||
|
| ||||||
| Education < 12 years | 40.4 | 45.3 | 47.3 | 42.7 | ns | ns |
|
| ||||||
| HCV-antibody positive | 20.2 | 32.1 | 21.4 | 18.3 | ns | ns |
|
| ||||||
| Heavy Smokinga | 25.0 | 50.9 | 17.7 | 52.4 | <0.01 | <0.01 |
|
| ||||||
| Heavy Drinkinga | 19.1 | 47.2 | 18.1 | 42.7 | <0.01 | <0.01 |
|
| ||||||
| Depression Profile | ||||||
|
| ||||||
| Antidepressant usea | 16.0 | 26.4 | 18.8 | 50.0 | ns | <0.01 |
|
| ||||||
| CES-D score > 16b | 23.4 | 37.7 | 26.8 | 46.3 | ns | <0.01 |
|
| ||||||
| HIV-Related Characteristics (%) | ||||||
|
| ||||||
| Baseline CD4 count (cells/mm3) | ||||||
| < 200 | – | – | 17.0 | 15.9 | – | ns |
| < 350 | – | – | 33.0 | 30.5 | ns | |
|
| ||||||
| Baseline viral load (plasma HIV RNA, copies/mL) | ||||||
| > 400 | – | – | 56.3 | 63.4 | – | 0.02 |
|
| ||||||
| Self-reported antiretroviral therapy use | ||||||
| Baseline | – | – | 64.3 | 58.5 | – | ns |
| > 95% of visits in study | – | – | 42.0 | 26.8 | – | <0.01 |
- Reported heavy use at ≥ 2 visits in study (corresponding to at least one year); Cocaine use defined as daily or weekly; Smoking defined as ≥ ½ pack cigarettes/day; Drinking defined as >14 alcoholic drinks/week
- Reported at > 50% of visits in study
- Wilcoxon rank sum or paired t-test; p<0.05, significant
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; ns, nonsignificant; SD, standard deviation; HCV, Hepatitis C Virus.
Latent class mixed models (LCMM) is an analytical method that clusters subjects with similar trajectories into latent classes, maximizing homogeneity within and heterogeneity across groups (53–56). In LCMM, the latent variable represented the trajectory of depressive symptoms over six years. Optimal data coverage for LCMM was from participants between ages 37–60 for CES-D. Model fit was determined by Bayesian Information Criterion (BIC), value of group membership probability and average posterior probability (entropy statistic), and distribution of subjects across latent classes. Each subject was assigned exclusively to the latent class for which the participant had the highest posterior probability of membership. Subjects with maximum posterior probabilities <0.65 were not classified into trajectory groups to maintain sufficient internal reliability (57); we set an a priori requirement for at least 5% of the total number of subjects to be classified in a trajectory group for inclusion in subsequent logistic regression analyses, a decision based on review of publications excluding minor groups not meeting an a priori population size estimate (58). The CES-D score, coded such that lower scores corresponded to worse depressive symptoms, was transformed by taking the square root of the score (to reduce skewness). Models were fit and trajectories for each latent class were plotted with 95% confidence intervals (CIs) using the lcmm package and supersmooth function in R.
The best-fitting model for the entire sample was determined to be a 5 class model. A logistic regression model and stepwise automated selection was then performed to assess significant clinical, demographic, and substance-related predictors associated with persistent high depressive symptoms versus low/moderate depressive symptoms class membership. Candidate variables were selected for multivariate analysis with p<0.2 (Wald test). Descriptive statistics and logistic regression was conducted in GraphPad Prism and SAS.
Results
Study Cohort
Clinical characteristics of study participants by HIV status and cocaine use are shown in Table I (n=341; 194 HIV-infected, 147 HIV-uninfected men). The median age was 42 years at study entry (IQR 37–45), and the majority were black (73.3%); HCV seropositivity was common (22%). After exclusions for using other drugs of abuse in subjects reporting daily or weekly cocaine use, crack cocaine was the most commonly used form of cocaine (84.8%). Cocaine use was associated with baseline heavy smoking and alcohol use (p<0.01). Groups were similar with respect to baseline age, race, education, HCV serostatus and length of follow up. While HIV-infected cocaine users had similar baseline CD4 cell counts, and CD4 nadir during follow-up compared to controls (Table I), users were more likely to have plasma HIV RNA ≥400 copies/mL (p=0.02) and report lower ART-adherence (p < 0.01).
Cocaine Use, HIV status, and Depressive Trajectories
HIV-infected cocaine users were more likely than HIV-infected controls to have CES-D scores ≥16, a cutoff suggestive of high depressive symptoms (p <0.01, Table I), and nearly half reported using an antidepressant at the baseline visit. Among all participants, HIV-infected cocaine users reported higher mean depression scores at baseline compared to any other group(p=0.03, Supplementary Table II). Using mixed-effects models, we investigated the association between cocaine use and HIV infection on baseline depressive symptoms and longitudinal depressive trajectories over an average of 4.8 years (±1.5 years) in participants with repeat CES-D scores (n=288; HIV+ cocaine nonusers n=125, HIV+ cocaine users n=37, HIV− cocaine nonusers n=100, HIV− cocaine users n=26). While heavy cocaine use was associated with a small increase in baseline depressive symptoms compared to nonusers (estimate 0.4314, p= 0.02), the modifying effect of HIV infection was associated with substantially higher depressive symptoms in cocaine users (estimate 5.7119, p= 0.03), independent of age, education level and cigarette smoking (Table II). Model estimates for time-related effects predicted an annual worsening of depression scores among cocaine users (estimate 0.017, p=0.03), with relative improvement over baseline in depressive symptoms for HIV-infected cocaine users (estimate −1.101, p= 0.02). Older age correlated with lower depressive symptoms; higher depressive symptoms correlated with lower education (Table II).
Table II.
Effect of Cocaine use and HIV infection on CES-D and Neurocognitive Scores
| CES-D (actual score; n=288) | Neurocognitive Summary (NPZ16; n=242) | |||||
|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | SE | p-value | Estimate | SE | p-value |
| Baseline age | −0.2754 | 0.1052 | 0.009 | −0.00250 | 0.006183 | 0.687 |
| Education < 12 years | 2.4459 | 1.1058 | 0.028 | 0.1064 | 0.08034 | 0.187 |
| Cocaine Use | 0.4314 | 2.0732 | 0.018 | −0.2806 | 0.1435 | 0.069 |
| HIV Infection | −0.6870 | 1.2460 | 0.105 | 0.08344 | 0.08905 | 0.039 |
| Smoker | 1.5933 | 1.1502 | 0.167 | 0.002895 | 0.08344 | 0.972 |
| Cocaine * HIV Infection | 5.7119 | 2.6552 | 0.032 | 0.2151 | 0.1836 | 0.243 |
| Years in Study | −0.1792 | 0.1730 | 0.004 | 0.04574 | 0.01262 | <0.001 |
| HIV Infection* Years in Study | 0.1884 | 0.2296 | 0.134 | −0.03825 | 0.01655 | 0.020 |
| Cocaine * Years in Study | 0.01700 | 0.3755 | 0.028 | 0.01033 | 0.02730 | 0.570 |
| Cocaine * HIV Infection * Years in Study | −1.1010 | 0.4823 | 0.023 | −0.00163 | 0.03340 | 0.961 |
Negative years in study estimates indicate a steeper slope of cognitive decline.
Abbreviations: CES-D, Center for Epidemiological Studies Depression Scale; SE, standard error; *, interaction term
Cocaine use, HIV status, and Cognitive Trajectories
Mean baseline raw scores for each cognitive test by group are listed in Supplementary Table II. There were no significant group differences observed in the z-scores for individual cognitive tests when all four groups were compared (HIV+ cocaine nonusers, HIV+ cocaine users, HIV− cocaine nonusers, HIV− cocaine users, two-way ANOVA and in pairwise comparisons between HIV− controls vs. HIV− cocaine users or HIV+ controls vs. HIV+ cocaine users; Supplementary Table II). In mixed-effects models among participants with at least two available neurocognitive tests (n=242; HIV+ cocaine nonusers n=101, HIV+ cocaine users n=35, HIV− cocaine nonusers n=83, HIV− cocaine users n=23, Table II) analyzed over an average of 4.1 years (± 1.7 years), cocaine use was marginally associated with worse neurocognitive summary scores at baseline (estimate − 0.28, p=0.07). While there was annual improvement in cognitive scores among HIV− uninfected controls, suggestive of a practice effect in this subgroup (estimate 0.04574, p <0.001), cocaine use was not associated with a change in cognitive performance over time (p=0.57). The annual improvement in cognitive scores observed in HIV− uninfected controls was attenuated among HIV-infected participants (estimate −0.03825, p= 0.02), and not further modified by cocaine use (p= 0.9).
Latent Class Analysis of Depressive Trajectories
Latent class analysis of longitudinal CES-D scores allowed for the examination of individual variability in depressive symptoms and potential identification of subgroups at high risk for persistent depressive symptoms. LCMM analysis clustered into five classes based on BIC and mean posterior probabilities; minor groups that did not meet the a priori defined population threshold (i.e. at least 5% of the original population) or posterior probability thresholds were removed from subsequent analysis (Supplementary Table III). Figure 1A shows the mean depressive trajectories and 95% confidence intervals of the remaining classes which made up 21%, 34%, and 37% of the total cohort analyzed (n=160). The largest cluster consisted of 66 subjects who exhibited persistently high depressive symptoms (i.e. CES-D ≥ 16) during the six years of follow up (Figure 1A and B).
Figure 1. Trajectories of Depressive Symptoms in HIV-Infected and HIV-Uninfected Participants Using Latent Class Mixed-effects Models.
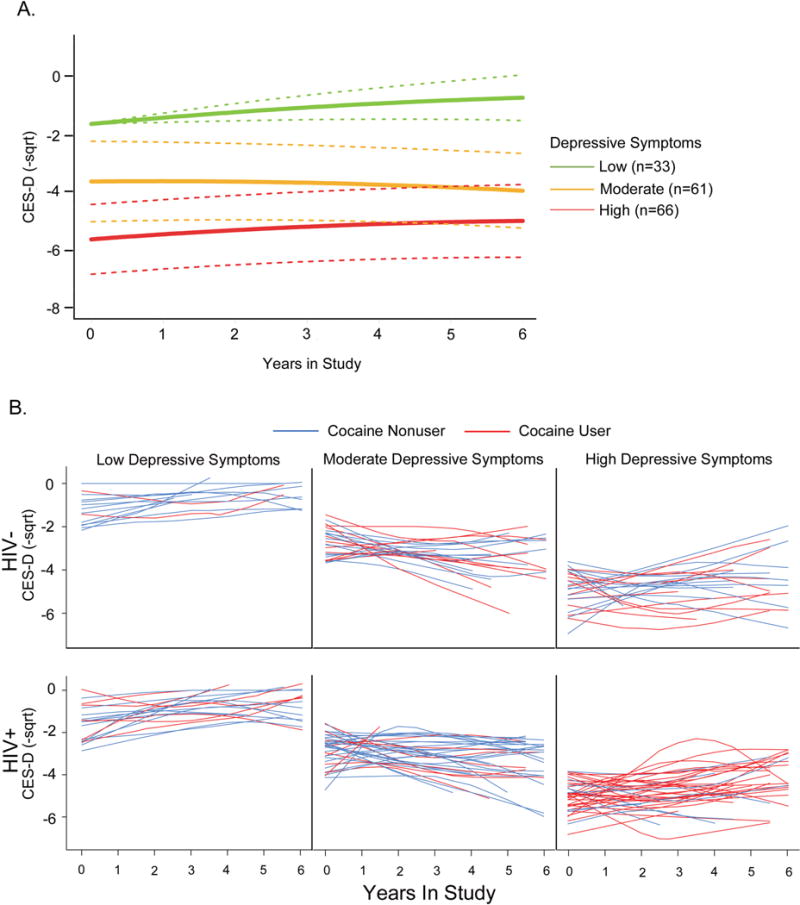
CES-D scores were square-root transformed and sign changed to negative values, so lower scores indicate worse depressive symptoms (i.e. CES-D square root values closer to zero are indicative of less depressive symptoms), and LCMM trajectories were estimated for the last six years enrolled in study for ages 37–60. The three group trajectories of CES-D performance that passed the posterior probability and population size thresholds are shown in Figure 1A. Individual depressive trajectories of cocaine users (red) and non-users (blue) are plotted, and separated by HIV-infection status (Figure 1B).
Using univariate and multivariate logistic regression, we evaluated predictors of persistently high depressive symptoms versus the remaining two latent classes (HIV+ cocaine nonusers n=70, HIV+ cocaine users n= 26, HIV− cocaine nonusers n= 50, HIV− cocaine users n=14, Table III). In univariate analysis, cocaine use (OR 3.71 [95% CI 1.75–7.88]), lower education (odds ratio (OR) 2.75 [95% CI 1.43–5.28]) and heavy drinking (OR 2.40 [95% CI 1.20–4.79]) were associated with high depressive symptoms among all participants. Cocaine use remained a predictor of high depressive symptoms after adjusting for lower education and heavy drinking (OR 3.04 [95% CI 1.38–6.69]). To investigate potential effects of HIV-disease characteristics on high depressive symptoms, we performed a secondary analysis among HIV-infected subjects only. In multivariate analysis, heavy cocaine use and baseline plasma viral load ≥ 400 copies/mL was associated with 8-fold [OR 8.41 (95% CI (2.73–25.83)] and 3-fold [OR 3.07 (95% CI (1.10–8.56)] higher odds of persistently high depressive symptoms, respectively. Figure 1B shows CES-D trajectories of individuals within these three latent classes, stratified by HIV and cocaine use, and illustrates the high prevalence of HIV-infected cocaine users in the predicted high depressive symptom group.
Table III.
Predictors of Depressive Trajectories in Latent Class Mixed Effects Models.
| CES-D | All participants (n=160) | HIV+ participants (n=86) | ||
|---|---|---|---|---|
| Unadjusted OR (95%Cl), p-value | Adjusted OR (95%Cl), p-value | Unadjusted OR (95%Cl), p-value | Adjusted OR (95%Cl), p-value | |
| Baseline Age | 0.96 (0.89–1.03), 0.30 | 0.89 (0.79–1.00), 0.05 | ||
| Educationa | 2.75 (1.43–5.28), <0.01 | 2.50 (1.26–4.97), <0.01 | 2.32 (0.99–5.40), 0.05 | |
| Cocaine useb | 3.71 (1.75–7.88), <0.001 | 3.04 (1.38–6.69), <0.01 | 5.92 (2.17–16.14), <0.001 | 8.41 (2.73–25.83), <0.001 |
| Heavy Drinking | 2.40 (1.20–4.79), 0.01 | 2.19 (1.04–4.59), 0.04 | 2.56 (1.03–6.36), 0.04 | |
| HIV Infection | 1.16 (0.61–2.21), 0.64 | |||
| Viral Load ≥ 400c | 1.79 (0.77–4.17), 0.17 | 3.07 (1.10–8.56), 0.03 | ||
| CD4 count < 350d | 1.06 (0.45–2.51), 0.88 | |||
Unadjusted and adjusted odds ratio (OR) for predictors of membership in the latent class representing persistent high depressive symptoms versus low/moderate symptoms
- OR for ≤12 years of education
- Daily/weekly use reported at first 2 visits in study interval
- Baseline viral load (plasma HIV RNA, copies/mL)
- Baseline CD4 count (cells/mm3)
Discussion
In this study, we used a prospective design with matched controls to evaluate the longitudinal effects of cocaine use on depressive symptoms and cognitive trajectories in a cohort of predominantly black men who have sex with men ages 30–60 years old. Using both mixed-effects models and subject-centered latent class analysis, we show that longitudinal depressive symptoms are influenced by the combined effects of cocaine use (primarily crack cocaine) and HIV infection. Heavy cocaine use was the most likely predictor for HIV-infected men to be classified in the persistently high depressive symptom group, and was a stronger determinant of depressive symptoms than unsuppressed plasma viral load. Social determinants such as education level and heavy drinking were also associated with depressive symptoms in all participants. Together, these findings suggest that depression and cocaine use, along with other psychosocial health challenges, are interconnected in MSM populations and potentially exacerbated in people with HIV infection, highlighting the critical need for integrated mental health and substance use treatments to address barriers to successful HIV-related care.
Black race has been associated with greater illicit drug use, higher depressive symptoms, and reduced ART adherence in previous studies of HIV-infected participants in the MACS cohort (32, 34, 59–61). Stigma undermines the health of MSM populations and limits access to health care (62), an effect that may be particularly accentuated for black MSM given multiple types of discrimination faced including discrimination based on race, sexual orientation, gender identity, and HIV status (61, 62). A study in a young black MSM cohort showed that individuals who reported higher levels of HIV stigma also had more unprotected sex while under the influence of drugs or alcohol (62), while another study showed that moderate depressive symptoms were associated with unprotected anal intercourse with casual male partners among black MSM (32). While stigma was not formally assessed in this study, other syndemic conditions including substance use and depression, in socially marginalized MSM populations are known to increase risk of HIV-infection acquisition and transmission and can lead to additional health disparities (27). Findings in this study, in which the majority of participants were black MSM, are consistent with other cohorts that report an association between high depressive symptoms in HIV-infected black MSM (63), and consistent with cross-sectional studies suggesting that cocaine use influences depressive symptoms among HIV-infected cohorts (27, 59, 64, 65). Thus, the co-occurrence of cocaine use and high depressive symptoms observed in this study provides further evidence of syndemic relationships that exist between depression and substance use (27), which may be amplified in black MSM (40, 59, 61, 66).
In this cohort, cocaine use was not associated with differences in baseline cognitive tests across multiple domains or cognitive decline after adjusting for other clinical covariates frequently associated with cognitive performance. While this finding was unexpected, our findings are consistent with previous longitudinal studies in the MACS and CNS HIV Antiretroviral Therapy Effects Research (CHARTER) cohorts that detected no differences in cognitive function associated with cocaine use (67, 68). In this study, we focused on the combined ART era, matched users to controls from the same cohort, controlled for differences in education level and other demographic covariates, focused on frequent cocaine users without polydrug use, and used mixed-effects models to isolate the independent effects of HIV infection and cocaine use on cognitive function. Despite these measures, we did not detect differences in individual baseline cognitive scores associated with cocaine use or cognitive decline in this cohort of 30–60 year old MSM. While depressive symptoms and cognitive impairment are common co-morbidities in HIV-infection (1, 28, 29, 45, 46), several studies suggest psychiatric and neurocognitive sequelae of HIV-infection are largely independent of each other (69–72). A recent report of depression in older people living with HIV on ART showed that despite greater overall depressive symptom burden among HIV-infected participants compared to HIV-uninfected controls, depressive symptoms were not associated with HIV-associated neurocognitive disorders, suggesting a disconnect between depressive and neurocognitive symptoms in HIV infection (72). Thus, findings in the present study along with studies from the literature highlight that in the context of HIV infection, etiological mechanisms underlying depressive symptoms may be distinct from mechanisms that influence cognitive decline. In contrast, findings in this study showed that HIV infection resulted in the attenuation of a practice effect observed in the control group in mixed-effects models, suggesting that HIV infection remains an important consideration when evaluating subtle changes in cognitive decline.
There are several key strengths of this study including longitudinal analysis with six years of follow-up data, stringent criteria to exclude subjects with polydrug use, and nested controls from a population with similar demographics, and psychosocial health factors. An additional strength is the focus on depressive trajectories in a predominantly black cohort, which stem from dedicated efforts of increased minority enrollment into the MACS. Black MSM are an underrepresented group in research studies, despite being disproportionately affected by HIV infection (14), and have the largest increases in rates of new HIV infection in the United States (13, 73), making findings in this study particularly relevant to a high-risk population for HIV-acquisition and critical for informing treatment interventions.
There are several important limitations in this study including those inherent to longitudinal observational studies such as selection, survivorship, and severity bias reflected in the MACS cohort. The study is restricted to men, predominantly with ≤12 years education, and requires replication in study populations with other demographic characteristics, including women. Longitudinal analyses were limited to visits within the last six years of enrollment in the study for maximal data coverage, restricting our ability to analyze longer follow-up periods. Given the limited number of heavy cocaine users over age 50, findings in this study could underestimate cognitive decline among older people living with HIV infection due to survivorship bias. Additionally, logistic regression analysis was performed for participants classified into latent classes, not among participants who did not meet posterior probability thresholds of latent class selection, which may introduce unintended bias among those individuals who for unknown reasons were not classified. While we considered our analysis of heavy cocaine users without polydrug use a strength of the study, this strategy also limits these findings to a subset of cocaine users and should be taken into consideration in future studies on cocaine use and depression in HIV-infected cohorts. Finally, subjects in this cohort were linked to care as part of their participation in the MACS, and it is possible that these findings underestimate the impact of cocaine use on depression within the larger MSM population.
In summary, the findings in this longitudinal study highlight the importance of cocaine use on depressive trajectories in the context of HIV infection. In two different longitudinal analyses examining within-subject changes in depressive symptoms, heavy cocaine use was associated with high depressive symptoms and had a profound impact in HIV-infected MSM. Achieving durable ART-mediated viral suppression is a challenging task in populations experiencing psychosocial barriers that includes depression and substance use. Given high rates of ART adherence failure among cocaine users infected with HIV, this study underscores the critical importance of HIV-care models that integrate mental health and substance use treatments in efforts to minimize HIV acquisition and optimize well-being, HIV treatment, and medical care in MSM populations.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Steven Wolinsky, Cecile Proust-Lima, and Dorene Rentz for discussion of primary data and the analysis, and Ms. Elizabeth Carpelan for assistance with manuscript preparation.
The data for this manuscript was obtained by the Multicenter AIDS Cohort Study (MACS) with centers at: Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, David Ostrow, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan.
FUNDING:
This work was supported by NIH grants to D.G. (R01 DA28994, R01 DA30985, R01 DA40391, R01 MH110259). The work was also supported in part by NIH funding to the Northwestern University Clinical Research Unit of the MACS (U01-AI35039, with additional co-funding from National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH)). Training and educational support for S.S.M and A.D. was provided by NIH T32-AG000222. Additional support for S.S.M included Harvard Catalyst Master’s Program in Clinical and Translational Investigation funded by the NIH Clinical and Translational Science Award Program (1UL1-TR001102), and Catalyst Biostatistical Consultation with contributions from Harvard Medical School and affiliated hospitals.
The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) [U01-AI35039, U01-AI35040; U01-AI35041; U01-AI35042; and UM1-AI35043], with additional co-funding from the National Cancer Institute (NCI), National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH) at the National Institutes of Health (NIH). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
CONFLICTS OF INTEREST
The authors reports no conflicts of interest
References
- 1.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the United States. Archives of general psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of acquired immune deficiency syndromes (1999) 2009;50(1):93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 3.Daskalopoulou M, Rodger A, Phillips AN, Sherr L, Speakman A, Collins S, et al. Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. The Lancet HIV. 2014;1(1):e22–e31. doi: 10.1016/S2352-3018(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 4.Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren Sa, Kitahata MM, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. American journal of public health. 2013;103:1457–67. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack Cocaine, Disease Progression, and Mortality in a Multi-Center Cohort of HIV-1 Positive Women. AIDS (London, England) 2008;22(11):1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Frontiers in bioscience: a journal and virtual library. 2007;12:1488–95. doi: 10.2741/2162. Epub 2006/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 7.Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. International journal of STD & AIDS. 2012 Aug;23(8):576–80. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cofrancesco J, Jr, Scherzer R, Tien PC, Gibert CL, Southwell H, Sidney S, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS (London, England) 2008;22(3):357. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palamar JJ, Davies S, Ompad DC, Cleland CM, Weitzman M. Powder cocaine and crack use in the United States: An examination of risk for arrest and socioeconomic disparities in use. Drug and alcohol dependence. 2015;149:108–16. doi: 10.1016/j.drugalcdep.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Administration DE. National drug threat assessment summary 2014. US Department of Justice. 2013:401–3. [Google Scholar]
- 11.Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal of behavioral medicine. 2011 Sep 21;34(2):128–38. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Grey JA, Sanchez TH, Sullivan PS. Rates of prevalent HIV infection, prevalent diagnoses, and new diagnoses among men who have sex with men in US States, metropolitan statistical areas, and counties, 2012–2013. JMIR public health and surveillance. 2016;2(1) doi: 10.2196/publichealth.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Control CfD, Prevention. Diagnoses of HIV infection among adolescents and young adults in the United States and 6 dependent areas, 2010–2014. HIV Surveillance Supplemental Report. 2016;2016(21):3. [Google Scholar]
- 14.Pathela P, Jamison K, Braunstein SL, Schillinger JA, Varma JK, Blank S. Incidence and Predictors of HIV Infection Among Men Who Have Sex with Men Attending Public Sexually Transmitted Disease Clinics, New York City, 2007–2012. AIDS and behavior. 2016:1–8. doi: 10.1007/s10461-016-1499-2. [DOI] [PubMed] [Google Scholar]
- 15.Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride: Are the differences myth or reality? Jama. 1996;276(19):1580–8. [PubMed] [Google Scholar]
- 16.Koblin BA, Chesney MA, Husnik MJ, Bozeman S, Celum CL, Buchbinder S, et al. High-risk behaviors among men who have sex with men in 6 US cities: baseline data from the EXPLORE Study. Am J Public Health. 2003 Jun;93(6):926–32. doi: 10.2105/ajph.93.6.926. Epub 2003/05/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colfax G, Vittinghoff E, Husnik MJ, McKirnan D, Buchbinder S, Koblin B, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. American journal of epidemiology. 2004 May 15;159(10):1002–12. doi: 10.1093/aje/kwh135. Epub 2004/05/07. eng. [DOI] [PubMed] [Google Scholar]
- 18.Colfax G, Coates TJ, Husnik MJ, Huang Y, Buchbinder S, Koblin B, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of san francisco men who have sex with men. Journal of urban health: bulletin of the New York Academy of Medicine. 2005 Mar;82(1 Suppl 1):62–70. doi: 10.1093/jurban/jti025. Epub 2005/03/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi K, Wood E, Kerr T, Dong H, Nguyen P, Puskas CM, et al. Factors associated with optimal pharmacy refill adherence for antiretroviral medications and plasma HIV RNA non-detectability among HIV-positive crack cocaine users: a prospective cohort study. BMC infectious diseases. 2016;16(1):455. doi: 10.1186/s12879-016-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of Active Drug Use on Antiretroviral Therapy Adherence and Viral Suppression in HIV-infected Drug Users. Journal of General Internal Medicine. 2002;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS and behavior. 2007;11(2):185–94. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34(5):1112–22. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. Journal of addiction medicine. 2014 Sep-Oct;8(5):368–76. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 24.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neuroscience & Biobehavioral Reviews. 2013;37(8):1838–59. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.WALDROP-VALVERDE D, Ownby RL, Kumar M. Influence of depression and HIV serostatus on the neuropsychological performance of injecting drug users. Psychiatry and clinical neurosciences. 2005;59(4):372–8. doi: 10.1111/j.1440-1819.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, et al. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Research. 2010;178(2):299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond ER, Lai S, Wright CM, Treisman GJ. Cocaine Use May be Associated with Increased Depression in Persons Infected with HIV. AIDS and behavior. 2016;20(2):345–52. doi: 10.1007/s10461-015-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meade CS, Towe SL, Skalski LM, Robertson KR. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug and alcohol dependence. 2015 Apr 01;149:128–35. doi: 10.1016/j.drugalcdep.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011 Feb;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millar BM, Starks TJ, Gurung S, Parsons JT. The Impact of Comorbidities, Depression, and Substance Use Problems on Quality of Life Among Older Adults Living With HIV. AIDS and behavior. 2016:1–7. doi: 10.1007/s10461-016-1613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clinical psychology review. 2005;25(4):433–57. doi: 10.1016/j.cpr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Reisner SL, Mimiaga MJ, Skeer M, Bright D, Cranston K, Isenberg D, et al. Clinically significant depressive symptoms as a risk factor for HIV infection among black MSM in Massachusetts. AIDS and behavior. 2009;13(4):798–810. doi: 10.1007/s10461-009-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilmer WS, Trivedi M, Rush A, Wisniewski S, Luther J, Howland R, et al. Factors associated with chronic depressive episodes: a preliminary report from the STAR-D project. Acta Psychiatrica Scandinavica. 2005;112(6):425–33. doi: 10.1111/j.1600-0447.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsao JC, Stein JA, Ostrow D, Stall RD, Plankey MW. The mediating role of pain in substance use and depressive symptoms among Multicenter AIDS Cohort Study (MACS) participants. Pain. 2011 Dec;152(12):2757–64. doi: 10.1016/j.pain.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54(12):2285–92. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- 36.Colzato LS, van den Wildenberg WPM, Hommel B. Reduced attentional scope in cocaine polydrug users. PloS One. 2009;4:e6043. doi: 10.1371/journal.pone.0006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrin L, Hull L, Unwin C, Wykes T, David A. Effects of depressed mood on objective and subjective measures of attention. The Journal of neuropsychiatry and clinical neurosciences. 2003;15:98–104. doi: 10.1176/jnp.15.1.98. [DOI] [PubMed] [Google Scholar]
- 38.You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007 Sep 26;27(39):10546–55. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010 Nov 01;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durvasula RS, Myers HF, Satz P, Miller EN, Morgenstern H, Richardson MA, et al. HIV-1, cocaine, and neuropsychological performance in African American men. J Int Neuropsychol Soc. 2000 Mar;6(3):322–35. doi: 10.1017/s1355617700633076. Epub 2000/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 41.Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, et al. HIV and recent illicit drug use interact to affect verbal memory in women. Journal of acquired immune deficiency syndromes. 2013 May 1;63(1):67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. Journal of neurovirology. 2013 Feb;19(1):65–74. doi: 10.1007/s13365-012-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richert L, Brault M, Mercie P, Dauchy FA, Bruyand M, Greib C, et al. Decline in locomotor functions over time in HIV-infected patients. AIDS. 2014 Jun 19;28(10):1441–9. doi: 10.1097/QAD.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 44.Gold JA, Grill M, Peterson J, Pilcher C, Lee E, Hecht FM, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS and Behavior. 2014;18(6):1124–32. doi: 10.1007/s10461-013-0688-5. [DOI] [PubMed] [Google Scholar]
- 45.Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry and clinical neurosciences. 2014;68(2):96–109. doi: 10.1111/pcn.12097. [DOI] [PubMed] [Google Scholar]
- 46.Anagnostopoulos A, Ledergerber B, Jaccard R, Shaw SA, Stoeckle M, Bernasconi E, et al. Frequency of and risk factors for depression among participants in the Swiss HIV Cohort Study (SHCS) PloS one. 2015;10(10):e0140943. doi: 10.1371/journal.pone.0140943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, et al. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. International journal of epidemiology. 2014 Apr;24:1–11. doi: 10.1093/ije/dyu092. Epub Apr 24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 49.Miller EN. California computerized assessment package. Encyclopedia of Clinical Neuropsychology: Springer. 2011:473–5. [Google Scholar]
- 50.Kupprat SA, Halkitis PN, Pérez-Figueroa R, Solomon TM, Ashman T, Kingdon MJ, et al. Age-and education-matched comparison of aging HIV+ men who have sex with men to general population on common neuropsychological assessments. Journal of health psychology. 2015;20(9):1175–85. doi: 10.1177/1359105313509844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of neurology. 2013 Sep;74(3):478–89. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadsworth LP, Lorius N, Donovan NJ, Locascio JJ, Rentz DM, Johnson KA, et al. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dementia and geriatric cognitive disorders. 2012;34(2):96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Philipps V, Amieva H, Andrieu S, Dufouil C, Berr C, Dartigues JF, et al. Normalized Mini-Mental State Examination for Assessing Cognitive Change in Population-Based Brain Aging Studies. Neuroepidemiology. 2014;43(1):15–25. doi: 10.1159/000365637. [DOI] [PubMed] [Google Scholar]
- 54.Fallu JS, Briere FN, Janosz M. Latent classes of substance use in adolescent cannabis users: predictors and subsequent substance-related harm. Frontiers in psychiatry. 2014;5(9):1–10. doi: 10.3389/fpsyt.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: results from the National Longitudinal Survey of Youth 1979 Cohort 1981–2006. International journal of epidemiology. 2011 Feb;40(1):240–50. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 56.Marioni RE, Proust-Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, et al. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. European journal of epidemiology. 2014 Mar;29(3):211–9. doi: 10.1007/s10654-014-9881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andruff H, Carraro N, Thompson A, Gaudreau P. Latent Class Growth Modelling: A Tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 58.Vistisen D, Witte DR, Tabák AG, Herder C, Brunner EJ, Kivimäki M, et al. Patterns of obesity development before the diagnosis of type 2 diabetes: the Whitehall II cohort study. PLoS Med. 2014;11(2):e1001602. doi: 10.1371/journal.pmed.1001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mimiaga MJ, Reisner SL, Fontaine YM, Bland SE, Driscoll MA, Isenberg D, et al. Walking the line: stimulant use during sex and HIV risk behavior among Black urban MSM. Drug and alcohol dependence. 2010 Jul 1;110(1–2):30–7. doi: 10.1016/j.drugalcdep.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedman MR, Stall R, Silvestre AJ, Wei C, Shoptaw S, Herrick A, et al. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS (London, England) 2015;29(9):1087–96. doi: 10.1097/QAD.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maulsby C, Millett G, Lindsey K, Kelley R, Johnson K, Montoya D, et al. HIV among Black men who have sex with men (MSM) in the United States: a review of the literature. AIDS and behavior. 2014 Jan;18(1):10–25. doi: 10.1007/s10461-013-0476-2. [DOI] [PubMed] [Google Scholar]
- 62.Radcliffe J, Doty N, Hawkins LA, Gaskins CS, Beidas R, Rudy BJ. Stigma and sexual health risk in HIV-positive African American young men who have sex with men. AIDS patient care and STDs. 2010;24(8):493–9. doi: 10.1089/apc.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh DL, Sarafian F, Silvestre A, Brown T, Jacobson L, Badri S, et al. Evaluation of adherence and factors affecting adherence to combination antiretroviral therapy among White, Hispanic, and Black men in the MACS Cohort. Journal of acquired immune deficiency syndromes. 2009 Oct 1;52(2):290–3. doi: 10.1097/QAI.0b013e3181ab6d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox HC, Jackson ED, Sinha R. Elevated cortisol and learning and memory deficits in cocaine dependent individuals: relationship to relapse outcomes. Psychoneuroendocrinology. 2009 Sep;34(8):1198–207. doi: 10.1016/j.psyneuen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wisniewski AB, Brown TT, John M, Cofranceso J, Jr, Golub ET, Ricketts EP, et al. Cortisol levels and depression in men and women using heroin and cocaine. Psychoneuroendocrinology. 2005;31(2):250–5. doi: 10.1016/j.psyneuen.2005.08.002. 2005. [DOI] [PubMed] [Google Scholar]
- 66.Dyer TP, Shoptaw S, Guadamuz TE, Plankey M, Kao U, Ostrow D, et al. Application of syndemic theory to black men who have sex with men in the Multicenter AIDS Cohort Study. Journal of Urban Health. 2012;89(4):697–708. doi: 10.1007/s11524-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine AJ, Reynolds S, Cox C, Miller EN, Sinsheimer JS, Becker JT, et al. The longitudinal and interactive effects of HIV status, stimulant use, and host genotype upon neurocognitive functioning. Journal of neurovirology. 2014 Jun;20(3):243–57. doi: 10.1007/s13365-014-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS. 2015 Jan 5;29 doi: 10.1097/QAD.0000000000000561. Epub Jan 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant I, Olshen RA, Atkinson JH, Heaton RK, Nelson J, McCutchan JA, et al. Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology. 1993;7(1):53. [Google Scholar]
- 70.Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, et al. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. Journal of clinical and experimental neuropsychology. 2006;28(3):420–37. doi: 10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society. 2007;13(01):1–11. doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- 72.Milanini B, Catella S, Perkovich B, Esmaeili-Firidouni P, Wendelken L, Paul R, et al. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS care. 2017:1–8. doi: 10.1080/09540121.2017.1281877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PloS one. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


