Key Clinical Message
We report here the clinical course of a Ph+ ALL patient who was treated with ponatinib 15 mg/day, as maintenance therapy, and developed a BCR‐ABL T315I mutation leading to ALL relapse. This clonal evolution was reversed, without adverse effects, by increasing ponatinib to 45 mg/day. To our knowledge, we have been confronted with the first clinical case of a T315I clonal selection of ALL caused by subeffective therapeutic level of the drug. This single patient experience highlights the risk of T315I clone selection in Ph+ ALL treated with reduced dose ponatinib.
Keywords: Acute lymphoblastic leukemia, T315I BCR‐ABL, treatment maintenance, tyrosine kinase inhibitor
Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) represents about 25% of B‐lineage ALL in adults. Chemotherapy combined with tyrosine kinase inhibitors (TKIs) and allogeneic hematopoietic stem cell transplantation (HSCT) in first remission has strongly improved patient survival, with a 5‐year overall survival rate about 60%. However, resistance to TKIs therapy represents a clinically major issue 1. Ponatinib is a third‐generation TKI approved for the treatment of chronic myeloid leukemia (CML) or Ph+ ALL, with T315I mutation or resistant/intolerant to other TKIs. On the basis of safety, pharmacokinetics and pharmacodynamics data of a phase 1 study 1, 45 mg/day were identified as the recommended dose for further clinical study. However, in a subsequent phase 2 study with ponatinib 45 mg once daily, serious vascular adverse events were reported 2 causing early study termination by the FDA. Additional cases of life‐threatening vascular thrombotic events were subsequently reported. Based on these data, experts recommended to minimize the use of ponatinib in Ph+ ALL patient with significant cardiovascular risk factors and to consider ponatinib dose reduction 3, 4.
Briefly, we report here the case of a 39‐year‐old patient with a Ph+ ALL, without cardiovascular risk factors, who presented after a second HSCT the emergence of a T315I mutant clone under maintenance therapy with reduced dose of ponatinib. The Ph+ ALL was initially treated with chemotherapy and imatinib followed by HSCT. Four years later, the Ph+ ALL relapsed and was treated with second‐generation TKIs (dasatinib, stopped because of erythroderma, and then nilotinib) and donor lymphocyte infusions (DLIs). Six months later, a second relapse was treated with FLAG salvage chemotherapy, and a second allogeneic HSCT followed by ponatinib 45 mg/day as maintenance therapy (Fig. 1). Ponatinib was chosen because of intolerance or resistance (sequencing did not identify any classical mutation) to other available TKIs (dasatinib, nilotinib, and bosutinib).
Figure 1.
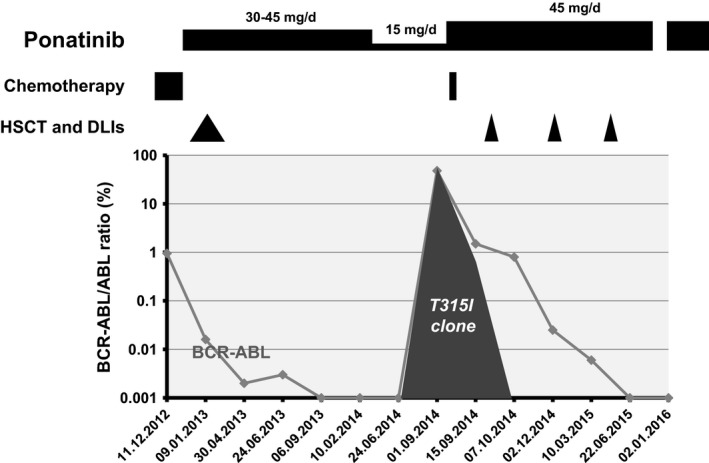
Disease course evolution after the 2nd Ph+ ALL relapse (1.12.2012) treated with FLAG salvage chemotherapy (■ fludarabine 30 mg/m2/day+ cytarabine 2 g/m2/day (days 1–5) and G‐CSF 5 mg/kg/day (FLAG)) followed by maintenance ponatinib (45 mg/day) after the second HSCT (▲). After 12 months of complete molecular remission, ponatinib was reduced to 15 mg/day. Six months later, ALL relapsed (1.9.2014) and T315I mutation were identified by Sanger sequencing. Ponatinib was immediately increased to 45 mg/day, and the patient received a unique dose of 2 mg of Vincristine ( ) and three DLIs (
) and three DLIs ( ) leading to rapid and persistent complete molecular response, bcr‐abl transcript remaining undetectable in bone marrow until today.
) leading to rapid and persistent complete molecular response, bcr‐abl transcript remaining undetectable in bone marrow until today.
To decrease the risks of vascular thrombotic events, the maintenance therapy was reduced to 15 mg/day, while ALL was in complete molecular remission after 12 months of treatment at the dose of 45 mg/day. Six months later, a molecular relapse was identified in bone marrow (BCR‐ABL/ABL ratio: 48%), and Sanger sequencing revealed a T315I mutation that was never documented in the previous analysis. The patient was treated with steroids, a single dose of vincristine (1.4 mg/m2) and ponatinib was increased to 45 mg once daily. Fifteen days later, BCR‐ABL/ABL ratio was reduced to 1.5% and sequencing showed the concomitant presence of wild type and T315I bcr‐abl transcripts. DLIs were started. Three months later, BCR‐ABL/ABL ratio decreased to 0.025%, and 6 months after the 2nd relapse, BCR‐ABL transcript was undetectable, without graft versus host disease (GVHD). As we write this report, 15 months after the 2nd relapse, the patient is still in complete molecular response. He presented a transient liver GVHD after the 3rd DLI. Ponatinib was stopped for 3 months and reintroduced at 30 mg/day due to thrombocytopenia and liver toxicity (Fig. 1). Aspirin 100 mg/day was introduced to try to reduce the risk of vascular complications, 3. All other cardiovascular risk factors were closely monitored and addressed if necessary 5. After more than 31 months of ponatinib therapy, the arterial assessment shows no sign of arteriopathy.
Primary or secondary ponatinib resistance is an extremely rare event in chronic phase‐CML. However, ponatinib IC50 is much higher for T315I clones, and intermediate dosage was shown to favor the outgrowth of resistant clones harboring this mutation in vitro 6. We postulate that our clinical observation correlates with these in vitro data. The appearance of a T315I clone under ponatinib 15 mg/day, and the achievement of a complete molecular remission and extinction of the T315I clone support this hypothesis and suggest that ponatinib 45 mg/day may be the appropriate dosage to treat T315I Ph+ ALL. Ph+ ALL is characterized with high cell proliferation and clone instability that highly increase the risk of mutated clone emergence, such as T315I clone. From our observation it may be not advisable to treat Ph+ ALL patient with low‐dose ponatinib. In this setting, the risks of vascular side effects should be viewed as acceptable considering the lack of other therapeutic options for these high risk patients. In the future, clinical dose finding trials are highly needed to identify the appropriate intensity of ponatinib treatment in induction and maintenance therapy. Dose titration and management of vascular events remain a major concern.
Authorship
JN: involved in manuscript redaction. MG: involved in manuscript redaction. Stavroula Masouridi‐Levrat : involved in manuscript revision. MD: involved in manuscript revision. OS: involved in manuscript revision.
Conflict of Interest
None declared.
The authors would like to thank Incyte for their contribution to publications costs.
References
- 1. Cortes, J. E. , Kantarjian H., Shah N. P., Bixby D., Mauro M. J., Flinn I., et al. 2012. Ponatinib in refractory Philadelphia chromosome‐positive leukemias. N. Engl. J. Med. 367:2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain, P. , Kantarjian H., Jabbour E., Gonzalez G. N., Borthakur G., Pemmaraju N., et al. 2015. Ponatinib as first‐line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2:e376–e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valent, P. , Hadzijusufovic E., Schernthaner G. H., Wolf D., Rea D., and le Coutre P.. 2015. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 125:901–906. [DOI] [PubMed] [Google Scholar]
- 4. Sanford, D. S. , Kantarjian H., O'Brien S., Jabbour E., Cortes J., and Ravandi F.. 2015. The role of ponatinib in Philadelphia chromosome‐positive acute lymphoblastic leukemia. Expert Rev. Anticancer Ther. 15:365–373. [DOI] [PubMed] [Google Scholar]
- 5. Herrmann, J. , Bell M. R., Warren R. L., Lerman A., Fleming M. D., and Patnaik M.. 2015. Complicated and advanced atherosclerosis in a young woman with philadelphia chromosome‐positive acute lymphoblastic leukemia: success and challenges of BCR/ABL1‐targeted cancer therapy. Mayo Clin. Proc. 90:1167–1168. [DOI] [PubMed] [Google Scholar]
- 6. O'Hare, T. , Shakespeare W. C., Zhu X., Eide C. A., Rivera V. M., Wang F., et al. 2009. AP24534, a pan‐BCR‐ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation‐based resistance. Cancer Cell 16:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]


