Abstract
α-Synuclein (αS) is a 140-residue neuronal protein that forms insoluble cytoplasmic aggregates in Parkinson's disease (PD) and several other neurodegenerative disorders. Two missense mutations (A53T and A30P) are linked to rare forms of familial PD. The normal function of αS is unknown, and cultured cell systems that model its modification from soluble monomers to aggregated forms have not been reported. Through a systematic centrifugal fractionation of mesencephalic neuronal cell lines and transgenic mouse brains expressing wild-type or A53T human αS, we observed unusual, previously unrecognized species of αS that migrate well above the 17-kDa monomeric form in denaturing gels. Incubation at 65°C of high-speed cytosols from cells or brains revealed a modified αS species migrating at ≈36 kDa and an extensive higher molecular mass αS-reactive smear. Extraction of the cytosols with chloroform/methanol or with a resin (Lipidex 1000) that binds fatty acids resulted in a similar pattern of higher molecular mass αS forms. On the basis of this effect of delipidation, we reexamined the primary structure of αS and detected a motif at the N and C termini that is homologous to a fatty acid-binding protein signature. In accord, we found that purified human αS binds oleic acid, with an apparent Kd of 12.5 μM. We also observed an enhanced association of A53T αS with microsomal membranes in both mesencephalic cells and transgenic mouse brains. We conclude that αS has biochemical properties and a structural motif that suggest it is a novel member of the fatty acid-binding protein family and may thus transport fatty acids between the aqueous and membrane phospholipid compartments of the neuronal cytoplasm.
Progress in characterizing insoluble protein aggregates in human neurodegenerative diseases has led to new insights into the molecular mechanisms of these previously obscure disorders (1). A salient example is the discovery of the protein α-synuclein (αS) as the principal constituent of the Lewy bodies that are the cytopathological hallmark of dopaminergic neuronal degeneration in Parkinson's disease (PD) (2, 3). This finding emerged from the observation of αS missense mutations in rare dominantly inherited forms of PD (4, 5). Lewy bodies, which are ubiquitylated cytoplasmic inclusions, and related neuritic alterations called Lewy neurites contain insoluble filamentous aggregates of the otherwise highly soluble αS protein (2, 6–8). Such aggregates also occur in diffuse Lewy body dementia, multiple systems atrophy, and some cases of Alzheimer's disease and Down's syndrome (9–12). Accordingly, there is now great interest in elucidating the normal structure–function relationships of αS in neurons and their modification in PD and the other “synucleinopathies.”
αS is a 140-residue soluble protein that is abundantly expressed in neurons, where it is localized in part to presynaptic nerve endings (13). Developmental studies in songbirds suggest that its expression in certain neurons is associated with the acquisition of learning (14). It may thus play a role in synaptic plasticity, perhaps by helping to maintain the size of the presynaptic vesicular pool (15). αS can inhibit in vitro the enzyme phospholipase D2, which hydrolyzes phosphatidylcholine to phosphatidic acid and may be involved in vesicle trafficking in the secretory pathway (16–18). Although αS is believed to be principally a soluble cytosolic protein, some portion of αS is associated with membranes (19–22). The N-terminal region of αS may interact reversibly with membranes via 7 repeated motifs of 11 amino acids each that are shared with the class A2 helices of apolipoproteins (9–12). αS appears to associate with membranes via not only this domain but also its C-terminal region, in which no such lipophilic sequence has been observed (21). In vitro studies suggest that αS binds acidic phospholipid vesicles in a way that markedly alters its secondary structure (23, 24).
Some transgenic (tg) mouse lines expressing wild-type (wt) human αS develop neuronal cytoplasmic inclusions reminiscent of Lewy bodies, loss of dopaminergic synapses, and associated motoric impairment (25). Expression of wt or mutant human αS in Drosophila leads to Lewy body-like inclusions in dopaminergic neurons associated with neuronal loss and impaired crawling (26). In vitro aggregation studies of purified αS show that its oligomerization into protofibrillar intermediates is accelerated by both the A53T and A30P missense mutations (8, 27, 28), although only A53T also increases the conversion of such intermediates into amyloid-like fibrils (29). The occurrence of the mutations in the region of homology to the class A2 helices of apolipoproteins suggests possible altered interactions with membrane lipids and mislocalization of αS. However, αS distribution in fractionated brain synaptic vesicles was found to be unaltered in wt vs. A30P tg mice (20), and no differences in membrane association were detectable in transfected primary cortical neurons by using fluorescence resonance energy transfer (21).
Here, we conducted a systematic biochemical fractionation study of αS in non-tg and tg mouse brains and in an αS-transfected rat mesencephalic (MES) neuronal cell line, MES 23.5 (30). The results reveal that αS shares biochemical and structural features with the fatty acid-binding proteins (FABPs) and binds fatty acids (FAs).
Materials and Methods
Cultured Cells.
The rodent mesencephalic neuronal cell line MES 23.5, which has dopaminergic properties (30), was stably transfected with wt or A53T αS cDNA in the pCDNA 3.1 vector. An unexpected and striking feature of these αS-transfected MES cells was that all stable clones gradually lost αS expression after being continuously passaged for 2–3 mos or more. The wt and A53T clones selected for the study expressed similar levels of αS.
Tg Mice.
Mice expressing human wt or A53T mutant αS were generated routinely, as will be described in detail elsewhere (M.S.G., C. A. Lemere, M. P. Frosh, P. T. Lansbury, and J.S., unpublished work ). Briefly, constructs containing the human wt or A53T mutant αS cDNA behind the platelet-derived growth factor (PDGF)-B promoter were injected into pronuclei of C57BL/6J mouse embryos, and resulting tg mice were maintained in the same background. Western and Northern analyses showed brain levels of human αS approximately half those of endogenous mouse αS, primarily in the cerebral cortex and hippocampus, as expected from the known expression pattern of the PDGF-B promoter. A complete description of the ongoing phenotypic analysis of these mice will be reported elsewhere.
Fractionation Protocol.
A protocol described previously (31) was modified to gain improved separation of microsomal vesicles (see Fig. 1A). All procedures were at 4°C. Briefly, whole mouse brains or cultured MES cell pellets (≈2 × 107 cells) were homogenized (20 up-and-down strokes with a Teflon homogenizer, followed by five passages through a 22-gauge needle) in 1:10 (wt/vol) homogenization (H) buffer [20 mM Hepes, pH 7.4/1 mM MgCl2/0.32 M sucrose/43 mM 2-mercaptoethanol/1× protease inhibitor mix (Sigma)]. The homogenate was centrifuged at 170 × g for 10 min. The resultant pellet (P1) was washed in H buffer and respun at 370 × g for 15 min. The pellet (P1*) was resuspended in H buffer, and the suspension was brought to 2.1 M sucrose and spun at 175,000 × g for 1 h. The pellet (consisting of nuclei) and the lipid-rich fraction floating to the top of this sucrose cushion were collected. S1 supernatant was centrifuged at 8,000 × g for 15 min to obtain P2 and S2. S2 supernatant was then subjected to sequential 60-min spins at 25,000 × g, 100,000 × g, 230,000 × g, and 370,000 × g. At each speed, the resultant supernatant (designated S25, S100, etc.) was transferred to a clean tube, and the resultant pellet was washed and then resuspended in homogenization buffer containing 1% Nonidet P-40. The pellets were extracted on ice for 20 min, cleared in a microfuge at 15,000 × g for 10 min, and the detergent-solubilized pellet extract was transferred to a clean tube. Samples were stored at −70°C for up to 6 mos. Protein concentrations were assayed by the Bradford method (32).
Figure 1.
Distribution of αS in subcellular fractions in normal and tg mouse brains and transfected MES cells. (A) Scheme of the centrifugal fractionation procedure. (B) Endogenous αS distribution in normal mouse brain. Whole normal mouse brain was fractionated as in A and subjected to quantitative Western blotting with H3C Ab. Results are presented as the ratio of the amounts of αS monomer (17 kDa) in each fraction to that of total αS monomer in the starting homogenate (100%) run in parallel on each gel (means ± SD of three brains). (C) Higher portion of A53T than WT αS is associated with P25 microsomes. Results are presented as the ratio of the amounts of αS monomer in P25 to that in S370 analyzed in parallel. [Means ± SD of n = 5 brains and n = 8 MES cultures each for wt (empty bars) and A53T (filled bars).]
Western Blotting for Quantitative Analysis of the Distribution of αS Monomer (17 kDa) in the Above Brain and Cell Fractionations.
To reduce experimental variation, experiments were conducted in pairs, i.e., wt and A53T samples were worked up in parallel on the same day, from harvesting the cells or sacrificing the mice through the fractionation, protein assay, and gel electrophoresis steps. Equal amounts of total protein (in duplicate for each fraction) were loaded on 8–16% Tris-glycine gels (NOVEX, San Diego), transferred to poly(vinylidene difluoride) membrane, and blotted by using an enhanced chemiluminescence detection kit (Amersham Pharmacia). The blots were scanned in a Umax Magic Scan (Eastman Kodak) and analyzed for density of the αS monomeric band by using un-scan-it gel 3.1 software (Silk Scientific). Results in the various fractions were normalized to the amount of αS monomer either in the corresponding total homogenate or in the S370 fraction run in parallel on each gel, as indicated. Although the stable lines analyzed express closely similar amounts of αS, results are presented as the ratio of αS in a specific fraction to total or S370 αS to eliminate small differences in expression levels. αS Abs and their epitopes were: LB509 (amino acid 115–122) (3);15G7 (amino acid 116–121) (20); Syn-1 (amino acid 15–123) (Transduction Laboratories, Lexington, KY); H3C (amino acid 126–140) (14); PER4 (amino acid 1–120) (33); affinity purified 7384 (amino acid 123–140) (this study).
Lipid Removal by Chloroform/Methanol Extraction.
S370 samples were extracted with chloroform/methanol to remove lipids attached to proteins, as originally described (34). In an Eppendorf tube, 2 vol of chloroform and 1 vol of methanol were mixed, and 0.25 vol of S370 was added. The mixture was vortexed for 30 sec and centrifuged at 10,000 × g for 5 min (room temperature). The resulting aqueous (upper) and organic (lower) phases were removed. The interface, containing all αS proteins (see Results), was lyophilized and resuspended in water or 2× Laemmli buffer.
FA-Binding Studies.
S370 samples were incubated at 37°C or 65°C overnight with Lipidex 1000 (5% wt/vol). The tube was spun at 10,000 × g for 5 min and the supernatant transferred to a clean tube. Proteins were eluted from the Lipidex pellet by boiling for 10 min in 2× Laemmli buffer.
Sequence Homology Search.
We searched through the ExPASy web site (http://expasy.cbr.nrc.ca) under “proteomic tools” for “pattern and profile searches,” by using scan Prosite. By allowing mismatches of up to 50%, we detected the “cytosolic FABP signature” motif in the αS sequence (see Results) by using the option “PROSITE scan” at the URL http://expasy.cbr.nrc.ca/tools/#pattern.
Oleic Acid (OA)-Binding Assays.
Assays were carried out as previously described (35). Briefly, purified human recombinant wt αS was resuspended in binding buffer (10 mM Tris⋅HCl, pH 7.5, 150 mM NaCl), filtered through Microcon YM-100 (Millipore), and its concentration determined both by OD 276 nm and Bradford (32). Samples containing wt αS (5 μM) and [14C] OA (NEN) (at the indicated concentrations) in 100 μl of binding buffer were processed as described previously for the Lipidex assay (35). Each value was corrected against a blank processed identically but without αS protein. Cold FA displacement experiments were performed by using the same assay. The reaction mix contained fixed concentrations of αS (5 μM) and [14C] OA (10 μM) and increasing concentrations of the unlabeled competitors: arachidonic acid (AA), docosahexaenoic acid (DHA), and OA. The radiolabeled OA and the competitors were added simultaneously to the protein. For quantifying FA binding directly to the S370, extracts (5 μg) from nontransfected or wt αS-transfected MES cells were treated with 0.5% SDS for 10′ at 37°C. SDS was diluted 1:10 with binding buffer to final reaction vol of 100 μl, and the S370 was subjected to the Lipidex assay (above).
Statistical Analyses.
The amounts of αS in P25 membrane pellets (Fig. 1 A and B) were assumed to follow a Poisson distribution (36) with rate given by 2*S370*μ. We analyzed the equality of the parameters μ between P25 αS values from A53T vs. wt αS and between tg mice vs. MES cells by using Poisson regression, adjusting for correlation from experiments conducted at the same time by including random effects for time of experiment. This was fit by using SAS software (SAS Institute, Cary, NC), release 8.1.
Results
The initial goals of this study were to determine the relative distribution of soluble and membrane-associated forms of αS in neural cells and to search specifically for forms of the protein that are aggregated or have unusual solubility properties and thus might be relevant to PD. To these ends, we carried out a systematic centrifugal fractionation of αS from two disease-relevant sources: human αS stable MES transfectants and human αS tg mouse brains. We expressed wt or A53T αS in the MES cells and selected stable clones with closely similar αS expression levels. To complement the data obtained in these neuronal cell lines, we examined C57BL/6J mice tg for either wt or A53T human αS. For some studies, we compared the results obtained in the wt and A53T human αS tg mice with the properties of endogenous αS in brains from non-tg littermates.
Fractionation of Brain and MES αS by Differential Centrifugation.
The distribution of endogenous αS was first examined in normal (non-tg) mouse brain by using quantitative Western blotting (see Materials and Methods). Approximately 50% of total brain αS was found to be cytosolic, i.e., was recovered in the final high-speed (370,000 × g) supernatant (Fig. 1B). The remaining ≈50% of brain αS was distributed among several membrane-enriched pellets, with the large majority of this noncytosolic protein being recovered in pellets P1 (170 × g) (≈25% of total) and P2 (8,000 × g) (≈20% of total). No significant αS-reactive protein was detected in the nuclear fraction, which was obtained from the P1 pellet (Fig. 1A). However, the lipid-rich material floating to the top of the sucrose cushion used to pellet the nuclei was highly αS immunoreactive (see below).
Because the primary structure of αS predicts that the N-terminal portion interacts with lipid molecules and thus with membranes, and because the two known PD-associated mutations are localized to this region, we were interested in the portion of total brain αS associated with various sized microsomal membranes. We therefore used differential centrifugation to collect four higher-speed membrane pellets from the S2 sup:P25 (i.e., 25,000 × g), P100, P230, and P370 (Fig. 1A). Taken together, these four pellets contained about 8% of total brain αS. Among these four higher-speed membrane pellets, the highest percentage of total brain αS was associated with P25 (mean ≈7%) (Fig. 1B). Western blotting with a panel of membrane protein markers revealed that P25 was particularly enriched in calnexin (an endoplasmic reticulum marker) and several markers of endosomes, including transferrin, syntaxin 6, and syntaxin 13, as well as synaptotagmin, a synaptic vesicle marker (data not shown).
Next, we carried out the same fractionation protocol on the brains of tg mice expressing wt or A53T human αS and on MES neuronal lines stably expressing these αS forms. Using the human αS-specific Ab, LB509 (3), we found that the overall distribution of both human isoforms among the tg brains and MES cell fractions was indistinguishable from that of endogenous mouse brain αS (Fig. 1B), i.e., was well within the range of experimental variation. Because P25 contained most of the αS immunoreactivity among the four microsomal pellet fractions, we compared the relative amounts of P25 αS immunoreactivity (normalized to the amount of S370 αS in the same fractionation) between wt and A53T expressors. Significantly more αS was associated with P25 microsomes in A53T than in wt αS expressors, both in the MES cells (P < 0.001) and in the tg mouse brains (P < 0.014) (Fig. 1C).
Identification of Higher Molecular Mass αS-Reactive Polypeptides in the Cytosol of Brain Tissue and Mesencephalic Neuronal Cells.
In the quantitative Western blot analysis of αS distribution described above, the vast majority of αS-reactive protein in all of the fractions migrated at ≈17 kDa, the expected molecular mass for the αS monomer (3, 14, 37). However, we also found variable amounts of αS-reactive protein that remained gel-excluded (i.e., in the sample well). In most fractionations, we further observed variable amounts of an ≈85-kDa αS-reactive protein specifically labeled by LB509 in the very high-speed pellet (P370) and supernatant (S370) of both the MES transfectants and tg mouse brains (not shown). In view of these small amounts of apparent higher Mr forms of human αS in the cytosol of mesencephalic neurons and brain, we undertook a concerted search for larger αS forms by attempting to dissociate or depolymerize such forms using a variety of chemical and physical treatments on the S370 supernatants.
Treatment of the S370 cytosol with 8 M urea, 2% SDS, 1.5% β-ME, divalent cations (10 mM MgCl2, 10 mM CaCl2 or 1 mM ZnSO4), 5 mM EDTA, 0.1 M NaOH, or 0.1 M HCl did not alter the αS electrophoretic pattern (not shown). We next incubated the S370 at either 37°C, 65°C, or 100°C for increasing time intervals to determine whether heat would alter the pattern of αS immunoreactivity (Fig. 2 A–D). Incubation at 37°C for 18 h revealed a αS-reactive 36-kDa band, which was not observed at 0°C (Fig. 2A). A dramatic change in the αS Western blot pattern was observed upon incubation at 65°C for 18 h. The change comprised a modest increase in the amount of αS monomer, the appearance of the 36-kDa band that became increasingly visible between 2 and 24 h of 65°C incubation, and the appearance of an extensive smear of αS immunoreactive material throughout the middle to higher Mr range of the gel (Fig. 2 A and B). This pattern was highly reproducible in both mouse brains (non-tg and tg) and MES transfectants. We observed no detectable difference in these changes between wt and A53T samples, both in brains and MES cells. This temperature-dependent change in the αS-reactive pattern of the S370 cytosols was not observed in any of the membrane pellets (P25 to P370), but it was observed in the lipid-rich floating material obtained during the sucrose cushion fractionation of the P1 pellet (not shown).
Figure 2.
αS forms higher Mr aggregated species in the cytosols of mouse brain and αS-transfected MES cells. (A) The appearance of higher Mr αS immunoreactive forms is temperature-dependent. Aliquots of S370 cytosol (20 μg) from normal mouse brain were incubated at the indicated temperatures for 18 h, loaded on an 8–16% Tris-Glycine SDS gel, and blotted with H3C Ab. *, Gel-excluded material. (B) Appearance of the higher Mr αS immunoreactive forms is time-dependent. Aliquots (20 μg) of S370 cytosol from human wt αS transfected MES cells were incubated at 65°C for the indicated times and blotted with human αS-specific Ab LB509. (C) The αS-reactive higher Mr material is immunospecific. The indicated αS Abs were preabsorbed (+) or not (−) at 4°C overnight with 5 μg purified αS per microliter Ab and used to blot duplicate aliquots (20 μg) of S370 (from wt human αS-transfected MES cells) that had been incubated either at 0°C or 65°C overnight. (D) αS monomer is shifted upwards by ≈1 kDa on 18 h incubation at 65°C (also visible in C). S370 aliquots (20 μg) from wt αS-transfected MES cells were blotted with LB509.
Incubation of S370 at 100°C for 18 h led to a αS-reactive pattern partially reminiscent of that at 65°C, but it was accompanied by a loss of the αS monomer and the appearance of gel-excluded αS in the sample well (Fig. 2A). Incubation of S370 at 37°C for 18 h did not reveal the higher Mr smear (Fig. 2A); however, longer (24- or 45-h) incubations at 37°C did produce a pattern closely similar to that seen after 65°C incubation (not shown).
To determine the specificity of the new αS-reactive species that appeared with 65°C treatment, we performed Western blotting with six different αS-specific Abs (see Materials and Methods) and found that all six Abs recognized the 17-kDa monomer, the 36-kDa band, and the mid-high Mr smear (Fig. 2C and data not shown). Preabsorption of H3C, LB509, or Syn-1 Abs with purified recombinant human αS protein markedly reduced the staining of the 17-kDa monomer and abolished the 36-kDa band and the higher Mr smear (Fig. 2C). By densitometry, the total amount of αS immunoreactivity detectable by Western blots was 10- to 100-fold higher in the 65°C-treated S370 fractions than in the same cytosol incubated simultaneously at 0°C. On very brief development of the Western blots, we noticed that the αS monomeric band migrating at 17 kDa before 65°C incubation shifted to a slightly higher molecular mass (18 kDa) after the 65°C treatment (Fig. 2D). This subtle but highly consistent shift in apparent Mr was observed in non-tg and tg mouse brains as well as in transfected MES cells. In some samples, only a portion of the αS monomer was up-shifted, resulting in a closely spaced doublet (17 + 18 kDa) containing the up-shifted and the conventional monomers (not shown).
Both Monomeric and Higher Mr αS Species in the Cytosol Are Bound to Lipids.
The striking increase in αS immunoreactive material effected by 65°C treatment suggested that heating exposed αS-reactive epitopes by denaturing the protein, releasing lipids or other bound molecules from it, and/or depolymerizing higher molecular weight forms and allowing them to enter the gel. In an attempt to distinguish among these explanations, we assessed the effects of delipidating agents on the αS immunoreactive pattern. Extraction of the S370 cytosol with chloroform/methanol (2:1) resulted in the recovery of αS-reactive monomer and abundant higher Mr species in the interface between the upper (aqueous) and lower (organic) phases (Fig. 3A, lane 2). This αS-reactive pattern after chloroform/methanol extraction was immunospecific (not shown) and resembled in part that observed when S370 was incubated at 65°C (Fig. 3A, lane 3).
Figure 3.
αS associates with lipids in brain and MES cells. (A) Aliquots (20 μg) of S370 cytosol from normal mouse brain were untreated (lane1), treated with chloroform/methanol and the interface between the upper and lower phases collected (lane 2) or incubated at 65°C overnight (lane 3) and then blotted with H3C. (B) Exposure of novel αS species by treatment with a FA-binding resin (Lipidex 1000), without or with heating. Aliquots (20 μg) of S370 from human wt αS-transfected MES cells were incubated overnight with or without Lipidex at the indicated temperatures, and samples were spun (10,000 × g for 5 min). The post-Lipidex supernatant (S-Lx) and the sample without Lipidex treatment (S370) were loaded directly, whereas the Lipidex pellet (P-Lx) was boiled for 10 min in 2× Laemmli buffer, and the extract was loaded. LB509 Western blots.
An alternative method of attempting to delipidate the various αS species is the use of lipid-binding resins, which can extract lipid bound to proteins (38–40). We therefore treated the S370 fraction with Lipidex 1000, a resin that principally binds FAs (see Materials and Methods). The Lipidex-treated supernatant again showed a temperature-dependent αS pattern. After Lipidex treatment at 0°C, we saw no effect on the αS-reactive pattern (Fig. 3B). Lipidex treatment at 37°C led to the recovery of some monomeric αS and a small amount of the 36-kDa species in the supernatant. However, greater amounts of both monomeric αS and the 36-kDa band were recovered in the Lipidex pellet (Fig. 3B). At 65°C, we again observed the marked enhancement of all αS species. At this temperature, the post-Lipidex supernatant and the Lipidex pellet contained more αS immunoreactivity than the S370 before Lipidex, and the higher Mr immunoreactive bands appeared more defined, i.e., not just as a smear (Fig. 3B). In this experiment, the post-Lipidex supernatant and Lipidex pellet originate from the same cytosol sample and contain the same amount of starting material as the parallel aliquot incubated without Lipidex (Fig. 3B). Therefore, much more αS-reactive material was recovered in the presence of Lipidex. We conclude that the recovery of novel higher Mr αS species does not depend on temperature alone but also occurs on removal of lipids, particularly FAs given the specificity of Lipidex.
αS Shares Sequence Homology with FABPs and Binds OA Stochiometrically.
Taken together, the effects in both brain and MES cells of the 65°C incubation, the chloroform/methanol treatment, and the delipidation with Lipidex all strongly support previous in vitro evidence that αS interacts with lipids (19, 23, 24). Further, these data suggest that the lipids mask immunoreactive epitopes in the αS protein. We therefore searched the αS amino acid sequence for motifs that might have homology with known lipid-binding proteins (see Materials and Methods). We found a significant regional homology to the family of FABP. We observed an 18-residue sequence at the extreme C terminus of αS that showed 67% homology (or 83% including the intervening space-holding residues) with a signature motif characteristic of the FABP (Fig. 4A). A stretch of 18 residues near the N terminus of αS also showed 55% homology (or 78% including intervening space-holding residues) with this same motif of the FABP. These previously unrecognized homologies suggest that αS shares some structure and properties with the FABP. Consistent with this hypothesis are the observations that the length of αS (140 aa) is closely similar to that of the large majority of FABPs (41) and that αS, like FABPs, is a cytosolic protein that associates with membranes and is subject to regulation by phosphorylation (42).
Figure 4.
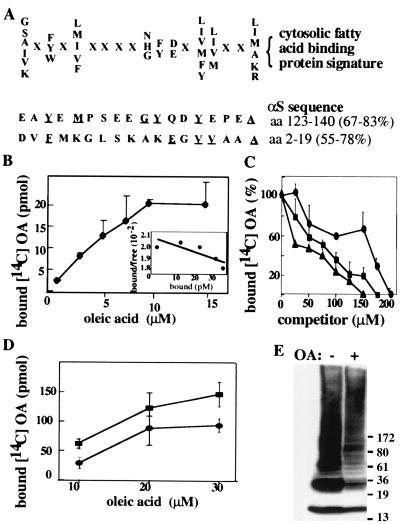
αS has characteristics of a FABP. (A) The cytosolic FABPs signature. The 18-residue motif comprises nine specific residues and nine undefined residues (represented by X). The homology percentage is calculated either by considering only the specific residues [67% in the amino acid 123–140 stretch (C terminus) and 55% in the amino acid 2–19 stretch (N terminus)] or by including the X residues needed to maintain proper spacing between the specific residues (83% at the C terminus and 78% at the N terminus). Underlined residues are shared with the FABP signature. (B) Purified human αS (5 μM) was incubated with increasing concentrations of 14C OA. Protein-bound OA was separated from free OA by using the Lipidex assay (see Materials and Methods). Data points are means of triplicates ± SD. (Inset) Scatchard plot. Estimated Kd and Bmax values were 12.5 μM and 1, respectively. (C) FA displacement curve: purified human αS (5 μM) was incubated with 10 μM [14C] OA and increasing concentrations of unlabeled OA (●) AA (■) or DHA (▴), as indicated. (D) Aliquots (5 μg) of S370 cytosols from untransfected or wt-αS-transfected MES cells were incubated with increasing concentrations of 14C OA, as in B. Nontransfected cells (●), αS transfected cells (■). (E) Aliquots of S370 (20 μg) were incubated at 65°C overnight without (−) or with (+) free cold OA (5 μM) and Western blotted with LB509.
In view of these similarities, we asked whether purified recombinant αS was capable of incorporating FA. We measured 14C OA binding by purified human wt αS in the Lipidex assay (see Materials and Methods). OA was chosen as a general FA ligand commonly used to characterize FABP and does not indicate the nature of any in vivo FA ligand(s). A dose-dependent binding of 14C OA up to saturation was observed when a constant amount (5 μM) of purified recombinant human wt αS was incubated with increasing OA concentrations (2–15 μM) (Fig. 4B). A Scatchard plot of the binding data yielded an estimate for the apparent Kd of 12.5 μM (mean of n = 3). The predicted molar ratio of αS to OA is 1:1, based on a Bmax of 1.
The specificity of the OA binding was then tested by displacement with unlabeled DHA, AA, and OA. DHA displaced 14C OA binding most potently (i.e., 100% at 150 μM), followed by AA (100% at 175 μM) and OA (100% at 200 μM) (Fig. 4C). Next we attempted to relate this binding of FA to purified human αS (Fig. 4 B and C) to the effect of delipidation of cytosolic human αS in mouse brains and MES neuronal cells (Figs. 2 and 3). We compared the amounts of 14C OA incorporation into the S370 cytosols of untransfected vs. human wt αS-transfected MES cells. The S370 cytosols of untransfected cells underwent a dose-dependent incorporation of OA (Fig. 4D). The αS-transfected S370 incubated simultaneously under identical conditions showed greater amounts of OA incorporation (Fig. 4D). To further link these findings to the αS-reactive species revealed by delipidation, we incubated S370 cytosol samples at 65°C without or with unlabeled OA and examined it by Western blotting. OA incubation substantially masked the 36 kDa and higher αS-reactive species, while only slightly decreasing the appearance of the 17-kDa monomer (Fig. 4E). Thus adding FA produces the opposite effect on the αS-reactive high Mr pattern than does removal of FAs by Lipidex 1000.
Discussion
αS is a principal constituent of Lewy bodies (2, 3), and recombinant αS assembles into filaments that are structurally and immunochemically highly similar to those of Lewy bodies (8, 27). These results indicate that αS accumulates abnormally and can aggregate into insoluble forms in PD. However, there have been no biochemical descriptions heretofore of altered or aggregated forms of wt or mutant αS in in vivo models, either in cell culture or in the brains of tg αS mice.
During a systematic biochemical fractionation of αS in mesencephalic neuronal cells and mouse brains expressing human αS, we discovered unusual, previously unrecognized species of αS that migrate well above the 17-kDa monomeric form in denaturing gels. A ≈36-kDa species is invariably present after 65°C incubation of the high-speed cytosol (S370) of mouse brain and mesencephalic neuronal cells. In addition, an extensive immunoreactive smear of αS material appears in the middle and upper Mr regions of the gels. The occurrence of these species not only in tg mice and stable MES transfectants but also endogenously in normal mouse brain suggests that modified and/or aggregated forms of αS occur normally in neurons.
Because the amount of αS monomer (17 kDa) was unchanged or modestly increased between the 0°C and 65°C incubations, it is unlikely that the newly appearing 36-kDa form and the higher smear of αS-reactive material arise from αS polymerization in vitro during the incubations. Rather, we hypothesize that this treatment exposes hitherto buried epitopes on an array of αS-reactive species in the cytosol that are not visualized under conventional Western blotting conditions and/or causes partial depolymerization of very high Mr aggregates of αS that cannot otherwise be detected by Western blotting. The reduced recovery of these species after incubating the cytosols with free OA (Fig. 4E) supports the first explanation of epitope exposure. We interpret the small upward shift in electrophoretic migration of the αS monomer consistently induced by 65°C incubation (Fig. 2D) as evidence of the unfolding of a partially SDS-resistant conformer of the αS monomer. Such subtle shifts in the electrophoretic migration of lipid-binding proteins on further denaturation have been described before, including in the brain proteolipid protein (43).
The effect of lipid extraction by using the Lipidex 1000 resin is strongly indicative of the presence of lipid molecules, most likely FAs, on αS, including on the 36-kDa and higher Mr αS-reactive species. The nature of the endogenous lipids that are attached to αS in mesencephalic neurons and in the brain is currently unknown. However, our unexpected observation of a regional homology between αS and a conserved motif characteristic of the FABP family, coupled with our demonstration that OA is incorporated stochiometrically into purified αS, leads us to hypothesize that αS may function as a FABP. Indeed two FAs that are abundant in brain tissue, AA and DHA, displace OA from purified αS in a dose-dependent fashion. Moreover, the enhanced binding of OA by the S370 cytosol of αS-overexpressing MES cells (Fig. 4D) is consistent with a role for αS as a FABP.
FABP are small (≈15 kDa) cytosolic lipid-transport proteins of diverse primary structure that include a large and growing list of tissue-specific proteins. The 14.5-kDa brain-specific (B)-FABP has been localized to glial cells, in particular to radial glia (44). B-FABP has not been described in neurons and Schwann cells. Because αS is principally expressed in the presynaptic terminals of neurons in the cerebral cortex, hippocampus, basal ganglia, and brainstem (13, 45), it appears that B-FABP and αS do not coexist in brain cells. As αS is expressed in very high abundance in the brain [≈0.5–1% of total cytosolic proteins (11)], our findings suggest that αS is a novel brain-specific FABP.
The temperature-dependent effects of Lipidex treatment we report (Fig. 3B) are similar to those described previously for other proteins (40). Importantly, the latter study found that Lipidex binds only free FA at 0°C, whereas it binds protein-bound as well as free FA at 37°C (40). On this basis, we propose that the presence of the 36-kDa αS-reactive species bound to the Lipidex pellet after 37°C incubation (Fig. 3B, lanes 4–6) represents a FA-bound form of the protein. The combined treatment of the S370 cytosol with Lipidex at 65°C markedly enhances the appearance of additional higher Mr αS-reactive species (Fig. 3B, lanes 7–9). We hypothesize that these forms are being visualized because of a dual effect: temperature-dependent partial denaturation exposes not only αS immunoreactive epitopes but also bound FA molecules, and the latter are captured by Lipidex.
The finding of αS association with various microsomal membranes is of special interest in light of its conserved FABP signature and its binding of FAs. FABP have been shown to transport free FAs from the aqueous phase of the cytoplasm to various membranes, during which the FABP transiently associates with the membrane to release a FA to or remove one from the membrane, a process called collisional transfer (41). The significant increase in the amount of αS associated with P25 microsomal membranes in both mouse brains and MES cells expressing the A53T familial PD mutation (Fig. 1C) raises the possibility that the function of αS in the FA transport process is partially altered by this mutation.
The accumulation of modified insoluble forms of wt αS in the Lewy bodies and Lewy neurites of idiopathic PD and in related cytoplasmic inclusions of other synucleinopathies leads us to speculate that a necessary FA transport function of cytosolic αS is altered in these disorders. Our mesencephalic neuronal cell model and tg mice should be useful in further testing this hypothesis.
Acknowledgments
We thank Drs. Wei-Dong Le and Stanley Appel (Baylor College of Medicine, Houston, TX) for providing the MES 23.5 cell line; Drs. Jean-Christoph Rochet and Peter Lansbury, Jr. (Brigham and Women's Hospital) for providing purified recombinant human αS, and Drs. Judith Storch, Marjorie Lees, and Omanand Koul for many helpful suggestions. This work was supported by the Morris K. Udall PD Center (National Institutes of Health Grant T50NS38375) at the Brigham and Women's Hospital.
Abbreviations
- αS
α-Synuclein
- PD
Parkinson's disease
- wt
wild type
- FABP
fatty acid-binding protein
- tg
transgenic
- FA
fatty acid
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- OA
oleic acid
- MES
rat mesencephalic neuronal cell line
References
- 1.Lansbury P T. Neuron. 1997;19:1151–1154. doi: 10.1016/s0896-6273(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini M G, Schmidt M L, Lee V M-Y, Trojanowski J Q, Jakes R, Goedert M. Nature (London) 1997;388:389–390. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Baba M, Nakajo S, Tu P-H, Tomita T, Nakaya K, Lee V M-Y, Trojanowski J Q, Iwatsubo T. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 4.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J T, Schols L, Riess O. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini M G, Crowther RA, Jakes R, Hasegawa M, Goedert M. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippa C F, Fujiwara H, Mann D M A, Giasson B, Baba M, Schmidt M L, Nee L E, O'Conell B, Pollen D, George-Hyslop P, et al. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway K A, Harper J D, Lansbury P T. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 9.Clayton D F, George J M. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 10.Hashimoto M, Masliah E. Brain Pathol. 1999;9:707–720. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger R, Muller T, Riess O. J Neural Transm. 2000;107:31–40. doi: 10.1007/s007020050002. [DOI] [PubMed] [Google Scholar]
- 12.Duda J E, Lee V M-Y, Trojanowski J Q. J Neurosci Res. 2000;61:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Iwai A, Masliah E, Ge N, Flanagan L, de Silva H A R, Kittel A, Saitoh T. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 14.George J M, Jin H, Woods W S, Clayton D F. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 15.Murphy D D, Rueter S M, Trojanowski J Q, Lee V M-Y. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenco J M, Rawlingson A, Daniels B, Morris A J. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 17.Exton J H. Physiol Rev. 1997;77:303–320. doi: 10.1152/physrev.1997.77.2.303. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y-G, Siddhanta A, Austin C D, Hammond S M, Sung T-C, Frohman M A, Morris A J, Shields D. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen P H, Nielsen M S, Jakes R, Dotti C G, Goedert M. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 20.Kahle P J, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, et al. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean P, Kawamata H, Ribich S, Hyman B T. J Biol Chem. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 22.Segrest J, Jones M, De Loof H, Brouillete C, Venkatachalapathi Y, Anatharamaiah G. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 23.Davidson W S, Jonas A, Clayton D F, George J M. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 24.Perrin R J, Woods W S, Clayton D F, George J M. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 25.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 26.Feany M B, Bender M W. Nature (London) 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 27.Narhi L, Wood S J, Steavenson S, Jiang Y, Wu G M, Anafi D, Kaufmann S A, Martin F, Sitney K, Denis P, et al. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 28.Conway K A, Lee S-J, Rochet J-C, Ding T T, Williamson R E, Lansbury P T. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway K A, Harper J D, Lansbury P T. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 30.Crawford G D J, Le W D, Smith R G, Xie W J, Stefani E, Appel S H. J Neurosci. 1992;12:3382–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto H, Buchner K, Beckmann R, Hilbert R, Hucho F. Neurochem Int. 1992;21:409–414. doi: 10.1016/0197-0186(92)90192-t. [DOI] [PubMed] [Google Scholar]
- 32.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Jakes R, Spillantini M G, Goedert M. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 34.Folch J, Lees M B, Sloane S G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 35.Smith A F, Tsuchida K, Hanneman E, Suzuki T C, Wells M A. J Biol Chem. 1992;267:380–384. [PubMed] [Google Scholar]
- 36.Wolfinger R D, O'Connel M. J Stat Comp Simul. 1993;48:233–243. [Google Scholar]
- 37.Maroteaux L, Campanelli J T, Scheller R H. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beijer K, Nystrpom E. Anal Biochem. 1972;48:1–8. doi: 10.1016/0003-2697(72)90164-9. [DOI] [PubMed] [Google Scholar]
- 39.Chojnacki T, Jankowski W, Jankowski T, Sasak W. Anal Biochem. 1975;69:114–119. doi: 10.1016/0003-2697(75)90572-2. [DOI] [PubMed] [Google Scholar]
- 40.Glatz J F C, Veerkamp J H. Anal Biochem. 1983;132:89–95. doi: 10.1016/0003-2697(83)90429-3. [DOI] [PubMed] [Google Scholar]
- 41.Storch J, Thumser A E A. Biochem Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 42.Veerkamp J H, Peeters R A, Maatman R G H J. Biochem Biophys Acta. 1991;1081:1–24. doi: 10.1016/0005-2760(91)90244-c. [DOI] [PubMed] [Google Scholar]
- 43.Chan D S, Lees M B. Biochemistry. 1974;13:2704–2712. doi: 10.1021/bi00710a008. [DOI] [PubMed] [Google Scholar]
- 44.Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. Development (Cambridge, UK) 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 45.Petersen K, Olesen O F, Mikkelsen J D. Neuroscience. 1999;91:651–659. doi: 10.1016/s0306-4522(98)00596-x. [DOI] [PubMed] [Google Scholar]