Abstract
Individuals with 22q11.2 deletion syndrome (22q11DS) are at markedly elevated risk for schizophrenia-related disorders. Stability, emergence, remission and persistence of psychosis-spectrum symptoms were investigated longitudinally. Demographic, clinical and cognitive predictors of psychosis were assessed. Prospective follow-up over 2.8 years was undertaken in 75 individuals with 22q11DS aged 8–35 years. Mood, anxiety, attention-deficit hyperactivity disorders and psychosis-spectrum symptoms were assessed with the Kiddie-Schedule for Affective Disorders and Schizophrenia and Scale of Prodromal Symptoms (SOPS). Four domains of cognition were evaluated with the Penn Computerized Neurocognitive Battery (executive functioning, memory, complex cognition and social cognition). Psychotic disorder or clinically significant SOPS-positive ratings were consistently absent in 35%, emergent in 13%, remitted in 22% and persistent in 31% of participants. Negative symptoms and functional impairment were found to be predictive of the emergence of positive psychosis-spectrum symptoms and to reflect ongoing deficits after remission of positive symptoms. Dysphoric mood and anxiety were predictive of emergent and persistent-positive psychosis-spectrum symptoms. Lower baseline global cognition and greater global cognitive decline were predictive of psychosis-spectrum outcomes but no particular cognitive domain stood out as being significantly more discriminating than others. Our findings suggest that negative symptoms, functioning and dysphoric mood are important predictors of psychosis risk in this population.
Introduction
Individuals with 22q11.2 deletion syndrome (22q11DS) are at markedly elevated risk for schizophrenia-related disorders, with 25% or more meeting criteria by adulthood.1, 2 Even greater proportions experience subthreshold symptoms of psychosis that are clinically significant and cause impairment but do not meet criteria for a psychotic disorder.3 The 22q11.2 deletion results in haploinsufficiency of 46 protein-coding genes and 90 total genes including several with effects on neurotransmission (COMT, PRODH), myelination (PI4KA), mitochondrial functioning (MRPL40, ZDHHC8) and dendritic spine morphology (DGCR8).4, 5, 6, 7 Onset and features across the psychosis-spectrum are largely comparable between individuals with 22q11DS and the general population.8, 9 Mouse models with homologous copy number variations have been created that demonstrate social behavioral deficits, and provide insights into gene function and sources of phenotypic heterogeneity.10 As such, 22q11DS is an important window for elucidating genetic and neurobiological substrates of psychosis risk.11
Longitudinal studies of psychopathology and cognition in 22q11DS have been conducted at several sites to determine clinical and cognitive predictors of psychosis. Replicated findings suggest that conversion to psychosis is predicted by clinical features including subthreshold symptoms, global functioning and ultra high-risk status.12, 13 There is also convergent evidence that cognitive features predict subthreshold psychotic symptoms and psychosis; implicated measures include lower baseline verbal intelligence quotient (VIQ), greater decline in VIQ, lower baseline full scale intelligence quotient (FSIQ) and greater decline in FSIQ.13, 14, 15, 16 Baseline anxiety has also been related to psychosis by multiple sites.13, 17, 18
Other results are more equivocal. The catechol O-methyltransferase low activity allele (COMT Met) was initially suggested to confer greater risk for psychosis,13, 16 but not confirmed in subsequent studies.18, 19 Some studies identified deficits in specific cognitive skills as predictive of psychosis, including executive function, visual learning, cognitive set shifting and emotion recognition.17, 19, 20
Elucidating psychosis predictors in 22q11DS is crucial for clinical care in this vulnerable population and understanding mechanisms that produce psychotic illness in some while sparing others. Waxing and waning symptoms of psychosis have been described in the literature for non-deleted at-risk youths and greater persistence of subthreshold psychotic symptoms appears to predict transition to threshold psychotic disorders.21, 22 Studies in 22q11DS have focused on conversion to psychosis or emergence of positive subthreshold symptoms but do not adequately investigate negative symptoms or other fluctuations in psychosis symptomatology like remission and persistence. Few studies of 22q11DS, measure multiple domains of cognition or provide detailed symptom-level information. It is also unknown whether identified predictors of psychosis are specific to psychotic processes or whether they predict psychiatric illness generally.
We address the following aims: (1) Demographic, clinical and cognitive measures were compared across four groups showing different patterns of psychosis-spectrum symptoms; namely, those with stable-nonpsychotic, emergent, remitted or persistent symptoms. Multiple domains of cognition were examined. (2) On a finer level of resolution, we investigated symptom-level data describing functional impairment, positive and negative symptoms, with a particular focus on features that may precede emergence or reflect ongoing impairment after remission. (3) We evaluate the predictive utility of demographic variables, multiple cognitive domains and clinical measures for psychosis-spectrum outcomes. (4) Anxiety, mood and attention-deficit hyperactivity disorders (ADHD) were evaluated against the same independent variables to determine the specificity of psychosis as predicted outcome.
Materials and methods
Sample
Participants were drawn from an ongoing study of brain and behavior in 22q11DS and represent the full psychosis-spectrum from total absence of psychosis symptoms to threshold psychotic disorders. They were recruited primarily through the ‘22q and You Center’ at the Children’s Hospital of Philadelphia. Inclusion criteria were pre-established and include: molecularly confirmed 22q11.2 deletion, ambulatory medical status, estimated IQ above 70 and ability to give informed consent/assent. Seventy-five individuals aged 8–35 years underwent repeated assessments and were included in this study. The size of the sample was limited by availability of affected patients and demands of repeated assessments. The sample (n=75) has 80% power to detect an effect size of 0.39.23 Sex was roughly evenly distributed with 48% female. The majority underwent two assessments (n=62), whereas 11 underwent three and 2 underwent four assessments. Mean follow-up interval was 2.8±1.2 years. To maximize the arc of symptom development available, the first and most recent clinical assessments were analyzed. The Institutional Review Boards of the University of Pennsylvania and the Children’s Hospital of Philadelphia approved all procedures. Informed consent/assent was obtained from each participant and accompanying parent/guardian at each time point. No changes were made in the participants’ medical and behavioral treatment. Thirty-one individuals had a lifetime history of receiving a psychotropic medication of any class. Antidepressants had been used by 20 individuals, stimulants by 11 and antipsychotic medications by 5. Further details on methods and neuropsychiatric measures in these participants have been published.2, 3, 8, 24, 25, 26
Clinical measures
Clinical phenotyping was completed using the Structured Interview for Prodromal Syndromes v.4.0 (SIPS)27, 28 and Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS).29 K-SADS sections assessed DSM-IV psychosis, mood, substance-related disorders and ADHD. Clinical assessments were administered by Bachelor’s and Master’s level interviewers who underwent formal training conducted by a clinical psychology faculty member (MEC). Participants and parents were also seen separately by an established clinical investigator. Elicited psychosis symptoms were rated according to the Scale of Prodromal Symptoms (SOPS); standardized anchors corresponded to a 7-point scale: 0=absent, 1=questionably present, 2=mild, 3=moderate, 4=moderately severe, 5=severe (but not psychotic) and 6=severe and psychotic/extreme.27, 28 A rating of 3 or higher is considered clinically significant. Only symptoms occurring in the last 6 months were considered for SOPS ratings. Narrative case summaries were presented at consensus case conferences where SOPS scores and diagnoses were finalized by consensus by 2 or more doctoral level clinicians. Threshold psychotic disorders were determined using DSM-IV-TR criteria.30 Global assessment of function (GAF) was determined according to SOPS anchors. Follow-ups covered the same scope but only intervening experiences were assessed.
There were four patterns of change in psychosis-spectrum symptoms: ‘Persistent’—threshold psychotic disorder or clinically significant rating on SOPS P1-P5 at both time points; ‘Remitted’—met these criteria only at baseline; ‘Emergent’—met criteria only at follow-up; and ‘Stable-Nonpsychotic’—never met criteria. Three participants completed only cognitive evaluations at baseline and were not grouped.
Cognitive measures
The cognitive domains of executive function, episodic memory, complex cognition and social cognition were assessed at all time points using the Penn Computerized Neurocognitive Battery (CNB), which includes 12 neurocognitive tasks that have been extensively characterized.31, 32, 33 Reading proficiency was evaluated using the Wide Range Achievement Test 4 reading subtest.34 Normalized scores were calculated from mean standardized accuracy then z-transformed against the entire sample over both time points. The global cognition score reflects aggregate performance in the entire CNB, whereas each domain is calculated from their three constituent tasks (Supplementary Table 1). Scores from the Wide Range Achievement Test and Penn Verbal Reasoning Test provide a verbal composite score that was constructed to reflect the inclusion of VIQ in prior studies. Baseline and change in global cognition and verbal composite score were included as independent variables in separate prediction models because they had been implicated in the literature as likely predictors of psychosis-spectrum outcomes.13, 14, 15
Analyses
Exploratory factor analyses were conducted on the SOPS items to derive empirical factors and condense variables included in prediction models (Table 1). Factor scores were calculated using the Thurstone method35 in the psych package in R.36 The number of factors to extract was determined by parallel analysis,37 the minimum average partial method,38 the minimum empirical Bayesian Information Criterion39 and visual examination of the scree plot, all of which suggested two factors as optimal. Least-square extraction and oblimin rotation were used. The two factors include one representing unusual thoughts and experiences (factor 1), and another representing impairment in social, occupational and daily functioning (factor 2). Exploratory factor analyses are sample dependent, and there was no expectation that our solution would be identical to others conducted in 22q11DS. Nevertheless, factor 1 came out to be equivalent to the ‘Positive’ factor from a three-factor solution in our previous publication3 and very similar to the ‘Attenuated Positive Symptoms’ factor from a three-factor solution published by a different group (which included P5 and not N4).40
Table 1. Two-factor solution for psychosis-spectrum symptoms.
| Items | Factor 1: Unusual thoughts and experiences | Factor 2: Impairment in social, occupational and daily functioning |
|---|---|---|
| P1—Unusual thought content/delusional ideas | 0.98 | |
| P4—Perceptual abnormalities/hallucinations | 0.77 | |
| D2—Bizarre thinking | 0.70 | |
| P3—Grandiosity | 0.61 | |
| P2—Suspiciousness/persecutory ideas | 0.53 | 0.33 |
| N4—Experience of emotions and self | 0.37 | |
| N6—Occupational functioning | 0.73 | |
| N3—Expression of emotions | 0.68 | |
| N2—Avolition | 0.63 | |
| G2—Dysphoric mood | 0.62 | |
| G3—Motor disturbances | 0.59 | |
| G4—Impaired tolerance to normal stress | 0.59 | |
| N1—Social anhedonia | 0.59 | |
| D4—Personal hygiene | 0.55 | |
| D1—Odd appearance or behavior | 0.36 | 0.54 |
| G1—Sleep disturbances | 0.43 | |
| P5—Disorganized communication | 0.42 | |
| N5—Ideational richness | 0.40 | |
| D3—Trouble with focus and attention | 0.35 |
Loadings <0.30 are not included. Bolded values represent primary factor loadings.
Continuous variables were compared using analysis of variance (ANOVA) and categorical variables were compared using Fisher’s exact tests.41 Variance was compared using F-tests and were dissimilar between groups. Non-parametric Kruskal–Wallis tests confirmed the results of the ANOVA for all comparisons.41 Other statistical assumptions were met. Income was estimated for each household based on the median yearly household income in the subject’s zip code, as reported by the American Community Survey. P-values for SOPS item-wise analyses were corrected for multiple comparisons using the false discovery rate (FDR) method.42 Effect sizes were calculated using the standard formula for Cohen’s d. Models were tested using linear (for continuous outcomes) and logistic (for categorical outcomes) regressions in R.43 Predictors initially included all available demographic, clinical and cognitive variables. However, standard errors were highly inflated because of collinearity. Race, estimated income, maternal education level and follow-up interval consistently failed to predict any outcome of interest and were omitted from the final models. For the same reason, cognition was treated as a single overall variable—that is, separate domain scores were not used. We selected emergence or persistence of psychosis as the categorical outcome based on the proneness–persistence–impairment model, which posits that conversion to psychosis results from accumulated persistence and worsening of symptoms.22 Other outcome measures included change in total SOPS score, and change in factor 1 and 2 scores to capture psychosis-related outcomes from a dimensional perspective.
Results
Comparisons among groups with stable/nonpsychotic, emergent, remitted and persistent-positive psychosis-spectrum symptoms
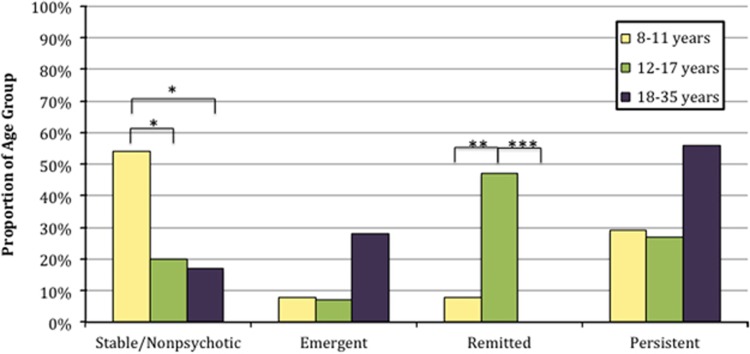
Psychosis-spectrum symptoms emerged in 9 individuals, remitted in 16, persisted in 25 and were stable-nonpsychotic in 22. Table 2 compares these groups across demographic, clinical and cognitive measures. Among demographic variables, groups differed in initial age (P=0.04) and sex (P=0.04). Pairwise comparisons reveal that mean initial age in the emergent group was older than in the remitted (P=0.02; d=1.17) and stable-nonpsychotic groups (P=0.04; d=0.86). Also, the remitted group included more males (81%) than the emergent (33% P=0.03) or persistent groups (40% P=0.01). There were no differences for follow-up interval, race, maternal education or estimated socio-economic status. A greater proportion of individuals 8–11 years old at baseline remained stable-nonpsychotic compared with the older individuals, whereas a greater proportion of individuals age 12–17 years old reported remission of psychosis-spectrum symptoms (Figure 1).
Table 2. Changes in psychosis-spectrum symptoms.
| Stable/nonpsychotic | Emergent | Remitted | Persistent | P-value | |
|---|---|---|---|---|---|
| n (%) | 22 (35%) | 9 (13%) | 16 (22%) | 25 (31%) | |
| Demographic | |||||
| T1 age (mean±s.d., yrs) | 13.5±6.4 | 19.3±7.7 | 14.4±2.2 | 17.5±7.2 | 0.04 |
| Follow-up interval (mean±s.d., yrs) | 2.5±1.2 | 2.6±1.4 | 3.1±1 | 3±1.2 | 0.42 |
| Sex (%female) | 41% | 67% | 19% | 60% | 0.04 |
| Race (%Caucasian) | 82% | 78% | 89% | 72% | 0.34 |
| Maternal education (mean±s.d., yrs) | 14.8±1.7 | 13.4±1.4 | 15±2.9 | 14.2±2.4 | 0.39 |
| Estimated household income (mean±s.d., USD) | 68k±21k | 69k±25k | 67k±16k | 72k±26k | 0.90 |
| Clinical | |||||
| T1 factor 1 (mean±s.d.) | −0.6±0.3 | −0.6±0.3 | 0.5±0.9 | 0.8±1.1 | 0.00 |
| T1 factor 2 (mean±s.d.) | −0.7±0.5 | −0.1±0.6 | 0.2±1 | 0.5±1 | 0.00 |
| T1 GAF (mean±s.d.) | 72.7±11.3 | 63.6±8.6 | 56.8±13.3 | 53.3±15.2 | 0.00 |
| Lifetime mood disorder (n, %) | 2 (9%) | 4 (44%) | 3 (19%) | 11 (44%) | 0.03 |
| Lifetime anxiety disorder (n, %) | 12 (55%) | 9 (89%) | 11 (67%) | 17 (68%) | 0.32 |
| Lifetime GAD (n, %) | 6 (27%) | 3 (33%) | 4 (25%) | 10 (40%) | 0.72 |
| Lifetime OCD (n, %) | 0 (0%) | 2 (22%) | 2 (13%) | 2 (8%) | 0.20 |
| Lifetime ADHD (n, %) | 12 (55%) | 5 (56%) | 9 (56%) | 9 (36%) | 0.49 |
| Lifetime substance-related (n, %) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0.28 |
| Lifetime psychotropic use (n, %) | 9 (41%) | 4 (44%) | 4 (25%) | 12 (48%) | 0.53 |
| Neurocognitive | |||||
| T1 verbal composite (mean±s.d.) | −0.3±1.1 | −0.4±0.8 | 0.1±1 | −0.3±0.8 | 0.51 |
| T1 global cognition (mean±s.d.) | −0.2±0.9 | −0.4±0.8 | 0.0±0.5 | −0.2±0.5 | 0.43 |
| Change in verbal composite (mean±s.d.) | 0.4±0.6 | 0.4±1 | 0.2±0.5 | 0.2±0.7 | 0.62 |
| Change in global cognition (mean±s.d.) | 0.4±0.7 | 0.2±0.9 | 0.3±0.3 | 0.3±0.4 | 0.86 |
Abbreviations: ADHD, attention-deficit hyperactivity disorder; factor 1, unusual thoughts and experiences; factor 2, impairment in social, occupational, and daily functioning; GAD, generalized anxiety disorder; GAF, global assessment of function; OCD, obsessive-compulsive disorder; s.d., standard deviation; T1, initial time point; USD, Unite States dollars; yrs, years.
Mood disorders include unipolar depression and bipolar disorders, as well as unspecified depressive and mood disorders and dysthymia. Anxiety disorders include GAD, OCD, unspecified anxiety disorder, social and separation anxiety. Substance-related disorders included alcohol abuse; there were no instances of drug abuse or dependence, including marijuana. Comorbidities may have been in full or partial remission, or met full criteria. Bolded P-values represent significant group effect.
Figure 1.
Changes in psychosis symptoms by age group. The proportion of participants in the indicated age group with stable/nonpsychotic, emergent, remitted and persistent symptoms is represented for each of the respective groups. Baseline ages were used. *P<0.05; **P<0.01; ***P<0.001.
Baseline factor scores (factor 1: unusual thoughts and experiences; factor 2: impairment in social, occupational and daily functioning) varied significantly by group, along with baseline functioning and prevalence of mood disorders (Table 2). Pairwise comparisons showed that factor 1 scores were higher in the remitted and persistent groups than in the stable/nonpsychotic (P<0.001 vs remitted; P<0.001 vs persistent) and emergent groups (P=0.003 vs remitted; P=0.001 vs persistent); meanwhile, factor 1 scores were not significantly different between stable/nonpsychotic and emergent, or between remitted and persistent groups. In contrast, baseline factor 2 scores were already higher in the emergent group compared with the stable/nonpsychotic (P=0.03; d=1.13), and did not significantly differ from the remitted or persistent groups. The emergent group was similarly indistinguishable from the remitted and persistent groups in baseline global functioning (GAF); there was a non-significant trend toward greater impairment in the emergent than stable-nonpsychotic (P=0.06; d=0.87). Compared with the stable/nonpsychotic (9%), lifetime mood disorders were more prevalent in the emergent (44% P=0.04) and persistent groups (44% P=0.01). There were no significant differences for lifetime anxiety disorders, ADHD, substance-related disorders or lifetime psychotropic use. Lifetime antipsychotic use did differ among groups (P=0.04), occurring in two individuals with emergent symptoms and three with persistent symptoms. There were no differences for antidepressant or stimulant use.
Regarding cognition, there were no significant differences between groups for baseline verbal composite score, baseline global cognition, change in verbal composite score or change in global cognition. Examining the separate cognitive domains, there were no group differences in executive functioning, episodic memory, complex cognition or social cognition.
Individuals who remained stable/nonpsychotic reflect resilience to psychosis compared with those who experienced psychosis-spectrum symptoms at either baseline or follow-up. This comparison is highlighted in Supplementary Table S4. In addition to lower baseline symptoms, they also demonstrated higher baseline global functioning (P<0.001, d=1.32) fewer lifetime mood disorders (9 vs 36% prevalence; P=0.02).
Symptom-level changes over time
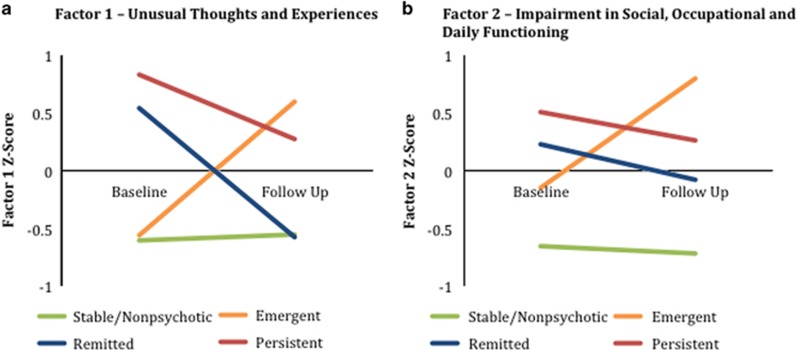
Figure 2 illustrates changes between baseline and follow-up for factor 1 and factor 2. On the basis of group definitions, a default pattern is expected where, at baseline, scores are similar for stable and emergent groups and higher for the remitted and persistent groups. At follow-up, factor scores are expected to be similar for stable and remitted groups and higher for the emergent and persistent groups. This pattern was reflected by changes in factor 1 scores, which corresponds to unusual thoughts and experiences. In contrast, factor 2 (impairment in social, occupational and daily functioning) was elevated at both time points for the emergent, remitted and persistent groups relative to the stable/nonpsychotic group.
Figure 2.
Factor scores at baseline and follow-up: individuals with stable/nonpsychotic, emergent, remitted and persistent psychosis were compared on mean scores for (a) factor 1 and (b) factor 2 at baseline and follow-up. Pairwise comparisons between groups for factor 1 show that at baseline, the remitted and persistent groups each score significantly higher than each of the stable/nonpsychotic and emergent groups. At follow-up, it is the persistent and emergent groups that score significantly higher on factor 1 than the stable/nonpsychotic and remitted groups. Pairwise comparisons for factor 2 at both baseline and follow-up show that the emergent, remitted and persistent groups do not differ significantly from one another but each score significantly higher than the stable/nonpsychotic group.
Initial and follow-up scores are shown for each of the SOPS items in Supplementary Figure 1.
Thirteen of the 19 SOPS items discriminated across groups at baseline, and fourteen discriminated at follow-up with significant group effects. These included all of the items represented by Factor 1, as well as P5—disorganized communication, N2—avolition, N3—expression of emotions, N5—ideational richness, D1—odd appearance or behavior, D4—personal hygiene, G1—sleep disturbances, G2—dysphoric mood, G3—motor disturbances and G4—impaired tolerance to normal stress. Pairwise comparisons reflect the default pattern for the majority of items. There were notable exceptions. At baseline, the emergent group already scored higher than stable-nonpsychotic in dysphoric mood (P=0.01; d=1.20) and sleep disturbance (P=0.002; d=1.43). Furthermore, remitted scored lower than persistent in dysphoric mood (P=0.04; d=−0.71) and ideational richness (P=0.04; d=−0.67). At follow-up, remitted individuals continued to be more impaired than stable/nonpsychotic in experience of emotions and self (P=0.05; d=0.80), expression of emotions (P=0.01; d=0.94), motor disturbance (P=0.01; d=0.92), personal hygiene (P=0.001; d=1.52) and ideational richness (P=0.008; d=0.96).
Prediction of psychosis
Threshold psychotic disorders were present in seven individuals at baseline, with an additional five converting to psychosis by follow-up. Age at conversion ranged from 14–31 years. Their diagnoses at follow-up included schizophrenia (n=3), psychotic disorder not otherwise specified and brief psychotic disorder. Three of the five had subthreshold psychosis at baseline, four had a history of anxiety disorder, and all had a history of mood disorder. Predictors of conversion to threshold psychosis were not formally assessed due to low incidence.
Prediction models tested four outcomes reflecting psychosis-spectrum symptoms at follow-up (Table 3): emergence or persistence of psychosis, change in total SOPS score, change in factor 1 score (unusual thoughts and experiences), and change in factor 2 score (impairment in social, occupational and daily functioning). Female sex was associated with emergence or persistence of psychosis and increased unusual thoughts and experiences. Higher baseline factor 1 score was predictive of the emergence or persistence of psychosis-spectrum symptoms. History of mood disorder contributed to greater increase in total SOPS and factor 1 scores, whereas history of anxiety disorder was predictive of emergence or persistence of psychosis. Lower baseline global cognition was predictive of all outcomes, whereas decline in cognition was predictive of all but emergence or persistence of psychosis.
Table 3. Predicting the psychosis-spectrum.
|
Outcomes |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors |
Emergence or persistence of psychosis |
Change in total SOPS score |
Change in factor 1: Unusual thoughts and experiences |
Change in factor 2: Impairment in social, occupational and daily functioning |
||||||||
| Coeff. | z | P | Coeff. | t | P | Coeff. | t | P | Coeff. | t | P | |
| T1 age | 0.15 | 1.76 | 0.08 | 0.02 | 1.25 | 0.22 | 0.01 | 0.31 | 0.76 | 0.03 | 1.53 | 0.13 |
| Sex | 2.12 | 1.99 | 0.05 | 0.25 | 1.35 | 0.18 | 0.59 | 2.92 | 0.01 | 0.01 | 0.04 | 0.97 |
| T1 factor 1 | 1.55 | 2.38 | 0.02 | −0.18 | −1.54 | 0.13 | −0.78 | −6.18 | 0.00 | 0.00 | 0.03 | 0.98 |
| T1 factor 2 | −0.17 | −0.23 | 0.82 | −0.42 | −2.65 | 0.01 | −0.23 | −1.38 | 0.18 | −0.50 | −2.75 | 0.01 |
| T1 GAF | −0.04 | −1.00 | 0.32 | 0.01 | 1.25 | 0.22 | 0.00 | −0.34 | 0.73 | 0.01 | 1.19 | 0.24 |
| T1 mood disorder | 1.31 | 1.06 | 0.29 | 0.70 | 2.55 | 0.01 | 1.12 | 3.79 | 0.00 | 0.50 | 1.61 | 0.11 |
| T1 anxiety disorder | 1.82 | 1.96 | 0.05 | 0.21 | 0.99 | 0.33 | 0.22 | 0.98 | 0.33 | 0.20 | 0.83 | 0.41 |
| T1 ADHD | −0.81 | −0.90 | 0.37 | 0.18 | 0.85 | 0.40 | −0.02 | −0.08 | 0.93 | 0.31 | 1.33 | 0.19 |
| Lifetime psychotropic use | 0.94 | 1.03 | 0.30 | −0.03 | −0.14 | 0.89 | −0.03 | −0.14 | 0.89 | 0.07 | 0.29 | 0.77 |
| T1 global cognition | −2.50 | −2.34 | 0.02 | −0.59 | −3.37 | 0.00 | −0.39 | −2.18 | 0.03 | −0.57 | −2.98 | 0.00 |
| Change in global cognition | −1.21 | −1.12 | 0.26 | −0.81 | −3.79 | 0.00 | −0.52 | −2.37 | 0.02 | −0.83 | −3.56 | 0.00 |
| CNB trials | −1.74 | −1.93 | 0.05 | 0.02 | 0.12 | 0.91 | 0.07 | 0.39 | 0.70 | 0.16 | 0.91 | 0.37 |
Abbreviations: ADHD, attention-deficit hyperactivity disorder; CNB, Computerized Neurocognitive Battery; Coeff, coefficient; GAF, global assessment of function; SOPS, Scale of Prodromal Symptoms; T1, time 1/initial evaluation.
CNB trials was added as covariate because of possible practice effects and non-uniform total number of trials administered to participants who are evaluated at 2–4 time points. Bolded entries highlight statistical significance.
Substituting verbal composite score for global cognition, psychosis continues to be predicted by initial factor scores, mood disorder, and anxiety disorders (Supplementary Table 2). Lower baseline verbal composite score is predictive of rise in total and factor 2 scores, but greater decline in verbal composite score was not predictive of any outcome.
Prediction of other psychiatric illnesses
The same regression models were conducted for mood, anxiety and ADHD disorders, omitting the diagnosis in question from the potential predictors (Supplementary Table 3). None of demographic, clinical or cognitive variables were predictive of these other psychiatric disorders.
Discussion
Implications for negative symptoms and functional deficits
Negative symptoms and functional deficits were found to be important both as predictors of the emergence of positive psychosis-spectrum symptoms and as areas of ongoing impairment for those who experience a remission of positive symptoms. At baseline, individuals who would experience emergent psychosis had higher factor 2 scores, which includes negative symptoms and manifestations of impairment in social, occupational and daily functioning such as anhedonia, asociality, amotivation, disorganization, impaired stress tolerance and decline in occupational functioning. At follow-up, individuals with remitted positive symptoms continued to show impairment in experience of emotions and self, expression of emotions, motor disturbances, personal hygiene and ideational richness. Furthermore, baseline global functioning was impaired at a similar extent in emergent, remitted and persistent groups.
To our knowledge, this the first report on detailing the predictive nature of negative and functional impairment symptoms for the emergence of psychosis-spectrum symptoms in 22q11DS and the first evidence that negative symptoms and functional impairment may continue after positive symptoms have diminished. Global functioning was predictive of conversion to psychosis in another 22q11DS sample.12 These results are consistent with findings in non-deleted community and help-seeking samples also suggesting that negative symptoms and functional impairment are predictors of psychosis.21, 44, 45, 46 Altogether, the consideration of negative and disorganized symptoms in determining psychosis-proneness appears to be warranted.
Dysphoric mood accompanies psychotic experiences
Psychotic experiences in individuals with 22q11DS are often accompanied by dysphoric mood, manifesting as mood or anxiety disorders. We found baseline mood and anxiety disorders to be predictive of measures of psychosis. Furthermore, mood disorders were less common in stable-nonpsychotic individuals. Baseline dysphoric mood also preceded the emergence of psychosis symptoms and portended subsequent remission. Sleep, typically disturbed in the presence of dysphoric mood, was more impaired prior to emergence of psychosis. Of the five individuals who developed a psychotic disorder, all had a history of mood disorders and four also had an anxiety disorder. Other studies in 22q11DS similarly show that conversion to psychosis is predicted by baseline anxiety disorder18 and anxious/depressed symptoms.13, 17 In samples without the deletion, dysphoric mood is elevated in individuals with psychosis-spectrum symptoms;21, 47 greater dysphoric mood is not predictive of conversion to psychosis21, 47 but decreased dysphoric mood is predictive of symptomatic and functional remission.48
On the other hand, the same predictors of psychosis did not predict other psychiatric disorders, including mood and anxiety disorders. This suggests that although psychosis is often accompanied by disturbances in mood and anxiety, mood and anxiety disorders need not be associated with psychotic experiences.
Consistent rates of emergent and persistent psychosis symptoms
Psychosis-spectrum symptoms persisted in 61% of individuals with psychotic disorder or significant positive subthreshold symptoms at baseline, remitted in 39%, and significant positive psychosis-spectrum symptoms emerged in 29% of non-spectrum. These rates are largely concordant with a 4-year longitudinal study in 22q11DS where subthreshold-positive psychotic symptoms also persisted in just over half (54%) of those with symptoms at baseline, remitted in under a half (45%) and emerged in around one third (39%) of individuals initially without psychosis-spectrum symptoms.49 To our knowledge, this is the first replication of these results. In non-deleted youths from the Philadelphia Neurodevelopmental Cohort (PNC), who were followed for 2 years and assessed with similar procedures, rates of persistence and remission are comparable (51 % and 49 %, respectively), but rates of emergence (16%) are around half of those reported in the 22q11DS studies.21
There are several important points to consider when interpreting these rates. Age, likely, has a role in observed rates of persistence and emergence. Reported rates of emergent psychosis-spectrum symptoms in 22q11DS may be relatively reduced compared with non-deleted samples due to inclusion of younger participants who have not reached the highest risk periods. Thirty-five percent of our sample was younger than 12 years at baseline. Criteria used to define the psychosis-spectrum outcome also influence results. The PNC criteria included both significant positive and negative/disorganized symptoms.21 If we adopt the same criteria, then the proportion of participants with persistent symptoms in our sample increases to 84% and rate of emergence increases to 56%. In addition, follow-up intervals differ across studies; rates of persistence are likely to drop with longer follow-up intervals, whereas rates of emergence are likely to increase. Despite these limitations in comparing rates of emergence, remission and persistence of psychosis-spectrum symptoms, both non-deleted and 22q11DS longitudinal studies clearly demonstrate instability and fluidity in psychosis symptoms for young participants.
Psychosis-spectrum symptoms may vary by age and sex
Psychosis symptoms emerge across a wide age-range from 8 to 28 years, but may be more likely to remit in younger individuals. Symptoms appear to be in the greatest flux during adolescence. With mean baseline age of 19 years, individuals with emergent psychotic symptoms were significantly older than those with stable or remitted symptoms (mean: 14 years). Children were the most likely to remain stable/nonpsychotic, whereas adolescents aged 12–17 years were the most likely to experience remitted psychosis symptoms. Other sites have also found that psychosis most commonly emerges in adolescence for individuals wit1h 22q11DS.18, 49
Regarding sex differences, being female predicted psychosis-spectrum symptoms, whereas being male was associated with remission. In an earlier publication, we showed that psychosis-proneness was more prevalent in males than females.8 However, prevalence of psychotic disorders does not appear to differ by sex in 22q11DS.1 One possible explanation is that males are more likely to experience transient symptoms of psychosis compared with females, therefore, leading female sex to be more predictive of lasting symptoms. Female sex may also confer risk through its association with higher rates of mood and anxiety disorders, which has been demonstrated in 22q11DS.1 Inadvertent over-sampling of female participants with psychosis may have a role and further study is clearly needed.
Cognitive deficit is predictive of psychosis-spectrum symptoms
When considered together with demographic and clinical variables, lower baseline global cognition, greater cognitive decline between initial and follow-up assessments, and lower baseline verbal cognition were predictive of psychosis. These findings are consistent with those of other studies in 22q11DS as well as in the non-deleted help-seeking population.13, 14, 15, 18, 20, 50, 51 In 22q11DS, no one cognitive domain appears to be most critical to developing psychosis. Both lower baseline performance and greater decline over time are implicated. It is unclear whether psychosis is truly associated with nonspecific or heterogeneous cognitive impairments, or whether current studies fall short of the resolution required to illuminate a finer pattern.
Limitations
There are several limitations to our study. By including young participants, we prospectively capture emergence of psychosis, but many participants classified as stable/nonpsychotic will go on to develop symptoms, whereas some with persistent symptoms will experience remission. As suggested by residual impairments in individuals who experienced a remission in positive psychosis-spectrum symptoms, for some individuals, the observed remission may represent a temporary quiescence of positive symptoms and may be followed by later re-emergence. For these reasons, we were not able to meaningfully study resilience with this design, though resilience to psychosis in this population is as important to understand as psychosis risk. Although this sample is sizable, small to moderate effects may be missed. Conversion to psychotic disorders occurred in too few individuals to be analyzed in depth. Although our definition for psychosis reflects norms in the 22q11DS field, that is, threshold psychotic disorder or clinically significant rating on SOPS P1-P5, any cut-off is somewhat arbitrary and may influence findings. Participants were also followed for irregular intervals, though this was not found to be predictive of any psychosis-spectrum outcome. Antipsychotic medications were used by a small proportion of participants (n=5) but may have affected outcomes, along with other interventions that may have been a part of the participants’ usual care.
Conclusions
Psychosis symptoms fluctuate in youth with 22q11DS, with approximately half of symptomatic individuals experiencing remission after 2.8 years and new symptoms emerging in one of ten individuals yearly. Functional deficits and negative symptoms precede emergence of positive symptoms and continue after they remit. The emergence and presence of psychosis symptoms is accompanied by dysphoric mood and anxiety. Psychosis symptoms may occur at any age, but positive symptoms often emerge in adolescence. Lower baseline cognition and greater cognitive decline over time were predictive of psychosis. These findings contribute to our understanding of the development of psychosis in 22q11DS and continue to suggest a fluctuating process that mirrors and may be generalizable to the general non-deleted population, though occurring at a higher rate and possibly with greater genetic and mechanistic homogeneity. The existence of mouse and other animal models for 22q11DS provides exciting opportunities to explore neurodevelopmental mechanisms simultaneously in humans and in models that allow for targeted genetic manipulations. Greater mechanistic understanding of the development of psychosis holds great potential for disease-modifying or disease-preventing therapeutics.
Acknowledgments
We thank the participants and their families, Kosha Ruparel, R. Sean Gallagher, Margaret Souders, Arielle Swenson, Allison Port, Amy Cassidy, Christian Kohler and Karin Borgmann-Winter. Funding was received from NIH grants: MH087626, MH087636; Doris Duke Charitable Foundation Clinical Research Fellowship (SXT); T32 MH019112 (JJY) and K08 MH079364 (MEC).
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Schneider M, Debbané M, Bassett AS, Chow EW, Fung WLA, van den Bree MB et al. Psychiatric disorders from childhood to adulthood in 22q11. 2 deletion syndrome: results from the international consortium on brain and behavior in 22q11. 2 deletion syndrome. Am J Psychiatry 2014; 171: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med 2013; 44: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA et al. Subthreshold psychotic symptoms in 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry 2014; 53: 991–1000.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA et al. 22q11. 2 deletion syndrome. Nat Rev Dis Primers 2014; 1: 15071–15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraju P, Zakharenko SS. Mitochondria in complex psychiatric disorders: Lessons from mouse models of 22q11.2 deletion syndrome: hemizygous deletion of several mitochondrial genes in the 22q11.2 genomic region can lead to symptoms associated with neuropsychiatric disease. BioEssays 2017; 39; doi: 10.1002/bies.201600177 [e-pub ahead of print 3 January 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MJ, Lazaro MT, Jalbrzikowski M, Bearden CE. Converging levels of analysis on a genomic hotspot for psychosis: insights from 22q11.2 deletion syndrome. Neuropharmacology 2013; 68: 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guna A, Butcher NJ, Bassett AS. Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J Neurodev Disord 2015; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Moore TM, Calkins ME, Yi JJ, Savitt A, Kohler CG et al. The psychosis spectrum in 22q11.2 deletion syndrome is comparable to that of nondeleted youths. Biol Psychiatry 2016; 82: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry 2003; 160: 1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry 2013; 18: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry 2014; 75: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Armando M, Pontillo M, Vicari S, Debbané M, Schultze-Lutter F et al. Ultra high risk status and transition to psychosis in 22q11.2 deletion syndrome. World Psychiatry 2016; 15: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry 2007; 164: 663–669. [DOI] [PubMed] [Google Scholar]
- Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry 2015; 72: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Curtiss K, Schoch K, Keshavan MS, Allen A, Shashi V. A longitudinal examination of the psychoeducational, neurocognitive, and psychiatric functioning in children with 22q11.2 deletion syndrome. Res Dev Disabil 2013; 34: 1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci 2005; 8: 1500–1502. [DOI] [PubMed] [Google Scholar]
- Kates WR, Russo N, Wood WM, Antshel KM, Faraone SV, Fremont WP. Neurocognitive and familial moderators of psychiatric risk in velocardiofacial (22q11.2 deletion) syndrome: a longitudinal study. Psychol Med 2015; 45: 1629–1639. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbané M, Frisch A, Glaser B et al. Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry 2013; 52: 1192–1203.e3. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry 2010; 49: 333–344. [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Ramanathan S, Kates WR. Predicting cognition and psychosis in young adults with 22q11.2 deletion syndrome. Schizophr Bull 2016; pii: sbw135 [e-pub ahead of print 25 October 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Satterthwaite TD, Wolf DH, Turetsky BI, Roalf DR et al. Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two-year follow-up. World Psychiatry 2017; 16: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez MDG, Wichers M, Lieb R, Wittchen H-U, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull 2011; 37: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champely S. pwr: Basic Functions for Power Analysis, R Package Version 2017.
- Yi JJ, Calkins ME, Tang SX, Kohler CG, McDonald-McGinn DM, Zackai EH et al. Impact of psychiatric comorbidity and cognitive deficit on function in 22q11.2 deletion syndrome. J Clin Psychiatry 2015; 76: e1262–e1270. [DOI] [PubMed] [Google Scholar]
- Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry 2014; 19: 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Tang SX, McDonald-McGinn DM, Calkins ME, Whinna DA, Souders MC et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet 2014; 165: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 2002; 159: 863–865. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J et al. Prodromal assessment with the structured interview for prodromal syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003; 29: 703–715. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988. [DOI] [PubMed] [Google Scholar]
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR®. American Psychiatric Publishing: Washington DC, 2000. [Google Scholar]
- Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 2015; 29: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 2012; 26: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods 2010; 187: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT4). Psychological Assessment Resources: Lutz, 2006. [Google Scholar]
- Thurstone LL. The vectors of mind. Psychol Rev 1934; 41: 1. [Google Scholar]
- Revelle Wpsych: Procedures for Personality and Psychological Research, R package version 2016; 1.6.9. Northwestern University: Evanston, IL, USA.
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika 1965; 30: 179–185. [DOI] [PubMed] [Google Scholar]
- Velicer WF. Determining the number of components from the matrix of partial correlations. Psychometrika 1976; 41: 321–327. [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6: 461–464. [Google Scholar]
- Schneider M, Van der Linden M, Glaser B, Rizzi E, Dahoun SP, Hinard C et al. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res 2012; 196: 277–284. [DOI] [PubMed] [Google Scholar]
- STATA. SE Version 13.0. StataCorp LP: College Station, TX, USA.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- R. R Core Team. Vienna, Austria, 2014.
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res 2012; 196: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox SI, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Perkins DO et al. Premorbid functional development and conversion to psychosis in clinical high-risk youths. Dev Psychopathol 2013; 25: 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A et al. Long-term follow-up of a group at ultra high risk ("prodromal") for psychosis: the PACE 400 study. JAMA Psychiatry 2013; 70: 793–802. [DOI] [PubMed] [Google Scholar]
- Addington J, Liu L, Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA et al. North American prodrome longitudinal study (NAPLS 2): the prodromal symptoms. J Nerv Ment Dis 2015; 203: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G et al. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr Bull 2012; 38: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Schaer M, Mutlu AK, Menghetti S, Glaser B, Debbané M et al. Clinical and cognitive risk factors for psychotic symptoms in 22q11.2 deletion syndrome: a transversal and longitudinal approach. Eur Child Adolesc Psychiatry 2014; 23: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des 2012; 18: 399–415. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA et al. Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American prodrome longitudinal study. JAMA Psychiatry 2016; 73: 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.