Abstract
Purpose of review
Primary sclerosing cholangitis (PSC) is a rare, idiopathic biliary disease often with an insidious onset, variable disease course, and premature death related to benign and malignant PSC-related sequelae. This review aims to discuss the epidemiology, clinical variants, and natural history of PSC, incorporating data from recent population-based studies.
Recent findings
PSC naturally leads to cirrhosis, cholangiocarcinoma, other hepatobiliary malignancies, dominant strictures, hepatic osteodystrophy and bacterial cholangitis. The incidence of PSC appears to be increasing, the reasons for which are unclear. The time from diagnosis to liver transplant appears to be longer in more recent studies compared to earlier studies which, suggesting a better overall prognosis than previously believed. In addition, with an increasing number of patients undergoing liver transplantation for PSC, the frequency of death due to liver failure has decreased while cancer-related deaths have increased among patients with PSC.
Summary
PSC is a heterogeneous disease with a variety of clinical outcomes, both fatal and non-fatal. The progression of liver fibrosis in an individual patient is difficult to predict and may vary from a relatively benign, non-progressive form to a rapidly progressive form with the need for liver transplantation.
Keywords: Outcomes, epidemiology, prognosis, biliary tract disease
Introduction
Primary sclerosing cholangitis (PSC) is a rare biliary disease, often with an insidious onset and highly variable natural history. Up to about half of the patients may be asymptomatic at diagnosis and can remain stable for many years, whereas others present with or advance relatively rapidly to liver failure (1, 2) or cholangiocarcinoma (CCA) (3-5). There is a significant association between PSC and inflammatory bowel disease (IBD) (5-9), though considerable geographic variation has been reported (10). Prior to the liver transplant era, death from liver failure was the leading outcome in PSC, but now it has been largely supplanted by liver transplant, and deaths due to CCA are relatively more common (4). In this review, we discuss the epidemiology, clinical variants, and natural history of PSC.
Epidemiology
North American and European prevalence and incidence of PSC range from 8.5-16.2 per 100,000 persons (7, 11, 12), and 0.9-1.3 per 100,000 person-years respectively (6, 7, 11, 12). These studies come predominantly from specialized centers or small populations, which due to the rarity of the disease and referral bias, may have resulted in inflated estimates of prevalence and incidence. A recent, larger population-based study of almost 8 million patients in the Netherlands found a lower prevalence and incidence rate of 6.0 per 100,000 persons and 0.5 per 100,000 person-years, respectively (4**). Also, a near population-based data set in northern California found a similar prevalence and incidence of 4.03 per 100,000 persons and 0.41 per 100,000 person-years, respectively (5). Lastly, United Kingdom's General Practice Research Database estimated the prevalence and incidence at 3.85 per 100,000 and 0.41 per 100,000 person-years (13). When examined over time, there has been a slow but significant increase in the prevalence of PSC cases (4, 7, 11, 13). One study reported an increase in average annual percentage change of 3.06 (11); in addition to reflecting an actual increase in incidence, this change may be due to improved survival, increased diagnosis due to increased disease awareness; increased testing of liver biochemistries, and/or increased use of magnetic resonance (MR) imaging.
In non-white populations, transplant data suggest that African Americans are affected with PSC at rates similar to whites, but tend to be younger and have higher model for end-stage liver disease (MELD) scores at the time of listing for liver transplantation compared to whites and Hispanics, suggesting a more severe disease phenotype. (14). In contrast, in Alaskan natives, PSC was not found in a large study of liver disease patients (15), and in Japan and Singapore, limited data suggest that PSC is rare in East Asia (16, 17).
Diagnosis
According to the American Association for the Study of Liver Diseases (AASLD), PSC is diagnosed in a patient with cholestatic laboratory values, bile ducts with multifocal strictures and segmental dilations on magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP), and when secondary causes of sclerosing cholangitis are excluded (18). MRCP has a sensitivity and specificity of 80% and 87%, respectively, and ERCP has a sensitivity and specificity of 89% and 80%, respectively (19). MRCP is preferentially utilized for diagnosis in order to avoid the potential complications of ERCP, whereas ERCP can facilitate therapeutic intervention and biliary specimen acquisition during the procedure (20, 21*). Liver biopsy is seldom used due to its low diagnostic value (20, 22). Indeed, the pathognomonic periductal fibrosis (“onion-skinning”) is only seen in 14% of patients (22). It should be noted that serum liver tests may spontaneously normalize in up to 20% of patients with PSC, and in rare instances, may be normal at the time of diagnosis (23).
Clinical Variation in PSC
The heterogeneity of PSC has become increasingly apparent with attempts to group patients into variants of PSC based upon clinical features. Whether these variants represent different pathogenic processes leading to similar outcomes or different manifestations of the same disease remains unclear. Although numerous variants have been described based upon sex, IBD presence or type, race, age of onset, serum alkaline phosphatase levels, and the presence of elevated serum IgG4 levels, the major differences among these variants are limited to the effects of the disease on the liver. Namely, whether large ducts or small ducts are primarily affected and if there is significant, concomitant hepatocyte injury, that is overlap with autoimmune hepatitis.
The diagnosis of PSC is generally based upon the presence of segmental intrahepatic and/or extrahepatic biliary strictures on cholangiogram in the absence of a secondary cause, such as infection, malignancy, ischemia, trauma, or infection. The median age at diagnosis of PSC is between 30 and 40 years (5, 6, 9) but can affect people of all ages; 7% of patients are diagnosed under the age of 18, 30% are diagnosed from 18-35 years, 50% are diagnosed from 36-65 years, and 13% are diagnosed at over 65 years. (5) Unlike most autoimmune diseases, there is a striking male predominance with the disease twice as prevalent in men than women. (5-7, 9, 11)
In the classic form of PSC, multifocal biliary strictures and dilation are present on cholangiogram and has come to be known as large-duct PSC. In contrast, small-duct PSC is diagnosed when the characteristic bile duct changes are absent on imaging, but liver biopsy shows characteristic biliary (periductal) fibrosis with a cholestatic serum biochemical profile (18). Compared to large-duct PSC, small-duct PSC is similar in male predominance and age at diagnosis (24) but is one-fifth as frequent with an incidence of 0.15 per 100,000 person-years, (6). Importantly, small-duct PSC patients have a favorable prognosis (4), although some patients with small-duct PSC progress to large-duct PSC (25). The more favorable prognosis is in part due to these patients having a lower rate of developing CCA compared to large-duct PSC. (25).
Four to fourteen percent of patients with PSC will have overlapping autoimmune hepatitis (AIH) (4, 6, 26). There remains controversy and a lack of consensus on the criteria that should be used to define PSC-AIH overlap, which may appear simultaneously or PSC may appear following a diagnosis of AIH. The distinction between PSC-AIH and PSC without AIH is important to make since the former may benefit from immunosuppression (27, 28). Another important, but often difficult, distinction is among a subset of patients with elevated IgG4. IgG4-related cholangiopathy can present with cholangiographic abnormalities similar to PSC, but is a distinct entity that is responsive to corticosteroids and other immunosuppressants. In contrast, elevated IgG4 levels can be found in roughly 10% of PSC (29, 30), but whether IgG4 alters disease course in PSC is unclear (29).
Importantly, PSC is found in association with inflammatory bowel disease (IBD) in 60-80% of cases, with a higher prevalence of IBD among men compared to women with PSC (3, 5-9, 31*). In roughly 70% of PSC-IBD patients, clinical recognition of IBD will precede the diagnosis of PSC by several years, while 10% of patients are diagnosed with PSC and IBD concurrently (7, 32). Over time, the frequency of IBD has been decreasing in PSC, which may reflect an increase in clinicians regarding PSC as a disease that can occur without the intestinal disease (33). Approximately 70-80% of the IBD associated with PSC are diagnosed as ulcerative colitis (UC), while the remainder are Crohn's disease (CD) or indeterminate colitis (9, 31*). Notably, the CD tends to present with colitis and rarely has a fibrostenosing phenotype. In fact, the IBD associated with PSC (PSC-IBD) in many cases has a unique phenotype with pancolitis or colitis restricted to the right side and a tendency for rectal sparing and backwash ileitis (34). Of note, women with PSC tend to have lower rates of IBD than men (61.4% vs 82.4%), with CD being proportionately more common in women (11). Conversely, PSC can be found in up to 8% of IBD patients (35**) with risk factors for PSC including male sex, nonsmoker status, pancolitis, and appendectomy (36). This prevalence was found after screening patients with 20 or more years of IBD with magnetic resonance cholangiography. This is a significant increase from the roughly 2% prevalence of PSC found in the Swiss Inflammatory Bowel Disease Cohort Study population (36) and may reflect an early or milder form of the disease, further complicating the study of PSC natural history. Although IBD alone increases the risk of colorectal carcinoma (CRC), a recent meta-analysis found that IBD with PSC had an increased risk for CRC (odds ratio of 3.24) and presented at a younger age compared to IBD patients without PSC (4, 37). Interestingly, this increased risk for CRC was not found in patients with PSC with CD (4, 37). Significantly, patients with PSC-CD may have milder disease and better survival than patients with PSC-UC (31*).
Natural History
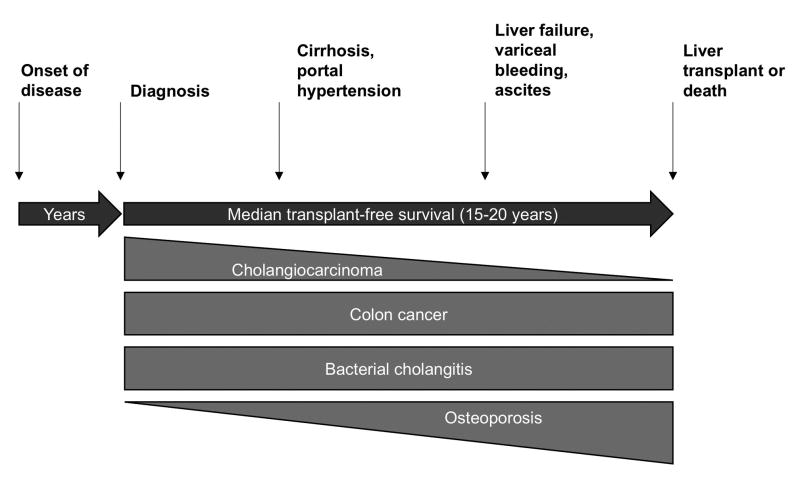
PSC follows a variable natural history (Fig 1). Initially, 10%-60% of patients are asymptomatic and may be diagnosed with PSC incidentally due to abnormal liver biochemistries (1, 6, 9, 32). Elevated alkaline phosphatase is the most common serum abnormality, although transaminases as well as gamma glutamyl transferase are often elevated as well (18). Bilirubin is normal initially in most cases, but like other serum liver test values, can fluctuate throughout the disease course. The elevated laboratory values may decrease after the diagnosis, as one study with over 300 patients showed that the average alkaline phosphatase, aspartate transaminase, alanine transaminase, and total bilirubin decreased from 1.99, 1.45, 2.11, 0.81 x the upper limit of normal to 1.22, 0.88, 1.00, and 0.65 x the upper limits of normal in one year (26). In fact, up to 40% of patients may experience normalized alkaline phosphatase levels in 1 year, and these patients have a higher rate of liver transplant-free survival (26, 38). Hepatomegaly and splenomegaly are present in 43.6% and 29.3% of patients, respectively, at presentation (9). A small subset of patients are diagnosed in the late stages of PSC with cirrhosis and ascites (6).
Fig 1.
Schematic representation of natural history of PSC.
When symptoms of PSC develop, abdominal pain, pruritus, diarrhea, jaundice, fatigue and fever are most common. (6, 9, 24). The disease may eventually lead to cirrhosis with portal hypertension, ascites, esophageal varices and liver failure in a similar fashion as other chronic liver disease (3, 7, 18). Clinicians can follow platelet levels, as platelets < 150 x 103/dL have an odds ratio of 6.3 for esophageal varices (39), among other clinical parameters (e.g. splenomegaly on imaging) which may herald the onset of portal hypertension and its sequelae.
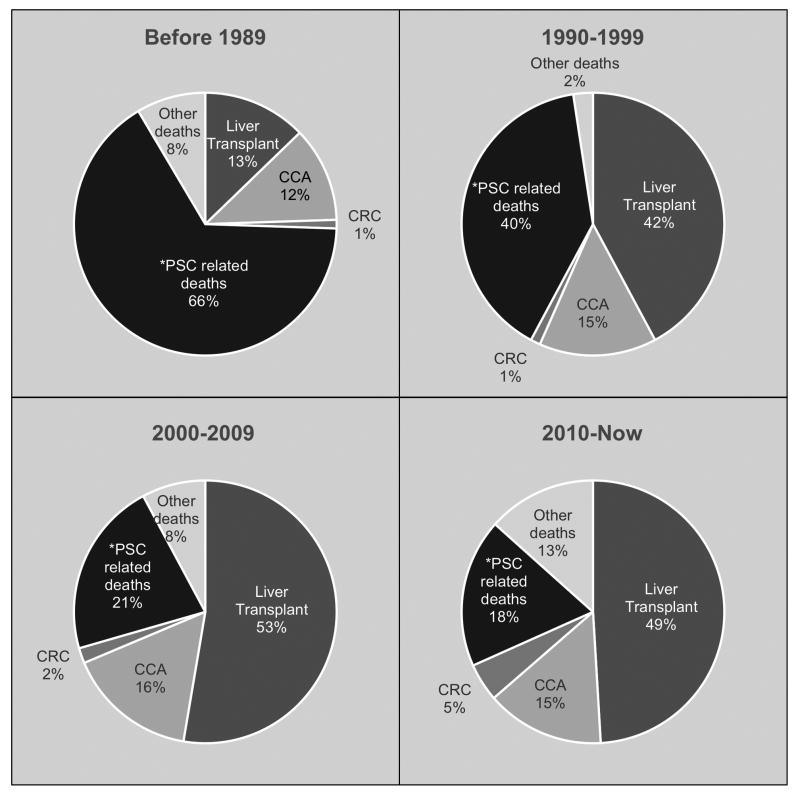
Before the 1990s, the majority of PSC patients died from PSC/liver-related complications; however in the past 25 years, liver transplantation has largely supplanted these outcomes. Unfortunately, the proportion of patients dying from CCA has changed minimally (Fig 2 (4, 5, 9, 33, 40-49)). In one study, patients with PSC were found to carry a four-fold increase risk of mortality than the general population and the most frequent cause of deaths was CCA (32%), liver failure (18%), liver transplant complication (9%), and CRC (8%) (4). The annual incidence of CCA, which often occurs prior to the onset of cirrhosis, is 0.5-1% per year, with a cumulative risk of 20% after 30 years (4). The median time between PSC diagnosis and CCA is 6 years (4), although a significant portion of these are diagnosed within the first year of PSC diagnosis (9, 44, 50). Eighty percent of patients who develop CCA die within 1 year (4). Additionally, the annual incidence rate of CRC in PSC patients is less than 0.5%, with a 30-year cumulative risk of 13%. Significantly, only 16% of patients who received an annual to biannual surveillance colonoscopy died from CRC compared to 50% of those who did not (4). Other causes of death include hepatocellular carcinoma, gallbladder carcinoma, pancreatic cancer, and non-malignant disease (5, 32, 50).
Fig 2.
Major clinical outcomes among patients with PSC over time. Shown are proportions of patients who experience PSC-related death, liver transplant, deaths from cholangiocarcinoma, deaths from colorectal carcinoma, and other deaths through four sequential time periods. PSC related liver failure was the leading cause of death prior to 1990s but has been largely supplanted by liver transplant (4, 5, 9, 33, 40-49). (Outcomes are charted according to the year they were published)
* PSC-related deaths include liver failure (most common), pancreatic/hepatic malignancy, esophageal varices, liver abscess/ sepsis, fulminant colitis.
Early estimates of median time from diagnosis to death or liver transplant were 9.6 -18 years (9, 24, 45). However, these studies were performed at centers specializing in PSC, which likely led to recruitment bias of more severe cases. A more recent population-based study from the Netherlands found a median transplant-free survival of 21.3 years among patients at non-transplant centers compared to 13.2 years among PSC patients at transplant centers (4). Thus, survival of patients with PSC is believed to be longer than initially perceived, an observation which may be related to transplant center referral bias in earlier studies and improved overall management of patients with chronic liver disease.
In summary, patients with PSC can have varying presentation from being asymptomatic to having symptoms of liver failure, exhibit variable serum liver chemistry values throughout the disease course, and progress at differing rates to need for liver transplant or death. In the absence of liver transplant, median survival is now estimated at 20 years; while some patients will present with advanced fibrotic or malignant disease, others will experience prolonged survival, while yet some others will not experience major adverse outcomes due to PSC during their lifetime
Non-Malignant Complications in PSC
In addition to transplant-free survival, other important clinical events occur as part of the natural history of PSC which greatly impact the lives of patients with PSC and in some cases may lead to fatal complications.
Dominant strictures
Dominant strictures (DS) are defined as a ≤1.5 mm stenosis of the common bile duct and/or ≤1.0 mm stenosis of hepatic duct within 2 cm of the bifurcation of the left and right hepatic ducts. (51) DS occur in 45%-63% of PSC patients over the course of disease and patients with elevation of serum bilirubin, worsening pruritis, increased dilatation of bile duct, and cholangitis should undergo examination (18, 52-54). Within patients with DS, Candida in bile appears to be associated with worse survival (53). DS has a poor prognosis and should raise suspicion of CCA (9, 18). In fact, patients with DS have a much worse survival rate than those without DS (13.7 years vs 23.0 years), and the difference was significantly reduced when patients with cholangiocarcinoma were excluded from those with benign DS (17.5 years vs 23.0 years) (54).
Bacterial cholangitis
In roughly 6% of patients, ascending cholangitis is present during the initial diagnosis of PSC. After PSC diagnosis, detecting acute cholangitis can be difficult since patients with PSC often already have abnormal serum liver tests and abdominal pain (6). Acute cholangitis should be suspected in patients who have an abrupt development of or worsening in these parameters and/or develop otherwise unexplained fever or leukocytosis. Although epidemiologic data regarding acute cholangitis in PSC are limited, in a cohort of PSC patients undergoing liver transplant, 38.6% had a history of bacterial cholangitis and 28% had acute bacterial cholangitis while on the waitlist. Of note, though, having a history of bacterial cholangitis did not alter waitlist survival significantly, and no waitlist patients died due to bacterial cholangitis over a study period of roughly 10 years (3).
Hepatic osteodystrophy
Bone loss is common in cholestatic liver diseases and can occur even in young men (an otherwise low-risk group) with PSC. When measured by dual-energy X-ray absorptiometry (DEXA) in patients, the risk of osteoporosis is increased 23.8-fold in PSC, with additional risk factors including age > 54 years, BMI < 24 kg/m2, and IBD duration > 19 years. (55) The rate of bone loss has been estimated to be 1% per year and does not appear to be affected by treatment with Vitamin D and calcium supplements, hormone replacement, or corticosteroids.
Conclusion
PSC is a rare disease with heterogeneous clinical features and a highly variable natural history. Indeed, given the variables of age, race, IBD, stricture location and degree, AIH overlap, and IgG4, there are in essence over 3000 possible different combinations of PSC phenotypes (56*). Overall, average liver transplant-free survival remains 15-20 years, and CCA remains a leading cause of death. A better understanding of distinct subsets of PSC may provide important insights into the disease and lead to novel therapies and better management strategies. With the recent increase in PSC incidence, we hope that awareness of the disease will likewise increase in order to accelerate research developments and improve patient care.
Key points.
PSC is a rare disease with a significant association with IBD, a rising incidence rate, a variable natural history and a transplant-free survival of roughly 20 years.
Variations in PSC exist and may be an important distinction to make for prognostication, clinical management, and disease-oriented research.
Complications of PSC includes cirrhosis and liver failure, cholangiocarcinoma, other hepatobiliary cancer, dominant strictures, bacterial cholangitis, and hepatic osteodystrophy
Acknowledgments
None
Financial Support and Sponsorship: None
Abbreviations
- PSC
Primary sclerosing cholangitis
- MELD
model for end-stage liver disease
- CCA
cholangiocarcinoma
- CRC
colorectal carcinoma
- IBD
inflammatory bowel disease
- UC
ulcerative colitis
- CD
Crohn's disease
- AIH
autoimmune hepatitis
- MRCP
magnetic resonance cholangiopancreatography
- ERCP
endoscopic retrograde cholangiopancreatography
- AASLD
American Association for the Study of Liver Diseases
- DS
dominant strictures
- DEXA
dual-energy X-ray absorptiometry
Footnotes
Conflicts of Interest: None
References and recommended reading
Papers of particular interest, published within the annual period of review, (18 months/ 2015- 2016) have been highlighted as:
* of special interest
** of outstanding interest
- 1.Porayko MK, Wiesner RH, LaRusso NF, Ludwig J, MacCarty RL, Steiner BL, et al. Patients with asymptomatic primary sclerosing cholangitis frequently have progressive disease. Gastroenterology. 1990;98(6):1594–602. doi: 10.1016/0016-5085(90)91096-o. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz A, Lemoinne S, Carrat F, Corpechot C, Chazouillères O, Arrivé L. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology. 2014;59(1):242–50. doi: 10.1002/hep.26620. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg DS, Camp A, Martinez-Camacho A, Forman L, Fortune B, Reddy KR. Risk of waitlist mortality in patients with primary sclerosing cholangitis and bacterial cholangitis. Liver Transpl. 2013;19(3):250–8. doi: 10.1002/lt.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045–55. doi: 10.1002/hep.26565. **Largest population based study of the prevalence and natural history of PSC showing differences in survival between transplant and non-transplant centers. [DOI] [PubMed] [Google Scholar]
- 5.Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. doi: 10.1186/1471-230X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102(5):1042–9. doi: 10.1111/j.1572-0241.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 7.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125(5):1364–9. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hadizadeh M, Abedi SH, Malekpour H, Radinnia E, Jabbehdari S, Padashi M, et al. Prevalence of inflammatory bowel disease among patients with primary sclerosing cholangitis in Iran. Arab J Gastroenterol. 2016;17(1):17–9. doi: 10.1016/j.ajg.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102(1):107–14. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 10.Franceschet I, Cazzagon N, Del Ross T, D'Incà R, Buja A, Floreani A. Primary sclerosing cholangitis associated with inflammatory bowel disease: an observational study in a Southern Europe population focusing on new therapeutic options. Eur J Gastroenterol Hepatol. 2016;28(5):508–13. doi: 10.1097/MEG.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 11.Lindkvist B, Benito de Valle M, Gullberg B, Björnsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52(2):571–7. doi: 10.1002/hep.23678. [DOI] [PubMed] [Google Scholar]
- 12.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33(1):99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 13.Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48(6):939–44. doi: 10.1016/j.jhep.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Bowlus CL, Li CS, Karlsen TH, Lie BA, Selmi C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: unique clinical and human leukocyte antigen associations. Liver Transpl. 2010;16(11):1324–30. doi: 10.1002/lt.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurlburt KJ, McMahon BJ, Deubner H, Hsu-Trawinski B, Williams JL, Kowdley KV. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97(9):2402–7. doi: 10.1111/j.1572-0241.2002.06019.x. [DOI] [PubMed] [Google Scholar]
- 16.Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002;17(8):908–13. doi: 10.1046/j.1440-1746.2002.02835.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Takikawa H. Geoepidemiology of primary sclerosing cholangitis: a critical review. J Autoimmun. 2013;46:35–40. doi: 10.1016/j.jaut.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 19.Berstad AE, Aabakken L, Smith HJ, Aasen S, Boberg KM, Schrumpf E. Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2006;4(4):514–20. doi: 10.1016/j.cgh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Lindor KD, Kowdley KV, Harrison ME, Gastroenterology ACo ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110(5):646–59. doi: 10.1038/ajg.2015.112. quiz 60. [DOI] [PubMed] [Google Scholar]
- 21.Tabibian JH, Visrodia KH, Levy MJ, Gostout CJ. Advanced endoscopic imaging of indeterminate biliary strictures. World J Gastrointest Endosc. 2015;7(18):1268–78. doi: 10.4253/wjge.v7.i18.1268. *Latest update on indeterminate billiary strictures and its management utilizing advanced ERCP techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol. 2003;98(5):1155–8. doi: 10.1111/j.1572-0241.2003.07401.x. [DOI] [PubMed] [Google Scholar]
- 23.Hilscher M, Enders FB, Carey EJ, Lindor KD, Tabibian JH. Alkaline phosphatase normalization is a biomarker of improved survival in primary sclerosing cholangitis. Ann Hepatol. 2016;15(2):246–53. doi: 10.5604/16652681.1193721. [DOI] [PubMed] [Google Scholar]
- 24.Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology. 2002;35(6):1494–500. doi: 10.1053/jhep.2002.33202. [DOI] [PubMed] [Google Scholar]
- 25.Björnsson E, Olsson R, Bergquist A, Lindgren S, Braden B, Chapman RW, et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology. 2008;134(4):975–80. doi: 10.1053/j.gastro.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 26.de Vries EM, Wang J, Leeflang MM, Boonstra K, Weersma RK, Beuers U, et al. Alkaline Phosphatase at Diagnosis of Primary Sclerosing Cholangitis and One Year Later: Evaluation of Prognostic Value. Liver Int. 2016 doi: 10.1111/liv.13110. [DOI] [PubMed] [Google Scholar]
- 27.Zenouzi R, Lohse AW. Long-term outcome in PSC/AIH “overlap syndrome”: does immunosuppression also treat the PSC component? J Hepatol. 2014;61(5):1189–91. doi: 10.1016/j.jhep.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54(2):374–85. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Benito de Valle M, Müller T, Björnsson E, Otten M, Volkmann M, Guckelberger O, et al. The impact of elevated serum IgG4 levels in patients with primary sclerosing cholangitis. Dig Liver Dis. 2014;46(10):903–8. doi: 10.1016/j.dld.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101(9):2070–5. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 31.Fevery J, Van Steenbergen W, Van Pelt J, Laleman W, Hoffman I, Geboes K, et al. Patients with large-duct primary sclerosing cholangitis and Crohn's disease have a better outcome than those with ulcerative colitis, or without IBD. Aliment Pharmacol Ther. 2016;43(5):612–20. doi: 10.1111/apt.13516. *Studied outcomes of CD and UC with PSC and observed a favorabable outcome for those with CD. [DOI] [PubMed] [Google Scholar]
- 32.Yanai H, Matalon S, Rosenblatt A, Awadie H, Berdichevski T, Snir Y, et al. Prognosis of primary sclerosing cholangitis in israel is independent of coexisting inflammatory bowel Disease. J Crohns Colitis. 2015;9(2):177–84. doi: 10.1093/ecco-jcc/jju013. [DOI] [PubMed] [Google Scholar]
- 33.Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126(7):1929–30. doi: 10.1053/j.gastro.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 34.Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54(1):91–6. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, et al. Prevalence of Sclerosing Cholangitis Detected by Magnetic Resonance Cholangiography in Patients With Long-term Inflammatory Bowel Disease. Gastroenterology. 2016;151(4):660–9.e4. doi: 10.1053/j.gastro.2016.06.021. **High prevalence of PSC detected by magnetic resonance cholangiography in a large IBD population. [DOI] [PubMed] [Google Scholar]
- 36.Fraga M, Fournier N, Safroneeva E, Pittet V, Godat S, Straumann A, et al. Primary sclerosing cholangitis in the Swiss Inflammatory Bowel Disease Cohort Study: prevalence, risk factors, and long-term follow-up. Eur J Gastroenterol Hepatol. 2016 doi: 10.1097/MEG.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 37.Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28(4):383–90. doi: 10.1097/MEG.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 38.Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43(4):309–13. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology. 2004;39(1):204–10. doi: 10.1002/hep.20029. [DOI] [PubMed] [Google Scholar]
- 40.Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, et al. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987;22(6):655–64. doi: 10.3109/00365528709011139. [DOI] [PubMed] [Google Scholar]
- 41.Lebovics E, Palmer M, Woo J, Schaffner F. Outcome of primary sclerosing cholangitis. Analysis of long-term observation of 38 patients. Arch Intern Med. 1987;147(4):729–31. [PubMed] [Google Scholar]
- 42.Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10(4):430–6. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 43.Jeffrey GP, Reed WD, Laurence BH, Shilkin KB. Primary sclerosing cholangitis: clinical and immunopathological review of 21 cases. J Gastroenterol Hepatol. 1990;5(2):135–40. doi: 10.1111/j.1440-1746.1990.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 44.Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38(4):610–5. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51(4):562–6. doi: 10.1136/gut.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka A, Takamori Y, Toda G, Ohnishi S, Takikawa H. Outcome and prognostic factors of 391 Japanese patients with primary sclerosing cholangitis. Liver Int. 2008;28(7):983–9. doi: 10.1111/j.1478-3231.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 47.Garioud A, Seksik P, Chrétien Y, Corphechot C, Poupon R, Poupon RE, et al. Characteristics and clinical course of primary sclerosing cholangitis in France: a prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22(7):842–7. doi: 10.1097/MEG.0b013e328331c2b7. [DOI] [PubMed] [Google Scholar]
- 48.de Valle MB, Björnsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population-based cohort. Liver Int. 2012;32(3):441–8. doi: 10.1111/j.1478-3231.2011.02614.x. [DOI] [PubMed] [Google Scholar]
- 49.Fevery J, Henckaerts L, Van Oirbeek R, Vermeire S, Rutgeerts P, Nevens F, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 2012;32(2):214–22. doi: 10.1111/j.1478-3231.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 50.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 51.Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36(2):151–6. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 52.Björnsson E, Lindqvist-Ottosson J, Asztely M, Olsson R. Dominant strictures in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2004;99(3):502–8. doi: 10.1111/j.1572-0241.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 53.Rudolph G, Gotthardt D, Klöters-Plachky P, Kulaksiz H, Rost D, Stiehl A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol. 2009;51(1):149–55. doi: 10.1016/j.jhep.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 54.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24(9):1051–8. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angulo P, Grandison GA, Fong DG, Keach JC, Lindor KD, Bjornsson E, et al. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology. 2011;140(1):180–8. doi: 10.1053/j.gastro.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkar S, Bowlus CL. Primary Sclerosing Cholangitis: Multiple Phenotypes, Multiple Approaches. Clin Liver Dis. 2016;20(1):67–77. doi: 10.1016/j.cld.2015.08.005. *Detailed descriptions of the multiple subsets of PSC. [DOI] [PMC free article] [PubMed] [Google Scholar]