Abstract
Axillary lymph nodes (axLN) are a rare site of nodal metastases in patients with lung cancer. BRAF mutated lung cancer is a genetically distinct subtype that occurs in 2–5% of non-small cell lung carcinomas (NSCLC). A recent study identified a highly unusual pattern of metastatic spread to axLN in patients with BRAF mutated colorectal cancer (CRC). The purpose of the study is to assess the incidence of axLN metastases in BRAF mutated NSCLC.
Baseline computed tomography (CT) imaging at diagnosis and all follow up CTs of patients with BRAF mutated NSCLC treated at our institution were retrospectively reviewed by two radiologists for evidence of axLN metastases. Positron emission tomography (PET)/CT was reviewed when available. A control group of patients with non-BRAF mutated NSCLC was assessed. Three criteria were used for the diagnosis of a metastatic node; pathologic confirmation, radiologic size greater ≥1.5 cm in short axis diameter or fluorodeoxyglucose avidity on PET/CT and radiologic size ≥1.0 cm in short axis diameter.
Forty-six patients with BRAF mutated NSCLC and CT images on the institutional PACS were identified. 7 (15%) patients with BRAF mutated NSCLC had axLN metastases using the proposed diagnostic criteria. One patient had a pathologic proven axLN metastasis, 3 had axLNs measuring ≥ 1.5cm in short axis, and 3 had nodes which were FDG avid on PET/CT and measured ≥1.0cm in short axis. By comparison, 1 of 46 (2%) control patients with non-BRAF mutated NSCLC had axLN metastases. Previous series have reported the prevalence of axLN metastases in patients with NSCLC as 0.61% –0.75%.
We have found a higher incidence of axLN metastases in BRAF mutated NSCLC patients than described in non-BRAF mutated NSCLC patients. Examination of the axilla should be a routine part of physical examination in this genetically distinct subgroup of lung cancer patients.
Introduction
Lung cancer is the leading cause of cancer related death worldwide and accounts for 13% of new cancer diagnoses(1). Axillary lymph nodes (axLN) are a rare site of nodal metastases in patients with lung cancer. Previous series have reported the prevalence of axLN metastases as 0.61% –0.75%(2, 3). BRAF mutated lung cancer is a genetically distinct subtype that occurs in 2–5% of non-small cell lung carcinomas (NSCLC)(4–6). Several subtypes of BRAF mutation exist. The most common is the BRAF V600E mutation which accounts for 50–81% of BRAF mutations and is associated with responsiveness to treatment with the targeted agents vemurafenib, dabrafenib and trametinib (7–9).
A recent study identified a highly unusual pattern of metastatic spread to axLN in patients with BRAF mutated colorectal cancer (CRC) (10). The aim of our study is to assess the incidence of axLN metastases in BRAF mutated NSCLC.
Materials and methods
Our institutional review board approved this retrospective study and waived the requirement for informed consent. This study was HIPAA compliant.
Patients
Patients were identified from a prospectively maintained institutional database of patients with a pathologic diagnosis of lung carcinoma with BRAF mutation. The date of pathologic diagnosis of BRAF mutation ranged from 4/21/2004 to 6/3/2013. Patients with a pathology report documenting lung cancer with a BRAF mutation and with CT images on the institutional Picture Archiving and Communication System (PACS) were included in the study. Baseline CT images were obtained between 5/9/2003 and 5/31/2013. Due to local referral patterns, some of the imaging studies included in the analysis were performed at outside institutions. CT studies obtained in our institution were performed on a variety of 16- and 64 slice multidetector CT scanners, with slice thicknesses ranging from 1.25 to 5 mm. Baseline CTs (at diagnosis, prior to treatment), and all follow-up CTs obtained were included for analysis. When a PET/CT was available, it was also reviewed. Images were retrospectively reviewed by 2 radiologists in consensus on commercially available PACS software (Centricity, GE Healthcare). Both readers were blinded to the clinical details at the time of image interpretation. Clinical information was extracted retrospectively from the institution’s electronic medical record, following analysis. Clinical parameters documented were age, sex and smoking status (either current/former smoker or never smoker). BRAF mutation subtype and TNM stage were also recorded. A control group of patients with a pathological diagnosis of lung carcinoma with non-BRAF mutations was selected for comparative assessment from an institutional database of lung cancer patient who had genetic profiling of their tumors. They were matched with the BRAF group for stage at diagnosis. They consisted of 4 ALK, 9 EGFR, 9 KRAS and 24 tumors without documented mutation.
Image Analysis
Imaging studies were assessed for evidence of axLN metastases. Three criteria were used for the diagnosis of a metastatic node: [1] pathologic confirmation, [2] radiologic size greater ≥1.5 cm in short axis diameter (based on response evaluation criteria in solid tumors (RECIST 1.1)) or [3] focal fluorodeoxyglucose (FDG) avidity on positron emission tomography (PET)/CT and radiologic size ≥1.0 cm in short axis diameter (i.e. a non-target RECIST lesion with PET positivity) (11). The presence or absence of additional thoracic metastases was also documented in patients with BRAF mutated lung cancer including mediastinal and supraclavicular nodal metastases, lung and pleural metastases, lymphangitis carcinomatosis (defined as irregular interlobular septal thickening) and chest wall invasion (defined as extension of the primary tumor through the chest wall).
Statistical analysis
Grey’s method was used to compare the risk of developing axLN metastases between BRAF and non-BRAF mutated lung cancer groups, treating death as a competing event.
Results
Patient characteristics
Forty-six patients with BRAF mutated lung cancer and CT images on the institutional PACS were identified. Twenty-four patients were male, 22 were female. The mean age of patients was 64 years (range 42–85). Forty-five of the 46 patients (98%) were current or former smokers. Twenty-six (57%) had a BRAFV600E mutation. The group contained 13 (28%) stage I tumors, 2 (4%) stage II tumors, 13 (28%) stage III tumors, and 18 (40%) stage IV tumors. Forty-six patients with non-BRAF mutated lung cancer and CT images available on the institutional PACS were identified for comparison. Median follow up for the 2 groups was 27.8 months (range: 0.7–133.6).
Imaging findings
Seven (15%) patients with BRAF mutated lung cancer had axLN metastases at diagnosis and/or during follow up using the proposed diagnostic criteria. One patient had a pathologic proven axLN metastasis, 3 patients had axLNs measuring ≥ 1.5cm in short axis (Figure 1), and 3 had nodes which were FDG avid on PET/CT and measured ≥1.0cm in short axis. Five of the seven patients with positive axLNs had a BRAF V600E mutation on sub-analysis.
Figure 1.
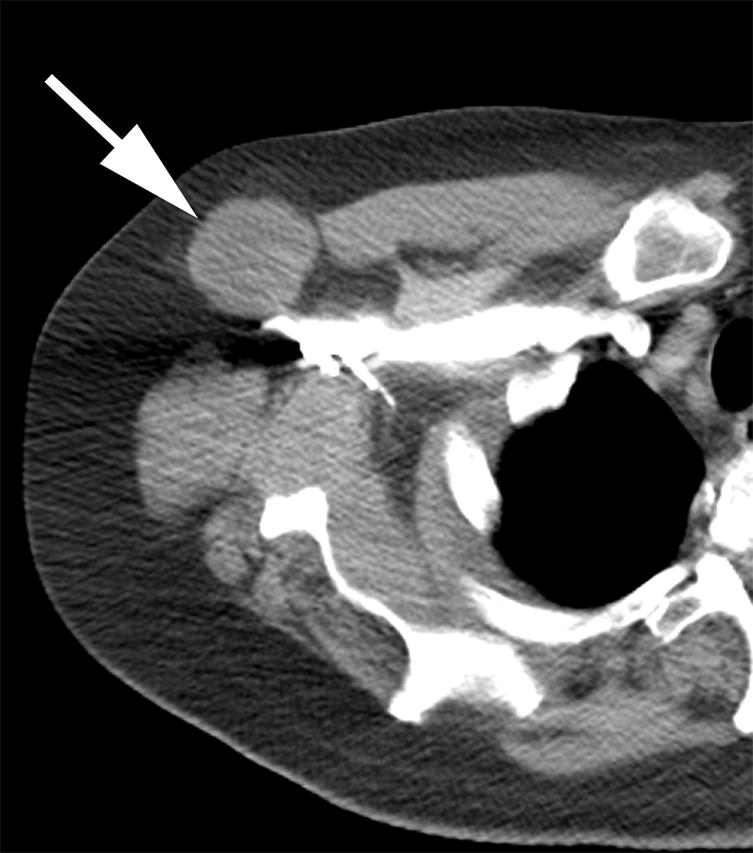
Axial contrast enhanced CT image of a patient with BRAF mutated NSCLC, with an enlarged right axillary node, measuring 3.5cm in short axis diameter (arrow).
Table 1 shows the incidence of thoracic metastases in patients with BRAF mutated lung cancer with and without axillary nodal metastases (Table 1). A higher percentage of patients with axLN metastases had supraclavicular and mediastinal nodal metastases. A single patient with chest wall invasion did not have axLN metastases.
Table 1.
Incidence of thoracic metastases in patients with BRAF mutated lung cancer with and without axillary nodal metastases. Abbreviations: LN = lymph node, axLN = axillary lymph node, +ve = positive, −ve = negative.
| BRAF Patients (N = 46) | axLN metastasis +ve | axLN metastasis −ve |
|---|---|---|
| Total number | 7 | 39 |
| Supraclavicular LN metastases | 2 (29%) | 7 (18%) |
| Mediastinal LN metastases | 7 (100%) | 24 (62%) |
| Pleural metastases | 3 (43%) | 13 (33%) |
| Chest wall invasion | 0 (0%) | 1 (3%) |
| Lung metastases | 3 (43%) | 22 (56%) |
| Lymphangitis carcinomatosis | 1 (14%) | 4 (10%) |
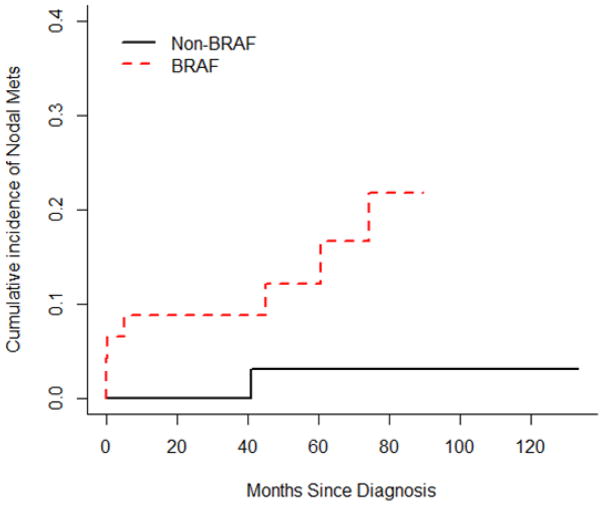
In the control group of 46 patients with non-BRAF mutated lung cancer, one patient had axLN metastases. This patient also had a large ipsilateral breast metastasis, which was presumably related to the axLN metastasis. The risk of developing axLN metastases was significantly higher in patients with BRAF mutated lung cancer than in patients with non-BRAF mutated lung cancer (p=0.023). The 2-year cumulative incidences of axLN metastases were 0 and 0.088 (95%CI: 0.005–0.171) in the two groups (Figure 2).
Figure 2.
Graph illustrating the cumulative incidence of axLN metastases in BRAF and non-BRAF mutated lung cancer patients.
Discussion
We describe the incidence of axLN metastases in a cohort of patients with BRAF mutated lung cancer patients. Using our proposed diagnostic criteria 7/46 patients with BRAF mutated lung cancer had axLN metastases (15%). This is a higher incidence than has been described previously in patients with lung cancer who had not undergone genetic profiling (<1%) (2, 3) and higher than that found in our control group of patients with non-BRAF mutated lung cancer.
Targeted molecular therapies have made a major impact on the treatment of NSCLC in recent years. The effectiveness of these therapies is dependent on the presence of specific genetic mutations in a tumor. The BRAF mutation was initially described in 2002 and its commonest subtype is the V600E mutation, which accounts for 50–81% of BRAF mutations in lung cancer (6). To date there are a limited number of studies evaluating the clinical features of patients with BRAF mutated lung carcinoma. BRAF mutated lesions are frequently associated with a smoking history (4, 6) although patients with a V600E mutations are more likely to be light/never smokers when compared to those without non-V600E mutations(12). Lesions with a V600E mutation are also associated with improved overall survival compared to non-V600E lesions(12). In many centers, molecular profiling of lung cancers has become routine clinical practice.
There are a growing number of studies describing the imaging features of lung tumors associated with specific genetic subtypes. For example, tumors with anaplastic lymphoma kinase gene (ALK) rearrangement have been associated with a solid appearance, and when compared to epidermal growth factor receptor (EGFR) mutated lesions are more likely to have multi-focal bulky lymphadenopathy or lymphangitic metastases(13). When compared to other genetically distinct subtypes of NSCLC, BRAF lesions are more likely to have pleural metastases and pleural effusions than lesions with KRAS mutations(14).
Satoh et al assessed the incience of axLN metastases in all lung cancer patients. They retrospectively studied 1340 patients and found axLN metastases in 10 patients (0.7%)(3). They concluded that the probability of developing axLN metastases was so low that no imaging procedures are specifically required in the absence of clinical evidence of axLN metastases. Riquet et al retrospectivley analyzed 1486 patients who had undergone surgical reesection of NSCLC and found that 9 developed subsequent axLN metastases (0.6%)(2).
Potential routes of axLN metastases have been discussed by others and include direct extension from chest wall invasion, retrograde spread from supraclavicular lymph node metastases, involvement of intercostal lymphatics via spread from mediastinum and development from systemic disease. Interestingly, downregulation of BRAF activated non-coding RNA found in specimens from patients with NSCLC has been recently correlated with increased LN metastases and poor survival rate, by affecting epithelial-mesenchymal transition(15). BRAF mutated CRC is an aggressive subset of CRC. Although AxLNs are an exceedingly rare site of metastatic involvement in wild-type CRC, Lipsyc et al, identified 9 patients with axLN metastases out of a cohort of 103 patients with BRAF mutated CRC. Several patients in their series developed axLN metastases in the setting of chest wall or breast involvement, suggesting initial spread and then local extension (9).
The retrospective nature of our study and its relatively small sample size are inherent limitations, both of which could be overcome by larger prospective multi-institutional studies. Older studies used for historical comparison may have included patients with BRAF mutation. Because of the retrospective nature of the study, the CT imaging protocols used were varied, resulting in differences in the images that were available for analysis, particularly with respect to the image slice thickness; nonetheless it was possible to evaluate the presence of axLN in all patients.
In this study we have found a higher incidence of axLN metastases in BRAF mutated NSCLC patients than in non-BRAF mutated NSCLC patients and a higher incidence than that described in previous series in patients who had not undergone genetic profiling. Examination of the axilla should be routine part of physical examination in this geneticaly distinct subgroup of lung cancer patients. Our findings extend our understanding of BRAF-mutated lung cancer as a distinct subset of lung cancer.
Axillary lymph nodes are a rare site of nodal metastases in lung cancer patients.
BRAF mutated NSCLC patients have a higher incidence of axillary nodal metastases.
Examination of the axilla should be routine in this distinct patient subgroup.
Acknowledgments
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Core Grant P30 CA008748.
Footnotes
Conflict of interest statement
Dr. Riely reports grants and personal fees from Novartis, grants and personal fees from Roche, grants from Pfizer, grants from Ariad. None of the other authors have any disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 Feb;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Riquet M, Le Pimpec-Barthes F, Danel C. Axillary lymph node metastases from bronchogenic carcinoma. Ann Thorac Surg. 1998 Sep;66(3):920–2. doi: 10.1016/s0003-4975(98)00556-6. [DOI] [PubMed] [Google Scholar]
- 3.Satoh H, Ishikawa H, Kagohashi K, Kurishima K, Sekizawa K. Axillary lymph node metastasis in lung cancer. Med Oncol Northwood Lond Engl. 2009;26(2):147–50. doi: 10.1007/s12032-008-9097-4. [DOI] [PubMed] [Google Scholar]
- 4.Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol Off J Am Soc Clin Oncol. 2011 May 20;29(15):2046–51. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol Off J Am Soc Clin Oncol. 2011 Sep 10;29(26):3574–9. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 6.Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer. 2015 Feb 1;121(3):448–56. doi: 10.1002/cncr.29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautschi O, Pauli C, Strobel K, Hirschmann A, Printzen G, Aebi S, et al. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2012 Oct;7(10):e23–4. doi: 10.1097/JTO.0b013e3182629903. [DOI] [PubMed] [Google Scholar]
- 8.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay J-Y, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015 Aug 20;373(8):726–36. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid S, Siano M, Joerger M, Rodriguez R, Müller J, Früh M. Response to dabrafenib after progression on vemurafenib in a patient with advanced BRAF V600E-mutant bronchial adenocarcinoma. Lung Cancer Amst Neth. 2015 Jan;87(1):85–7. doi: 10.1016/j.lungcan.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Lipsyc MD, Yaeger R, Dengel LT, Saltz L. Axillary Lymph Node Involvement, a Unique Pattern of Metastasis in BRAF-Mutant Colorectal Cancer. JAMA Oncol. 2015 Aug;1(5):686–7. doi: 10.1001/jamaoncol.2015.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer Oxf Engl 1990. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Litvak AM, Paik PK, Woo KM, Sima CS, Hellmann MD, Arcila ME, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2014 Nov;9(11):1669–74. doi: 10.1097/JTO.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpenny DF, Riely GJ, Hayes S, Yu H, Zheng J, Moskowitz CS, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer Amst Neth. 2014 Nov;86(2):190–4. doi: 10.1016/j.lungcan.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpenny D, Riely G, Plodkowski A, Litvak A, Zheng J, Sosa R, et al. Radiogenomic evaluation of lung cancer-are there imaging characteristics associated with lung adenocarcinomas harboring BRAF mutations?. Radiological Society of North America 2015 Scientific Assembly and Annual Meeting; November 29 - December 4, 2015; Chicago IL. [Accessed September 14, 2016]. archive.rsna.org/2015/15012076.html. [Google Scholar]
- 15.Sun M, Liu X-H, Wang K-M, Nie F, Kong R, Yang J, et al. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68. doi: 10.1186/1476-4598-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]