Abstract
Background:
Herbal drug delivery is limited by poor solubility and bioavailability which can be overcome with suitable nanomaterials that will enhance their pharmacokinetics and performance.
Objective:
This study aimed to analyze the synthesis, characterization, and biological activities of silver nanoparticles (AgNPs) from three spices.
Materials and Methods:
AgNPs were prepared using 0.1 M silver nitrate and aqueous extracts of Allium sativum L. (garlic), Zingiber officinale Rosc. (ginger), and Capsicum frutescens L. (cayenne pepper). The AgNPs were characterized using ultraviolet-visible (UV-Vis) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray, X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy.
Results:
The AgNPs were formed within an hour of the reaction and showed maximum UV-Vis absorption in the 375–480 nm range. SEM and TEM revealed well-dispersed spherical particles with little agglomeration, average sizes of 3–6 nm, 3–22 nm, and 3–18 nm for garlic, ginger, and cayenne pepper, respectively. FTIR showed that amine, protein, phenolic, aromatic, and alkynes groups contributed to AgNP synthesis and XRD confirmed their crystalline and face-centered cubic nature. Antibacterial action of the AgNPs was in the following order: ginger (minimum inhibitory concentration [MIC] <25 μg/mL) > garlic> cayenne pepper (MIC 125 μg/mL). Antioxidant action showed cayenne pepper > ginger > garlic (inhibitory concentration 50% [IC50]: 40, 240, and 250 μg/mL, respectively) against 2,2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) and garlic > cayenne pepper > ginger (IC50: <31.25, 40, and 120 μg/mL, respectively) against 1,1-diphenyl-2-picrylhydrazyl.
Conclusion:
Optimization of this green synthesis would support the production of AgNPs with great therapeutic potentials.
SUMMARY
The synthesis, characterization, and biological activities of silver nanoparticles (AgNPs) from garlic, ginger and cayenne pepper were evaluated
The AgNPs formed were characterized using UV-Vis spectroscopy, SEM and TEM microscopy, as well as EDX, XRD and FTIR spectroscopy AgNPs were well dispersed with spherical shapes and average sizes of 3-6nm, 3-22nm and 3-18 nm for garlic, ginger and cayenne pepper respectively
Amine, protein, phenolic and alkyne groups were revealed as the capping agents for the nanoparticles
The silver nanoparticles were confirmed to be crystalline with characteristic face centred cubic nature
The antibacterial and antioxidant activities of the AgNPs confirmed the therapeutic potential of the AgNPs.
Abbreviations used: AgNPs: Silver nanoparticles; UV-Vis: ultraviolet-visible; SEM: Scanning electron microscopy; TEM: Transmission electron microscopy; EDX: Energy dispersive X-ray; XRD: X-ray diffraction; FTIR: Fourier transform infrared; GaNPs: Garlic nanoparticles; GiNPs: Ginger nanoparticles; C.PeNPs: Cayenne pepper nanoparticles; FCC: Face centred cubic; SPR: Surface Plasmon resonance; ABTS-2: 2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid); DPPH-1: 1-diphenyl-2-picrylhydrazyl.
Keywords: Antimicrobial, antioxidant, characterization, silver nanoparticles, spices
INTRODUCTION
Nanotechnology is the synthesis, characterization, fabrication, and manipulation of structures, devices, or materials that have at least one dimension (or contain components with at least one dimension) that is approximately 1–100 nm in length. Particle size below this threshold results in materials with physical and chemical properties that are significantly different from the properties of macroscale materials composed of the same substance.[1]
In the last decade, biosynthesis of nanoparticles has received increasing attention due to a growing need to develop environmentally friendly technologies in material synthesis. The biosynthetic method employing plant extracts has received some attention as a simple and viable alternative to chemical procedures and physical methods synthesizing metal nanoparticles only in recent years. Nanotechnology is becoming increasingly important for the food and health sectors. Promising results and applications are already being developed in the areas of nutrient and drug delivery systems through bioactive nanoencapsulation, biosensors to detect and quantify pathogens, as well as novel resources for the evaluation and development of newer, safer, and effective drug formulations.
Recently, the use of biological molecules as templates for “green nanotechnology” is increasing and plants, plant wastes, bacteria, and fungi are frequently been used for the synthesis of nanoparticles.[2,3] According to Krithiga et al.,[4] plants are better options for nanoparticle synthesis because they are mostly not toxic, provide natural capping agents, and reduce the cost of microorganism isolation and culture media.
Silver nanoparticles (AgNPs) are of current importance because of easy preparation process and unique optical, electrical, and thermal properties which enhances electrical conductivity, near infrared absorption, and effective charge separation.[5]
Plant extracts are preferred over other biological sources due to their ample availability and wide array of reducing metabolites. Plant secondary products provide rich resources as potential drugs, nutraceuticals, and food additives. Plant polyphenols are one of the largest groups of natural antioxidant secondary metabolites.[6] The reduction properties of these antioxidant metabolites have been linked with the higher potential ability of plant extracts to synthesize nanoparticles with improved characteristics.[7] Biosynthesis of AgNPs using plant sources offers several advantages such as cost-effectiveness, eco-friendliness, and the elimination of high pressure, energy, temperature, and toxic chemicals necessary in the traditional synthesis methods.[8] Among the various inorganic metal nanoparticles, AgNPs have received substantial attention as preservatives, effective antimicrobial and anticancer agents, and biomedical sensors and detectors that exhibit low toxicity for in vitro and in vivo applications.[6,7,8] Other researchers have reported the larvicidal,[9,10] anticoagulant, thrombolytic,[11,12] and anti-inflammatory[13,14] applications of AgNPs.
Medicinal plants with known therapeutic properties and no side effects have now occupied lead positions in the pharmacopoeia. However, the delivery of plant/herbal therapeutic molecules as drugs is problematic due to poor solubility, poor permeability, low bioavailability, instability in biological milieu, etc. These limitations of herbal drugs can be overcome by attaching or encapsulating them with suitable nanomaterials which can significantly enhance the pharmacokinetics and greatly improve their performance.[15] Medicinal plants are of special concern since they control the size and shape of nanoparticles by providing capping layers.[16] Several medicinal plants including spices have been utilized for the production of AgNPs.[8,17,18,19]
Allium sativum (garlic), Zingiber officinale (ginger), and Capsicum frutescens (cayenne pepper) are important spices with medicinal properties common to most cuisines all over the world. These spices are individually considered as general remedies for many diseases. Various biological activities attributed to these spices include antimicrobial, anticancer, antioxidant, antidiabetic, antiemetic, antihypertensive, hypoglycemic, hypolipidemic, and immunomodulatory.[20,21,22,23,24,25] Phytochemical studies of these spices have shown that they are rich in alkaloids, tannins, carotenoids, saponins, phenols, and flavonoids, and they have been reported to exhibit high antioxidant activities[26,27] and may be considered as potential factors for reducing silver to silver nanoparticles.
In continuation of our research on the medicinal, nutraceutical, and economic usage of these spices, the present work is aimed at the synthesis, characterization, and evaluation of antibacterial and antioxidant potentials of nanoparticles from aqueous silver nitrate (AgNO3) and extracts of A. sativum, Z. officinale, and C. frutescens.
MATERIALS AND METHODS
Materials
Fresh garlic, ginger, and cayenne pepper were purchased from the local fruit and vegetable outlet in Alice, South Africa. The spices were identified and authenticated with the previously collected herbarium specimens available. AgNO3 (Sigma-Aldrich, USA) and all other reagents used in this study were of analytical grades. The individual spices were dried (60°C, 72 h), pulverized into powder with a coffee grinder, and stored in air-tight bottles at 4°C till needed. Deionized water was used throughout the experiment.
Preparation of silver nanoparticles
Twenty grams each of A. sativum, Z. officinale, and C. frutescens was weighed into individual 250 mL conical flasks and was extracted with 100 mL of deionized water at 60°C for 10 min in a water bath. The extracts were cooled and filtered using Whatman filter paper no. 1 under vacuum. Exactly 15 mL of the prepared extracts was added to 45 mL of aqueous AgNO3 (0.1 M solution) at room temperature and stirred continuously with a magnetic stirrer for 15 min. The solution obtained was kept in dark to prevent auto-oxidation of silver.
Characterization of silver nanoparticles
After 24 h, the solution containing AgNPs was centrifuged at 3000 rpm for 10 min, the resulting pellets were dried in an oven at 100°C for 24 h. The purified AgNPs were characterized using the following techniques.
The formation of AgNPs was monitored by visual assessment of the color changes of the solutions. The reduction of silver was measured periodically at a wavelength range of 300–700 nm using a UV-Vis spectrophotometer (UV-3000 PC, UK). The UV-Vis spectra of AgNPs produced were plotted and recorded as a function of bioreduction time (15 min intervals) at room temperature at a resolution of 0.5 nm. Size, shape, and morphology of the nanoparticles were determined by scanning electron microscopy (SEM) (JEOL JSM-6390 LV) and transmission electron microscopy (TEM) (Zeiss LIBRA® 120), while the presence of silver was confirmed by energy dispersive X-ray (EDX) spectrometry analysis: the samples for SEM and EDX assays were sonicated for 5 min to make a suspension of AgNPs in distilled water. A drop of the suspension was then placed on double-sided-coated carbon stubs, allowed to dry, and observed using SEM at a voltage of 15–20 kV at different magnifications. EDX analysis was also performed for the confirmation of elemental silver. Both SEM and EDX analyses were carried out on the same instrument (JEOL JSM-6490A). The samples for TEM were prepared by sonicating the pellet of centrifuged AgNPs in methanol. A drop of the suspension was placed on a carbon-coated copper grid and allowed to dry at room temperature.
TEM analysis was performed using a TEM operating at 80 kV. X-ray diffraction (XRD) measurement of the nanoparticles was recorded on an X-ray diffractometer (Brucker D8 Advanced) operated at a voltage of 45 kV and a current of 30 mA equipped with a proportional counter Cu Kα radiation (δ =1.5405 Ắ, nickel filter). Fourier transform infrared (FTIR) analysis was used to determine the possible biomolecules responsible for the reduction of silver ions to AgNPs. The samples were analyzed using a JASCO FT-IR 4100 spectrometer. Spectra were collected from 50 scans at a resolution of 4 cm−1 in the transmission mode of 4000–440 cm−1.
Antimicrobial assay
The comparative antibacterial activities of the AgNPs from the respective spices were assessed against two Gram-negative and two Gram-positive bacterial strains. The organisms Streptococcus faecalis (ATCC: 29212), Bacillus cereus (ATCC: 10702), Escherichia coli (ATCC: 25922), and Shigella flexneri (KZN) were chosen primarily on the basis of their importance as opportunistic pathogens of humans and food deterioration and were obtained from the Microbiology Unit of the Medicinal Plants and Economic Development Research Centre, Botany Department, University of Fort Hare.
Agar dilution method was used for evaluating the minimum inhibitory concentration (MIC) of the AgNPs against the bacterial strains. In brief, Mueller–Hinton agar was prepared by autoclaving and allowed to cool at 55°C. A stock solution of each synthesized AgNPs was prepared in dimethyl sulfoxide (Sigma). The agar medium containing the nanoparticles at final concentrations of 0.015625–0.25 mg/mL was poured into Petri dishes, swirled gently until the agar began to set, and left over night for solvent evaporation. Organisms were streaked in radial pattern on the agar plates. The inoculum of each test strain was standardized at 5 × 105 cfu/ml using McFarland Standard as described by the National Committee for Clinical Laboratory Standards.[28] The plates were incubated under aerobic conditions at 37°C and examined after 24 h. Each treatment was performed in triplicate, and complete suppression of growth at a specific concentration of the nanoparticles was taken as the MIC. Ciprofloxacin was used as positive control in this experiment.
Antioxidant assay
The antioxidant activities of the spices and AgNPs were evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assays.
2,2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical scavenging assay
The modified method described by Re et al.[29] using microtiter well plates was adopted for the determination of ABTS scavenging activity. In brief, the stock solutions consisting of 7 mM ABTS solution and 2.4 mM potassium persulfate solution were prepared. The working solution was prepared by mixing the two stock solutions in equal proportions (1:1 v/v) and allowing them to react for 12 h at room temperature in dark. The solution was then diluted by mixing 1 mL ABTS + solution with 60 mL of methanol to obtain an absorbance of 0.708 ± 0.001 units at 734 nm using the spectrophotometer. A volume of 100 μL of methanol was added into all the wells with the exception of second (B) and third (C) rows. Exactly 200 μL of the nanoparticles (0.5 mg/ml) or standards (Rutin) prepared in methanol were added in triplicates to the third row (C). A 2-fold serial dilution was done by mixing the contents in each well of the third row (starting from the first column) and transferring 100 μL into the second well of the same column, and the procedure was repeated up to the 7th well of the same column, and the last 100 μL from the 7th well was discarded. A 2-fold dilution yielding concentrations of the plant extracts and standards ranging from 0.01 to 0.5 μg/mL was thus prepared in the wells. The AgNPs (100 μL) and the control were allowed to react with 100 μL of the ABTS + solution, and the absorbance was measured at 734 nm after 7 min using the spectrophotometer. The ABTS + scavenging capacity of the extract was then compared with that of the standards, and the percentage inhibition was calculated as follows:
ABTS scavenging activity (%) = [1− (Abssample)/(Abscontrol)] × 100
where, Abscontrol is the absorbance of ABTS radical + methanol and Abssample is the absorbance of ABTS radical + sample (nanoparticles/standard drugs).
1,1-diphenyl-2-picrylhydrazyl radical scavenging assay
To assess the scavenging ability on DPPH radicals, the method described by Zou et al.[29] using micro-well titer plates was used. In brief, a stock solution of 0.135 mM DPPH radical was prepared in methanol. A volume of 100 μL of methanol was added into all the wells with the exception of second (B) and third (C) rows. Exactly 200 μL of the nanoparticles (0.5 mg/ml) or standards (rutin) prepared in methanol were added in triplicates to the third row (C). A 2-fold serial dilution was done by mixing the contents in each well of the third row (starting from the first column) and transferring 100 μL into the second well of the same column, and the procedure was repeated up to the 7th well of the same column, and the last 100 μL from the 7th well was discarded. A 2-fold dilution yielding concentrations of the plant extracts and standards ranging from 0.01 to 0.5 mg/mL was thus prepared in the wells. The reaction mixture was then vortexed thoroughly and left in dark at room temperature for 30 min, after which the absorbance was measured in a spectrophotometer at 517 nm. The DPPH radical scavenging ability of the nanoparticles was calculated as follows:
DPPH scavenging activity (%) = [(Abscontrol – Abssample)/(Abscontrol)] × 100
Where, Abscontrol is the absorbance of DPPH + methanol and Abssample is the absorbance of DPPH + sample (nanoparticles/standards).
Statistical analysis
Results were expressed as mean ± standard deviation of three replicates and were subjected to analysis of variance using Minitab release version 12, Windows 95. Significant levels were tested at P < 0.05.
RESULTS
Synthesis and characterization of silver nanoparticles
Addition of the spice extracts to aqueous AgNO3 solution resulted in changes of color of the mixtures from faint yellow to colloidal brown indicating AgNP formation [Figure 1].
Figure 1.

Spice extracts before and after reaction with silver nitrate. (a) Allium sativum (garlic), (b) Zingiber officinale (ginger), and (c) Capsicum frutescens (cayenne pepper)
The ultraviolet-visible (UV-Vis) spectrum of AgNPs [Figure 2] was recorded from the reaction medium as a function of time intervals of 15, 30, 45, and 60 min. The AgNPs from the spices gave absorbance peaks ranging between 375 and 480 nm, with a strong resonance centered at 375, 400, and 480 nm at 60, 15, and 60 min for garlic, ginger, and cayenne pepper, respectively.
Figure 2.
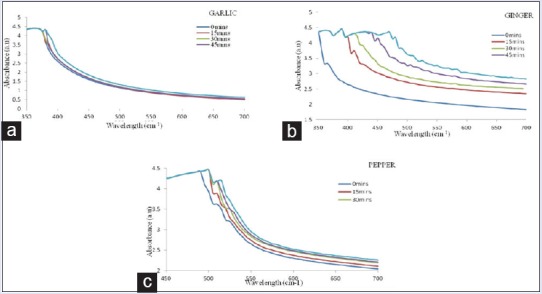
Ultraviolet-visible spectra of silver nanoparticles as a function of time intervals of 15, 30, 45, and 60 min
The UV-Vis spectra also revealed that the AgNPs formed rapidly within 60 min and remained stable even after 24 h. The most intense Plasmon bands were observed for ginger after 15 min between 400 and 435 nm, while for cayenne pepper, it was at 480 nm, an indication of high-reducing ability. The UV-Vis absorption of AgNPs for garlic was at 375 nm. In all the cases, the peak due to silver ion at 300 nm was found missing.
Scanning electron microscopy analysis of silver nanoparticles
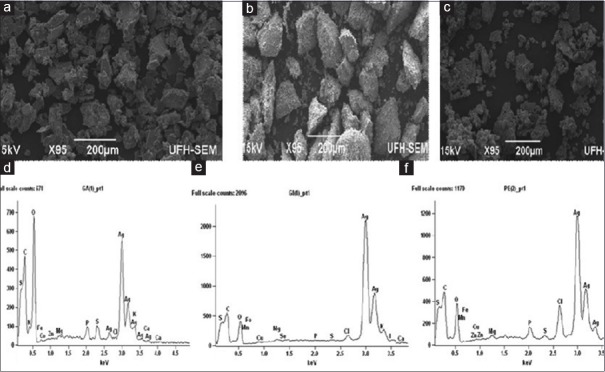
SEM analysis of the AgNPs revealed that well-dispersed, highly crystalline AgNPs were formed [Figure 3]. Morphological examination also revealed that the nanoparticle crystals were cuboidal and not agglomerated.
Figure 3.
Scanning electron micrographs of nanoparticles derived from (a) garlic, (b) ginger, and (c) cayenne pepper; (d-f) Energy dispersive absorption spectra of silver nanoparticles derived from (d) garlic, (e) ginger, and (f) cayenne pepper
Energy dispersive X-ray analysis of silver nanoparticles
EDX further confirmed the formation of AgNPs from the individual spices as there was a strong signal in the silver region confirming the formation of AgNPs [Figure 3]. From the EDX spectra, AgNPs reduced by the spices have the weight percentage of silver as 45.84%, 79.27%, and 57.16% for garlic, ginger, and cayenne pepper, respectively.
Transmission electron microscopy analysis of silver nanoparticles
Representative TEM images of AgNPs of the three spices are shown in Figure 4. The TEM imaging of AgNPs synthesized from the spice extracts revealed spherical shapes with uniform particle size distribution and average sizes of 5.28, 12.97, and 10.86 nm for garlic, ginger, and cayenne pepper, respectively.
Figure 4.
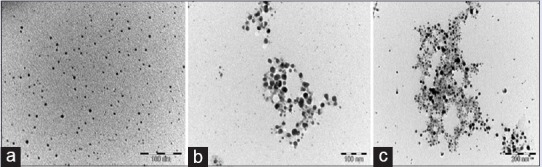
Transmission electron microscopy micrographs of silver nanoparticles from (a) garlic, (b) ginger, and (c) cayenne pepper
Fourier transform infrared microscopic analysis of silver nanoparticles
FTIR spectral measurements were carried out at a resolution of 4 cm−1 to identify the potential functional groups of biomolecules in the spice extracts responsible for reducing and capping the bio-reduced AgNPs. The FTIR analysis revealed different stretches of bonds at different peaks for each of the spices [Figure 5].
Figure 5.
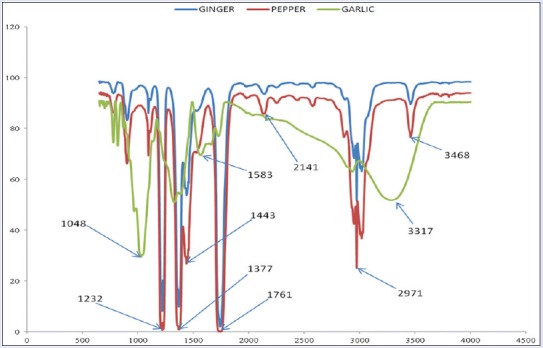
Fourier transform infrared spectra of silver nanoparticles from garlic, ginger, and cayenne pepper
The nanoparticles from garlic showed peaks at 1048, 1331, 1583, 2946, and at 3317 characteristic of C-O (acid and ester stretch), C-N (amine), and O-H stretch/bends, respectively; ginger showed the sharp, less intense peaks at 1238, 1386, 1439, 1475, 2970, and 3443 of C-O (acid and ester stretch), C-N (nitrile), C-H, C=C (aromatic), and the C-H/O-H stretch. The FTIR of nanoparticles from cayenne pepper was characterized by peaks at 1232, 1377, 1443, 1761, 2141, 3019, and 3468 assigned to the C-N (amine), C-H, C=O, C≡C/CN (alkyne/nitrile), C=O (carbonyl), O-H, and N-H stretches, respectively.
X-ray diffraction analysis of silver nanoparticles
The dry powder XRD pattern of the AgNPs synthesized from the spices is shown in Figure 6. XRD provides information about the arrangement of atoms within a crystalline material. All diffraction peaks corresponded to the characteristic crystalline face-centered cubic (FCC) silver lines compared with the standard powder diffraction card of the Joint Committee on Powder Diffraction Standards (JCPDS), silver file No. 04-0783.
Figure 6.
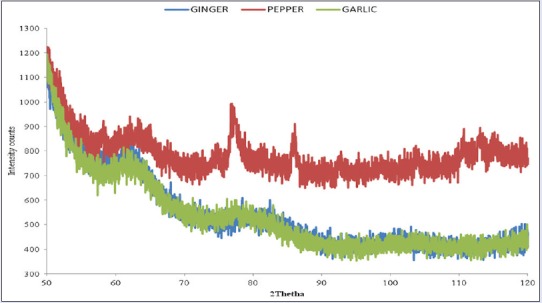
X-ray diffraction pattern of silver nanoparticles from garlic, ginger, and cayenne pepper
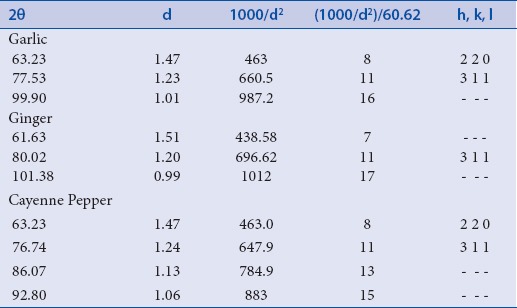
Strong Bragg reflections which correspond to the reflections of silver are shown for each of the spice nanoparticles. The powder diffraction pattern and Miller Indices (hkl) to each peak are assigned and shown in Table 1. Three peaks at 2θ values of (63.23, 77.53, 99.90), (61.63, 80.02, 101.38) corresponding to (220), (311) planes of silver were observed for garlic and ginger, respectively, while four peaks at 2θ values of (63.23, 76.74, 86.07, 92.80) corresponding to (220) and 311 planes of silver were observed for cayenne pepper.
Table 1.
Powder diffraction pattern and Miller Indices (h k l) from d–spacing
Antibacterial activities of silver nanoparticles
Antibacterial activities of the AgNPs were evaluated by Agar dilution method using Mueller–Hinton agar. Agar dilution is one of the most commonly used techniques to determine the MIC of antimicrobial agents, including antibiotics and other substances with bactericidal or bacteriostatic activities. The MIC for each spice nanoparticle is shown in Figure 7.
Figure 7.
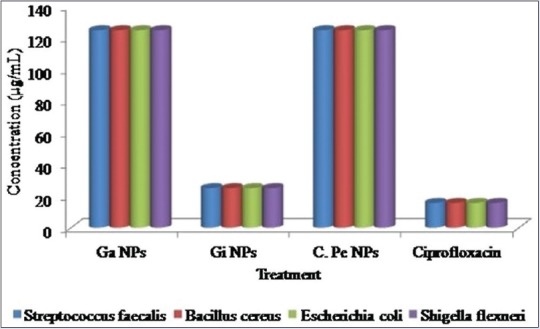
Minimum inhibitory concentration (MIC, μg/mL) of silver nanoparticles against bacterial isolates. Note-GaNPs: Garlic nanoparticles, GiNPs: Ginger nanoparticles, C.PeNPs: Cayenne pepper nanoparticles
Antioxidant activity of silver nanoparticles
The antioxidant activity of synthesized AgNPs was evaluated by DPPH and ABTS radical scavenging assay, and Rutin was used as a positive control. The AgNPs exhibited potential free radical scavenging activity against both radicals as shown in Figure 8. The maximum scavenging activity against ABTS was exhibited by nanoparticles from cayenne pepper (inhibitory concentration 50% [IC50] = 31.25 μg/mL), while ginger and garlic nanoparticles exhibited a concentration-dependent scavenging activity with IC50 of 240 and 250 μg/mL, respectively. Garlic nanoparticles exhibited the highest scavenging activity against DPPH (IC50: <31.25 μg/mL), followed by cayenne pepper (IC50: 40 μg/mL) and ginger (IC50: 120 μg/mL).
Figure 8.
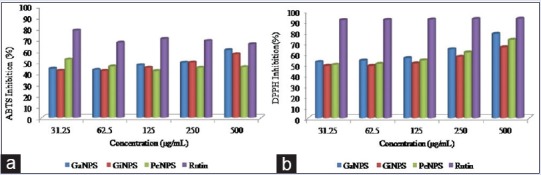
Inhibition of (a) 2,2-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) and (b) 1,1-diphenyl-2-picrylhydrazyl radicals by synthesized silver nanoparticles
DISCUSSION
The chemical reduction of aqueous solution of AgNO3 is one of the most widely used methods for the synthesis of AgNPs. According to reports by Lalitha et al.[30] and Hyllested et al.,[31] color change is an important factor for the synthesis of AgNPs. AgNPs appear brown in aqueous medium as a result of surface Plasmon vibrations.[32] Other studies have reported similar color changes which confirm the formation of AgNPs.[33,34] Several authors have reported color formations ranging from light yellow, yellowish brown, to dark brown for colloidal AgNPs.[4,35,36] The nanoparticles were primarily characterized by UV-Vis spectroscopy, which has proved to be a very useful technique for the analysis of nanoparticles. Reduction of Ag+ ions in the aqueous solution of silver complex during the reaction with the ingredients present in the spice extracts observed by the UV-Vis spectroscopy revealed that AgNPs in the solution may be correlated with the UV-Vis spectra. This has been attributed to excitation of Plasmon vibrations of AgNPs in the solution which indicates the reduction of silver ions to AgNPs. Researchers have reported surface Plasmon resonance for colloidal silver ranging from 320 to 390 nm when sorghum bran was used,[37] while others[10,35] have reported 420 nm when soluble starch and Annona squamosa leaves were used, respectively. These tend to suggest that the nature of the capping agent could affect the SPR of colloidal AgNPs.
The UV-Vis absorption for garlic implies that AgNPs were coated and stabilized by bioactives such as proteins which prevented the transition of electrons from the surface of AgNPs. All these results cumulatively suggest that the colloidal silver system so obtained was ideally monodispersed. This clearly shows that only AgNPs are present in the colloidal dispersion.38. SEM is commonly used to provide images about the morphology, size, shape, and organization of the surface topography of specimens.[39] According to Theivasanthi and Alagar,[40] most metals tend to nucleate and grow into twinned and multiply twinned particles with their surfaces bounded by the lowest energy facets. The observation of some larger nanoparticles may be attributed to the fact that AgNPs have the tendency to agglomerate due to their high surface energy and high surface tension of the ultrafine nanoparticles. The fine particle size results in a large surface area that, in turn, enhances the nanoparticle activity.
EDX micro-analysis is performed by measuring the energy and intensity distribution of X-ray signals generated by a focused electron beam on a specimen. EDX spectra recorded from the AgNPs confirmed the synthesis of AgNPs by the extracts. According to previous reports, AgNPs typically absorb in the region of 3 keV owing to surface Plasmon resonance.[41] Other weak peaks observed in the EDX spectra may have originated from biomolecules bound to the surface of the AgNPs.[34,42]
TEM is the most suitable method for providing detailed information about very fine structures in nanoparticles.[43] The magnification factor for TEM is typically in the range of about 102–106 times, which enables very fine structures to be observed.[44] There were no obvious particle aggregations in the TEM imaging. The identical shapes observed for the three NPs could be attributed to similarity in the reductive agents present in the three spices.[45] AgNPs of various sizes (ranging from 5 to 100 nm) and morphologies (spherical, nanorods, hexagonal, etc.) have been synthesized using various plant extracts such as soluble starch, coffee, green tea, fungal, spices, and other biological extracts.[46,47,48,49]
FTIR spectral measurements are used to identify the potential functional groups of biomolecules responsible for reducing and capping the bio-reduced AgNPs. According to Sasidharan et al.,[50] FTIR has proven to be a valuable tool for the characterization and identification of compounds or functional groups, and the spectra of pure compounds are usually so unique that they are like a molecular “fingerprint.” In this study, the bands observed denote stretching vibrations responsible for compounds such as flavonoids, phenols, terpenoids, and proteins,[17] and could confirm that these biomolecules in the spice extracts were responsible for reducing, capping, and stabilizing of the AgNPs.[34,51] The reduction and capping of silver ion into AgNPs in the present study could be attributed to flavonoids, phenols, and proteins, which abound in the three spices. Several studies have reported similar observations from FTIR analysis of AgNPs synthesized from plant extracts such as Cycas circinalis, Ficus amplissima, Commelina benghalensis and Lippia nodiflora,[52] Pedalium murex,[53] Urtica dioica,[49] and Azadirachta indica.[54]
XRD provides information about the chemical composition and arrangement of atoms within a crystalline material. The observed arrangement in this study confirmed that the main composition of the nanoparticles was silver.[55] All the peaks in XRD pattern were readily indexed to a FCC structure of silver as per the available literature (JCPDS, File No. 4-0783), and the particles are crystalline in nature. The intensity of peaks reflected the high degree of crystalline nature of the AgNPs. However, the diffraction peaks which are broad indicate that the crystal sizes are very small.[56]
Further, the Bragg reflections revealed that both ginger and garlic were assigned to the same space group. Some unassigned peaks have also been observed, suggesting the crystallization of other biomolecules responsible for silver ion reduction and stabilization of resultant nanoparticles.[57,58,59] This is in agreement with the results obtained by other researchers on Morganella spp. and Elaeagnus latifolia.[60,61]
The AgNPs showed strong antibacterial activity against all the tested bacterial strains. Ginger AgNPs exhibited the maximum inhibition for both Gram-positive and Gram-negative bacteria, while garlic and cayenne pepper nanoparticles exhibited equipotent antibacterial property. This implies that the ginger nanoparticles had the smallest diameter compared to those from garlic and cayenne pepper. The biocidal activity of the spice nanoparticles could be due to the high affinity of Ag+ for thiols, leading to disruption of enzyme function responsible for nutrient uptake and cellular energy production/storage processes, thereby killing the microbes.[49,62] Most AgNPs obtained from medicinal plants have been credited with high antimicrobial properties, especially against drug-resistant human pathogenic and food spoilage microbes.[63,64] The results from this study correlate with other reports that most plant extract AgNPs exhibit enhanced broad-spectrum antibacterial activities.[65] This suggests that nanoparticles from spice extracts could yield valuable alternative as antibacterial drugs.
DPPH and ABTS are widely used for testing preliminary radical scavenging activity of compounds or nanoparticles and provide easy and rapid evaluation. In the present study, the synthesized AgNPs exhibited potential free radical scavenging activity against both radicals. Polyphenolic compounds such as flavonoids, flavonols, proanthocyanidin, and phenolics in plants have been reported to have strong antioxidant activities which help to protect cells against oxidative damage by free radicals. The antioxidant activities were enhanced by conversion into AgNPs. The enhanced antioxidant activities of AgNPs from plant sources such as Chenopodium murale,[66] Piper longum[67] as well as zinc nanoparticles from A. sativum, Rosmarinus officinalis, and Ocimum basilicum[66] have been reported.
CONCLUSION
AgNPs were successfully synthesized and characterized from AgNO3 and aqueous extracts of garlic, ginger, and cayenne pepper. Due to the varying properties of the three spices, variations in size of the synthesized AgNPs were observed. The synthesis of AgNPs using garlic, ginger, and cayenne pepper was a rapid, large-scale, size- and shape-controlled process. All the nanoparticles exhibited potent antibacterial and antioxidant activities, with ginger nanoparticles being the most active. Further optimization of the current green-synthesis method would help in the production of monodispersed AgNPs having great potential for the treatment of several diseases and food preservation. Thus, the AgNPs synthesized from these spices could be promising candidates for use in nanomedicine and other areas where such applications are needed, especially with drug-resistant bacteria.
Financial support and sponsorship
This work was supported by the National Research Foundation (NRF), South Africa, under Grant NRF: 85294 (Govan Mbeki Research and Development Centre [GMRDC], University of Fort Hare, South Africa) under Grant GMRDC: C127.
Conflict of Interest
There are no conflicts of interests.
REFERENCES
- 1.Duncan TV. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garima S, Riju B, Kunal K, Ashish RS, Rajendra PS. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nano Res. 2011;13:2981–8. [Google Scholar]
- 3.Suganya TR, Devasena T. Exploring the mechanism of anti-inflammatory activity of phyto-stabilized silver nanorods. Dig J Nanomater Biostruct. 2015;10:277–82. [Google Scholar]
- 4.Krithiga N, Rajalakshmi A, Jayachitra A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J Nanosci. 2015:8. doi: 10.1155/2015/928204. [Google Scholar]
- 5.Khan MA, Kumar S, Ahamed M, Alrokayan SA, Alsalhi MS. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nano Res Lett. 2011;6:434. doi: 10.1186/1556-276X-6-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cieśla L, Kowalska I, Oleszek W, Stochmal A. Free radical scavenging activities of polyphenolic compounds isolated from Medicago sativa and Medicago truncatula assessed by means of thin-layer chromatography DPPH˙ rapid test. Phytochem Anal. 2013;24:47–52. doi: 10.1002/pca.2379. [DOI] [PubMed] [Google Scholar]
- 7.Kharissova OV, Dias HV, Kharisov BI, Pérez BO, Pérez VM. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–8. doi: 10.1016/j.tibtech.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Geetha AR, George E, Srinivasan A, Shaik J. Optimization of green synthesis of silver nanoparticles from leaf extracts of Pimenta dioica (Allspice) ScientificWorldJournal 2013. 2013:362890. doi: 10.1155/2013/362890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmugasundaram T, Balagurunathan R. Mosquito larvicidal activity of silver nanoparticles synthesised using actinobacterium, Streptomyces sp. M25 against Anopheles subpictus, Culex quinquefasciatus and Aedes aegypti. J Parasit Dis. 2015;39:677–84. doi: 10.1007/s12639-013-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velayutham K, Ramanibai R. Larvicidal activity of synthesized silver nanoparticles using isoamyl acetate identified in Annona squamosa leaves against Aedes aegypti and Culex quinquefasciatus. J Basic Appl Zool. 2016;74:16–22. [Google Scholar]
- 11.Raja S, Ramesh V, Thivaharan V. Antibacterial and anticoagulant activity of silver nanoparticles synthesised from a novel source-pods of Peltophorum pterocarpum. J Ind Eng Chem. 2015;29:257–64. [Google Scholar]
- 12.Lateef A, Akande MA, Ojo SA, Folarin BI, Gueguim-Kana EB, Beukes LS. Paper wasp nest-mediated biosynthesis of silver nanoparticles for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. 3 Bitech. 2016;6:140. doi: 10.1007/s13205-016-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David L, Moldovan B, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E, et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf B Biointerfaces. 2014;122:767–77. doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Aparna Mani KM, Seethalakshmi S, Gopal V. Evaluation of in-vitro anti-inflammatory activity of silver nanoparticles synthesised using Piper nigrum Extract. J Nanomed Nanotechnol. 2015;6:575–81. [Google Scholar]
- 15.Okafor F, Janen A, Kukhtareva T, Edwards V, Curley M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int J Environ Res Public Health. 2013;10:5221–38. doi: 10.3390/ijerph10105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari SH, Islam F, Sameem M. Influence of nanotechnology on herbal drugs: A review. J Adv Pharm Technol Res. 2012;3:142–6. doi: 10.4103/2231-4040.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauwel P, Küünal S, Ferdov S, Rauwel E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv Mater Sci Eng. 2015:9. doi:10.1155/2015/68274. [Google Scholar]
- 18.Huang J, Li Q, Sun D, Lu Y, Su Y, Xin Y, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104–15. [Google Scholar]
- 19.Lal SS, Nayak PL. Green synthesis of gold nanoparticles using various extract of plants and spices. Int J Sci Innov Discov. 2012;2:325–50. [Google Scholar]
- 20.Muthukumaran U, Govindarajan M, Rajeswary M, Hoti SL. Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol Res. 2015;114:1817–27. doi: 10.1007/s00436-015-4368-4. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja KD, Robertson IK, Geraghty DP, Ball MJ. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr. 2006;84:63–9. doi: 10.1093/ajcn/84.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Petrovska BB, Cekovska S. Extracts from the history and medical properties of garlic. Pharmacogn Rev. 2010;4:106–10. doi: 10.4103/0973-7847.65321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubra IR, Rao LJ. An impression on current development in the technology, chemistry and biological activities of ginger (Zingiber officinale roscoe) Crit Rev Food Sci Nutr. 2012;52:651–88. doi: 10.1080/10408398.2010.505689. [DOI] [PubMed] [Google Scholar]
- 24.Haniadka R, Rajeev AG, Palatty PL, Arora R, Baliga MS. Zingiber officinale (Ginger) as an anti-emetic in cancer chemotherapy: A review. J Altern Complement Med. 2012;18:440–4. doi: 10.1089/acm.2010.0737. [DOI] [PubMed] [Google Scholar]
- 25.Yiming L, Tran VH, Duke CC, Roufogalis BD. Preventive and protective properties of Zingiber officinale (Ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: A brief review. Evid Based Complement Alternat Med. 2012:10. doi: 10.1155/2012/516870. doi:1155/2012/516870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. 2013;16:1031–48. [PMC free article] [PubMed] [Google Scholar]
- 27.Otunola GA, Oloyede OB, Oladiji AT, Afolayan AJ. Comparative analysis of the chemical composition of three spices-Allium sativum L., Zingiber officinale Rosc and Capsicum frutescens L. commonly consumed in Nigeria. Afr J Biotechnol. 2010;9:6927–31. [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard. NCCLS Document M7-A6. 6th ed. Wayne, PA: NCCLS; 2003. [Google Scholar]
- 29.Re R, Pellegini N, Proteggente A, Pannala A, Yeung M, Rice-Evans C. Antioxidant activity: Applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 30.Zou Y, Chang SK, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 2011;59:2268–76. doi: 10.1021/jf104640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalitha A, Subbaiya R, Ponmurugan P. Green synthesis of silver nanoparticles from leaf extract of Azadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol Appl Sci. 2013;2:228–35. [Google Scholar]
- 32.ᴁrøe Hyllested J, Espina Palanco M, Hagen N, Mogensen KB, Kneipp K. Green preparation and spectroscopic characterization of plasmonic silver nanoparticles using fruits as reducing agents. Beilstein J Nanotechnol. 2015;6:293–9. doi: 10.3762/bjnano.6.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces. 2010;76:50–6. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, et al. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces. 2010;81:81–6. doi: 10.1016/j.colsurfb.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess. 2014;1:3. [Google Scholar]
- 36.Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341:2012–8. doi: 10.1016/j.carres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr Res. 2004;339:2627–31. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Rani SU, Pandian KJ, Reddy BS. Syntheses and characterisation of silver nanoparticles in the acrylate copolymer. J Exp Nanosci. 2009;4:285–99. [Google Scholar]
- 39.Egerton R. Physical Principles of Electron Microscopy: An Introduction to TEM, SEM, and AEM. New York: Springer Science; 2008. [Google Scholar]
- 40.Theivasanthi T, Alagar M. Electrolytic synthesis and characterizations of silver nanopowder. Nano Biomed Eng. 2012;4:58–65. [Google Scholar]
- 41.Magudapatty P, Gangopadhyayrans P, Panigrahi BK, Nair KG, Dhara S. Electrical transport studies of Ag nanoparticles embedded in glass matrix. Physica B: Condensed Matter. 2001;299:142–6. [Google Scholar]
- 42.Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32:79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 43.Dudkiewicz A, Tiede K, Loeschner K, Jensen LH, Jensen E, Wierzbicki R, et al. Characterization of nanomaterials in food by electron microscopy. Trends Analyt Chem. 2011;30:28–43. [Google Scholar]
- 44.Klang V, Valenta C, Matsko NB. Electron microscopy of pharmaceutical systems. Micron. 2013;44:45–74. doi: 10.1016/j.micron.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces. 2009;68:55–60. doi: 10.1016/j.colsurfb.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Nadagouda MN, Varma RS. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008;10:859–62. [Google Scholar]
- 47.Siemieniec J. Synthesis of silver and gold nanoparticles using methods of green chemistry. CHEMIK. 2013;67:842–7. [Google Scholar]
- 48.Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6:399. [Google Scholar]
- 49.Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 2016;9:217–27. [Google Scholar]
- 50.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 51.Mehmood RA, Murtaza G, Bhatti TM, Kausar R, Ahmed MJ. Biosynthesis, characterization and antimicrobial action of silver nanoparticles from root bark extract of Berberis lyceum. Pak J Pharm Sci. 2016;29:131–7. [PubMed] [Google Scholar]
- 52.Liu X, Atwater M, Wang J, Huo Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B Biointerfaces. 2007;58:3–7. doi: 10.1016/j.colsurfb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Khanna PK, Nair CK. Synthesis of silver nanoparticles with fish oil: A novel approach to nano-biotechnology. Int J Green Nanotechnol Physics Chem. 2009;1:3–9. [Google Scholar]
- 54.Ahmed S, Saifullah MA, Babu LS, Saiqa I. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9:1–7. [Google Scholar]
- 55.Mubayi A, Chatterji S, Rai PM, Watal G. Evidence based green synthesis of nanoparticles. Adv Mater Lett. 2012;3:519–25. [Google Scholar]
- 56.Roopan SM, Madhumitha RG, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 2013;43:631–5. [Google Scholar]
- 57.Sathiya CK, Akilandeswari S. Fabrication and characterization of silver nanoparticles using Delonix elata leaf broth. Spectrochim Acta A Mol Biomol Spectrosc. 2014;128:337–41. doi: 10.1016/j.saa.2014.02.172. [DOI] [PubMed] [Google Scholar]
- 58.Parikh RY, Ramanathan R, Coloe PJ, Bhargava SK, Patole MS, Shouche YS, et al. Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One. 2011;6:e21401. doi: 10.1371/journal.pone.0021401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phanjom P, Sultana A, Sarma H, Ramchiary J, Goswami K, Baishya P. Plant mediated synthesis of silver nanoparticles using Elaeagnus latifolia leaf extract. Dig J Nanomater Biostruct. 2012;7:1117–23. [Google Scholar]
- 60.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci. 2014;5:73–81. [Google Scholar]
- 62.Stan M, Popa A, Toloman D, Silipas TD, Vodnar DC. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metallurgica Sin (Eng Lett) 2016;29:228. [Google Scholar]
- 63.Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18:356–63. [Google Scholar]
- 64.Reddy NJ, Nagoor Vali D, Rani M, Rani SS. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater Sci Eng C Mater Biol Appl. 2014;34:115–22. doi: 10.1016/j.msec.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 65.Abdul-Rehman P, Qamar A, Attarad A, Hussain R, Song JK, Muhammad Z, et al. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J Pharm Sci. 2016;2:31–6. [Google Scholar]
- 66.Ojha AK, Behera S, Rout J, Dash MP, Nayak PL. Green synthesis of silver nanoparticles from syzygium aromaticum and their antibacterial efficacy. Int J Pharm Bio Sci. 2012;1:335–41. [Google Scholar]
- 67.Vivekanandhan S, Christensen L, Misra M, Mohanty AK. Green process for impregnation of silver nanoparticles into microcrystalline cellulose and thin antimicrobial bionanocomposite films. J Biomater Nanobiotechnol. 2012;3:371–6. [Google Scholar]