Abstract
Aims:
The study is conducted to evaluate the immunomodulatory, cytotoxicity, and antioxidant potential of Ziziphus mauritiana (Rhamnaceae). Phytochemical analysis of Z. mauritiana revealed the presence of alkaloids, anthraquinone glycoside, cardiac glycoside, saponin, tannin, and flavonoids.
Methodology:
The cytotoxicity of the plant Z. mauritiana was evaluated by brine shrimp lethality test. Antioxidant parameters such as superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) levels were calculated in the plasma of rats after chronic administration of 400 mg/kg of Z. mauritiana for 6 weeks.
Results:
The dichloromethane extract of the plant exhibited significant immunomodulatory activity, with inhibitory concentration 50% of 55.43 ± 7.9. The dichloromethane extracts of the plant showed 70% mortality at concentration 1000 μg/ml. SOD and T-AOC levels were increased while MDA level in the plasma was reduced in the plasma of rats treated with dichloromethane Z. mauritiana.
Conclusion:
This can be deduced that the root of Z. mauritiana has immunomodulatory, cytotoxic, and antioxidant potential.
SUMMARY
Roots of Z. mauritiana was exhibited immunomodulator, cytotoxic and antioxidant activities
Z. mauritiana showed potential antioxidant activity in rats
Abbreviations used: SOD: Superoxide dismutase; T-AOC: Total antioxidant capacity; MDA: Malondialdehyde; ZMRD: Z. mauritiana root extract of dichloromethane fraction; LD50: Z. mauritiana root extract of methanol fraction ZMRM, lethal dose 50.
Keywords: Cytotoxicity and antioxidant potential, immunomodulatory, Ziziphus mauritiana
INTRODUCTION
There is a lot of archaeological evidence that humans used medicinal plants for better living, reducing disease, and improving quality of life. Several studies have described the use of plants in traditional medicine for many years and have recently gained much importance in the field of pharmacological industries. The knowledge and assessments of the biological properties of extracts from plants can serve as a source of future drug candidates in many areas of health.[1] The relevant literature shows that many plants or plant products have been used as an alternative source for the treatment of different diseases. Several of these herbs have been used to exert immunomodulatory effect in the treatment of various diseases. Medicinal plants with immunomodulatory activity are used for cases of organ transplant rejection or for the treatment of various autoimmune diseases.[2] Medicinal plants can inhibit or stimulate immune response which could be useful in the treatment of various human diseases. In particular, medicinal plants capable of inhibiting the cellular and humoral responses could have useful applications in the treatment of immunological disorders.[3]
The plant Ziziphus mauritiana belongs to the family Rhamnaceae. The family comprises 58 genera and almost 900 species.[4] Many species of the genus Ziziphus are found in Pakistan, Iran, Europe, America, India, and Afghanistan.[5] The plant is commonly known as Berry and Chotta Ber in Pakistan.[6] The plant is used to cure wide variety of diseases such as fever, ulcer, asthma, depression, anxiety, and inflammation.[7,8,9] Plant is enriched with biological and phytochemical properties. The Z. mauritiana showed antioxidant, antimicrobial, antitumor, antidiabetic, and anticancer activities.[6,10,11]
The various species of genus Ziziphus showed antipyretic, antidiuretic, anti-inflammatory, and analgesic activities.[12,13] The genus Sisyphus has found many photochemical constituents, cyclopeptide alkaloids such as Abssenine B, Zazyphine D, amphibine C, and oxyphylline A.[14] The polysaccharides such as rhamnose and arabinose also reported from genus Ziziphus.[15]
MATERIALS AND METHODS
Plant collection
The roots of Z. mauritiana was collected from surroundings of Bahauddin Zakariya University in 2015 and identified by Dr. Zafrulla Ullah Zafar, Plant Taxonomist, Institute of Pure and Applied Biology, Bahauddin Zakariya University Multan, Pakistan. A voucher specimen number Stewart 469 (6) for Z. mauritiana was submitted to the department.
Extraction
The freshly collected roots of Z. mauritiana (20 g) was taken and shade dried, ground, and extracted successively with solvents dichloromethane and methanol (3 × 6 L) at room temperature for 24 h. The dichloromethane and methanolic extract was concentrated under vacuum on Rotavapor model number Buchi-Rotavapor R.200. The dichloromethane extract was obtained brown crude extract (1.64 g) which was labeled as ZMRD. The other extract of methanol was obtained as dark brown crude extract (13.37 g) which was labeled as ZMRM. Results of the extraction of the plant are mentioned in Table 1.
Table 1.
Result of extraction of roots of Zaziphus maritiana

Phytochemical tests
Detection of alkaloids, anthraquinone glycosides, cardiac glycosides, tannin, catechin, flavonoids, and saponin was done using the gold standard methods as reported.[16]
Brine shrimp lethality assay
10 mg of each plant extract was dissolved in 1 ml of dimethyl sulfoxide to prepare a stock solution. It was used to prepare further dilutions. 34 g of sea salt (Sigma) was mixed in a liter of distilled water. It was continuously stirred for 2 h. We used a rectangular tray having two compartments with sea salt saline for the hatching of brine shrimps. One compartment was covered for darkness. Eggs of brine shrimps were sprinkled in this dark compartment and placed for hatching for 24 h. Larvae were collected after 24 h with the help of pipette from lightened side. 100 μl (1000 μg), 10 μl (100 μg), and 1 μl (10 μg) of the plant extract stock were taken up and added in drum vials used in the assay. In each vial, we added 2 ml of saline and then with the help of pipette transferred 10 shrimps in each vial, raised the volume of saline in each vial up to 5 ml, and incubated at 25°C. After 24 and 48 h of incubation, number of survivors was counted using magnifying glass.
Then, using Abbot's formula calculations were made.
Percentage death = (control - sample)/control × 100 (Meyer et al. 1982).
Immunomodulatory activity
The estimation of oxidative burst activity performed by chemiluminescence assay. Luminol-enhanced chemiluminescence assay was conducted following procedure.[17] The brief steps are given below:
Place 25 μl Hanks’ balanced salt solution (HBSS)++ to a 96-well flat bottomed plate in a final volume of 0.1 ml
Add sample (drug or compound to be tested) and prepare serial dilutions to obtain very low level of activity
Add HBSS++ with no compound or drug to be used as control
Place 25 μl whole blood (1:50 dilution) or PMNCs (1 × 106/ml) or MNCs cells (1 × 106/ml) or macrophages (1 × 106/ml) in suspension of HBSS++ to each well, except B* and blank
The mixture will be placed in incubator for 30 min in Luminoskan chamber about 37°C
After incubation, add 25 μL of luminol into each well
Place 25 μL serum opsonized zymosan, except A* and blank
The luminometer used to monitor the phagocytosis studies for 50 min in the repeated scan mode. Peak and total integral chemiluminescence reading were expressed in the relatively light unit (RLU). The RLU was used to express the peak and total chemiluminescence.
Antioxidant activity
Totally, 18 Wistar–Kyoto rats were recruited and acclimatized for 5 days before starting the chronic administration of Z. mauritiana. Animals were divided into 3 groups: control group receiving water, standard group receiving Vitamin C 1 g/kg, and third group receiving 400 mg/kg dose of Z. mauritiana for 6 weeks as reported.[18] These animals were given free access to water and food containing ad libitum.
Measurement of oxidative stress parameters
Oxidative stress parameters such as superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) levels were measured after 6 weeks of chronic administration by drawing the blood by nipping the tail and collecting the plasma by centrifugation the blood at 10,000 rpm for 5 min from the plasma using kits (NJJC Bio Inc., Nanjing, China).
RESULTS AND DISCUSSION
The preliminary phytochemical analysis of the plant showed the presence of alkaloids, flavonoids, tannins, saponin, and cardioglycosides [Table 2]. The dichloromethane and methanol extracts were obtained and subjected to investigate the cytotoxicity and immunomodulatory activity. The dichloromethane extract of the plant exhibited significant inhibitory of immunomodulatory activity [Table 3]. The dichloromethane extracts of the plant showed 70% mortality at concentration 1000 μg/ml [Tables 4 and 5]. The cytotoxicity of the plant showed due to the presence of flavonoids.
Table 2.
Results of phytochemical screening of Zaziphus mauritiana

Table 3.
Results of immunomodulatory studies dichloromethane and methanolic extracts of roots of Zaziphus mauritiana
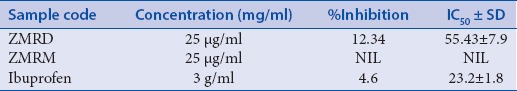
Table 4.
Result of Brine Shrimp (Artemiasalina) lethality bioassay of dichloromathane extracts of roots of Zaziphus mauritiana
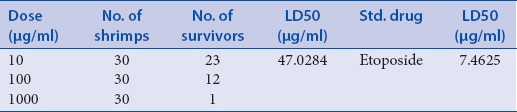
Table 5.
Result of brine shrimp (Artemiasalina) lethality bioassay of methanolic extracts of roots of Zaziphus mauritian
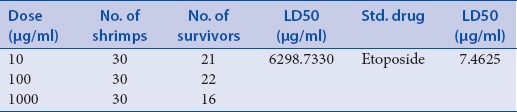
Many natural products have played a significant role in maintaining and improving the quality of human life. The modulation of immune response to alleviate many diseases has been one mechanism of interest for pharmacology studies. A great number of medicinal plants have been shown to stimulate or inhibit immune response. In the present study, dichloromethane and methanolic extract obtained from roots of this plant was investigated for its cytotoxic effects and immunomodulatory activity. For this purpose, at first, the present study evaluated the cytotoxic activity of the extract of the plant by brine shrimp lethality activity. It was observed that ZMRD extract showed highest activity at 1 g dose having LD50 values 47 μg/ml when compared to standard drug etoposide 7.46 μg/ml. In contrast, methanolic extract was 50% less effective at same dose, indicating that the dichloromethane extract is better in cytotoxicity.
Environmental pollutants and dietary habits cause disturbances in immune activities, and diet containing micronutrients and antioxidants is known to prevent these alterations.[19] The use of herbs as an immunomodulator can modulate the body's defense system. With this point, both extracts were also employed to explore immunomodulatory activity. It was observed that methanolic extract ZMRM did not show any activity when compared to standard drug ibuprofen while dichloromethane extract ZMRD showed 12.35% inhibition with inhibitory concentration 50% 55.43 ± 7.9 when compared to standard drug brufen having 4.6% inhibition. This immunomodulatory activity of ZMRD opens a gateway to explore the different activities such as anti-inflammatory, antiangiogenic, antileukotriene, and immunosuppressant of extract.
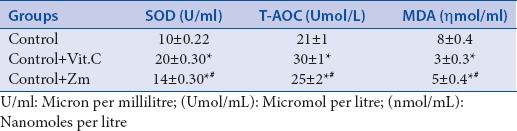
Oxidative stress plays an imperative role in cardiac and vascular abnormalities in different types of cardiovascular diseases that are why any antioxidant therapy may be beneficial for combating these diseases.[18] Natural plants have been used as antioxidant extensively to combat with different diseases. In the human body, mostly SOD and MDA are in the balance, while most of the time, SOD is predominated in normal conditions. Increased MDA indicates the presence of free radicals and onset of diseases left ventricular hypertrophy.[20] These free radicals are culprit for the onset of many diseases where MDA is elevated locally and systemically. Antioxidants are responsible to counteract these free radicals and increase the antioxidant mechanisms of the body such as SOD and T-AOC. It has been observed in present study that exogenous administration of Z. mauritiana roots after 6 weeks of oral treatment significantly reduced (P < 0.05) the plasma levels of MDA and significantly increased (all P < 0.05) the levels of SOD and T-AOC in the plasma when compared to control group as shown in Table 6. These findings are in line with the findings of previously reported data,[21] which explored antioxidant role of Z. mauritiana in liver protection. These findings indicate the emerging role of this medicinal plant as an antioxidant in different pathological ailments.
Table 6.
Oxidative stress parameters investigated after 6 weeks. *Denotes significant difference P<0.05 in comparison vs. Control while #represents comparison with Control + Vit.C
CONCLUSION
The present study has explored that Z. mauritiana roots extract, particularly dichloromethane, exhibited cytotoxicity, immunomodulatory, and antioxidant activities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bose U, Bala V, Ghosh TN, Gunasekaran K, Rahman AA. Antinociceptive, cytotoxic and antibacterial activities of Cleome viscosa leaves. Braz J Pharmacol. 2011;21:165–9. [Google Scholar]
- 2.Hajra S, Mehta A, Pandey P. Immunostimulating activity of methanolic extract of Swietenia mahagoni seeds. Int J Pharm Pharm Sci. 2012;4:442–5. [Google Scholar]
- 3.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaleem W, Muhammad N, Khan H, Rauf A. Pharmacological and phytochemical studies of Genus Ziziphus. Middle East J Sci Res. 2014;21:1243–63. [Google Scholar]
- 5.Razi MF, Anwar R, Basra S, Khan MM, Khan IA. Morphological characterization of leaves and fruit of jujube (Ziziphus mauritiana Lamk.) germplasm in Faisalabad, Pakistan. Pak J Agric Sci. 2013;50:211–6. [Google Scholar]
- 6.Ashraf A, Sarfraz RA, Anwar F, Shahid SA, Alkharfy KM. Chemical composition and biological activities of leaves of Ziziphus mauritiana L. native to Pakistan. Pak J Bot. 2015;47:367–76. [Google Scholar]
- 7.Bhatia A, Mishra T. Hypoglycemic activity of Ziziphus mauritiana aqueous ethanol seed extract in alloxan-induced diabetic mice. Pharm Biol. 2010;48:604–10. doi: 10.3109/13880200903218935. [DOI] [PubMed] [Google Scholar]
- 8.Siddarth P, Kailash P, Niraj V, Karuna M, Vimal P, Bharadia P, et al. Antiulcer activity of methanolic extract of Ziziphus mauritiana Stem Bark. Int J Pharmacog Phytochem Res. 2010;2:6–11. [Google Scholar]
- 9.Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012;12:59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisse A, Ndiaye A, Lopez-Sall P, Seck F, Faye B, Faye B. Antidiabetic activity of Ziziphus mauritiana Lam (Rhamnaceae) Dakar Med. 2000;45:105–7. [PubMed] [Google Scholar]
- 11.Borgi W, Recio MC, Ríos JL, Chouchane N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Ziziphus lotus (L.) Lam. South Afr J Bot. 2008;74:320–4. [Google Scholar]
- 12.Nisar M, Adzu B, Inamullah K, Bashir A, Ihsan A, Gilani AH. Antinociceptive and antipyretic activities of the Ziziphus oxyphylla Edgew. leaves. Phytother Res. 2007;21:693–5. doi: 10.1002/ptr.2139. [DOI] [PubMed] [Google Scholar]
- 13.Adzu B, Amos S, Wambebe C, Gamaniel K. Antinociceptive activity of Ziziphus spina-christi root bark extract. Fitoterapia. 2001;72:344–50. doi: 10.1016/s0367-326x(00)00289-6. [DOI] [PubMed] [Google Scholar]
- 14.Gournelis DC, Laskaris GG, Verpoorte R. Cyclopeptide alkaloids. Nat Prod Rep. 1997;14:75–82. doi: 10.1039/np9971400075. [DOI] [PubMed] [Google Scholar]
- 15.Renault JH, Ghedira K, Thepenier P, Lavaud C, Zeches-Hanrot M, Le Men-Olivier L. Dammarane saponins from Ziziphus lotus. Phytochemistry. 1997;44:1321–7. doi: 10.1016/s0031-9422(96)00721-2. [DOI] [PubMed] [Google Scholar]
- 16.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 17.Helfand SL, Werkmeister J, Roder JC. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J Exp Med. 1982;156:492–505. doi: 10.1084/jem.156.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bafna A, Mishra S. Antioxidant and immunomodulatory activity of the alkaloidal fraction of Cissampelos pareira linn. Sci Pharm. 2010;78:21–31. doi: 10.3797/scipharm.0904-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad A, Sattar MA, Rathore HA, Abdulla MH, Khan SA, Azam M, et al. Up Regulation of cystathione γ lyase and Hydrogen sulphide in the myocardium inhibits the progression of isoproterenol-caffeine induced left ventricular hypertrophy in wistar Kyoto Rats. PLoS One. 2016;11:e0150137. doi: 10.1371/journal.pone.0150137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahiru D, Obidoa O. Evaluation of the antioxidant effects of Ziziphus mauritiana Lam. Leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr J Tradit Complement Altern Med. 2007;5:39–45. doi: 10.4314/ajtcam.v5i1.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]


