Abstract
Saraca asoca (Roxb.) De Wilde (Ashoka) is a highly valued endangered medicinal tree species from Western Ghats of India. Besides treating cardiac and circulatory problems, S. asoca provides immense relief in gynecological disorders. Higher price and demand, in contrast to the smaller population size of the plant, have motivated adulteration with other plants such as Polyalthia longifolia (Sonnerat) Thwaites. The fundamental concerns in quality control of S. asoca arise due to its part of medicinal value (Bark) and the chemical composition. Phytochemical fingerprinting with proper selection of analytical markers is a promising method in addressing quality control issues. In the present study, high-performance liquid chromatography of phenolic compounds (gallic acid, catechin, and epicatechin) coupled to multivariate analysis was used. Five samples each of S. asoca, P. longifolia from two localities alongside five commercial market samples showed evidence of adulteration. Subsequently, multivariate hierarchical cluster analysis and principal component analysis was established to discriminate the adulterants of S. asoca. The proposed method ascertains identification of S. asoca from its putative adulterant P. longifolia and commercial market samples. The data generated may also serve as baseline data to form a quality standard for pharmacopoeias.
SUMMARY
Simultaneous quantification of gallic acid, catechin, epicatechin from Saraca asoca by high-performance liquid chromatography
Detection of S. asoca from adulterant and commercial samples
Use of analytical method along with a statistical tool for addressing quality issues.
Abbreviations used: HPLC: High Performance Liquid Chromatography; RP-HPLC: Reverse Phase High Performance Liquid Chromatography; CAT: Catechin; EPI: Epicatechin; GA: Gallic acid; PCA: Principal Component Analysis.
Keywords: Adulteration, chemical fingerprinting, high-performance liquid chromatography, Polyalthia longifolia, Saraca asoca
INTRODUCTION
Saraca asoca (Roxb.) De Wilde (Caesalpiniaceae) is one of the highly traded medicinal plant used in Ayurveda and traditional systems of medicine. Genus Saraca comprises ~ 20 species, out of which, India is endowed with four species S. asoca (Roxb.) Willd., Saraca declinata Miq., Saraca indica L., and Saraca thaipingensis Prain.[1,2] Among them, S. asoca is only the wild species and the remaining are grown in botanical gardens. Bark of the plant is greatly valued for its use in gynecological disorders, and as a consequence, it is immensely exploited by the pharmaceutical industry.[3,4,5,6] The bark of this tree is used as main ingredient in several commercial, ayurvedic preparations such as “Ashokrishtam” and “Ashokaghritham.” The bark extract has been reported to have antitumor/anticarcinogenic activities.[7] Besides, antimicrobial, larvicidal, antidiabetic, antioxidant, oxytocic, anti-estrogenic, and anti-inflammatory properties of this plant have also been reported.[8,9,10,11]
According to the National Medicinal Plant Board, New Delhi, and Foundation for Revitalization of Local Health Traditions, Bengaluru, India (2007), domestic demand of “Asoka” bark is more than 100 metric tons per year.[12] However, wild populations of the plant is scattered in small patches, mostly in Western Ghats, India. These populations are so small that it is likely that they never fulfilled the commercial demands earlier nor they can do so in near future. The plant is red listed in vulnerable category and is reported to be endangered.[13,14] This implies existence of rampant substitution/adulteration of the crude drug to match the ever increasing demand. However, it is reportedly substituted/adulterated with various plant materials.[2] Another plant, Polyalthia longifolia (Sonnerat) Thwait. (Annonaceae) also known as “Ashoka” is largely reported to be the putative adulterant for S. asoca bark.[2,11,15,16,17] It is perhaps because of this adulteration/substitution in the market, scarcity is not observed.
Phytochemical studies have shown the presence of (+)-catechin (CAT), (−)-epicatechin (EPI), procyanidin B-2, 11’-deoxyprocyanidin B4, leucocyanidin, etc., to be the major compounds in S. asoca.[6,15,18,19] Although these compounds have not been analyzed in P. longifolia, other compounds such as liriodenine, noroliveroline, oliveroline-f3-N-oxide, duorene alkaloids, polyfothine, and clerodane diterpenoids have been reported.[20,21,22] Among these, CATs are well reported for various kinds of biological activities and are reported to be useful for the symptomatic treatment of several gastrointestinal, respiratory and vascular diseases.[18,23] Chemoprofiling is now an essential and standard practice for quality control.[24,25] Chemistry of plant drugs may vary with factors such as growing stage, harvest time, locality, storage condition, processing and manufacturing procedures, but they can yield robust information to address adulteration issues for quality assurance in herbal industry. The recent nuclear magnetic resonance spectroscopy-based assessment of S. asoca market samples revealed the widespread adulteration and therefore the development of simple, robust quality control parameters are essential.[26] For detecting adulteration in Ginkgo biloba products, a simple method is reported in which quercetin, kaempferol and isorhamnetin used as markers.[27] Similarly, major bioactive compounds (gallic acid [GA], CAT, EPI, proanthocyanidin unit, and galloyl unit) as criteria to standardize quality and composition, especially for detection of adulterants in commercial grape seed-derived products, have been reported.[28]
Thus, the present study was undertaken with the aims of understanding and comparing chemoprofiles of S. asoca with its putative adulterant P. longifolia and also with the commercial market samples by using phytochemical markers (GA, CAT and EPI).
MATERIALS AND METHODS
Plant material collection
Five samples each of S. asoca (Roxb.) De Wilde bark (Code: SAZ-1 to SAZ-5; Sirsi: N.14°48.167’ E.074°44.502’; Code: SAV-1 to SAV-5; Siddapur: N.14°21.418’ E.074°43.433’) along with its adulterant P. longifolia bark (Code: PLZ-1 to PLZ-5; Sirsi: N.14°37.416’ E.074°50.268’; PLV-1 to PLC-5; Siddapur: N.14°21.453’ E.074°45.690’) were collected from two populations each from Western Ghats regions of India. Flowering twigs of the plants were authenticated by qualified taxonomist and the voucher specimens were deposited in the herbarium at Regional Medical Research Centre, Belagavi, India (voucher numbers: S. asoca [Roxb.] De Wilde – RMRC 996 and P. longifolia [Sonnerat] Thwaites-RMRC 1256).
Commercial samples of raw bark samples sold as S. asoca were collected from various Indian markets of Belagavi (MAR-1) and Bengaluru in Karnataka (MAR-2); Chennai (MAR-3) and Coimbatore in Tamil Nadu (MAR-5), and Mumbai in Maharashtra (MAR-4).
Chemicals and reagents
Deionized water was used from in-house Milli-Q system (Millipore, USA). All the solvents used were of high-performance liquid chromatography (HPLC) grade, methanol (Merck, Mumbai, India), acetonitrile (RANKEM, RFCL Ltd., Haryana, India) and acetic acid (RANKEM, Avantor™, India). HPLC grade standard compounds of CAT (95%), EPI (95%) and GA (95%) were procured from Natural Remedies, Bengaluru, India.
Standard preparation
Different concentration of GA (0.05, 0.1, 0.5, 1, 5, 10 μg/mL), CAT (0.1, 0.5, 1, 5, 10 μg/mL), and EPI (0.1, 0.5, 1, 5, 10 μg/mL) were prepared in HPLC grade methanol and used to obtain calibration and linearity data.
Sample preparation
The collected bark samples were dried and powered. Powdered samples of 250 mg were soaked in deionized water overnight with shaking and filtered through Whatman No. 1 filter paper. The filtrate was evaporated to dryness. Extract of 2 mg/mL were weighed and dissolved in the solvent system (acetonitrile, 12:water, 85:glacial acetic acid, 3) to obtain mg/mL concentration samples. Similarly, all test samples were passed through 0.20 μ nylon filter (Sartorius, Germany) before injecting into HPLC.
Reversed phase high-performance liquid chromatography analysis of gallic acid, catechin, and epicatechin
Reversed phase-HPLC (RP-HPLC) analysis was performed on a Shimadzu chromatographic system (Model no. LC-20AD) consisting of a quaternary pump, auto-injector (SIL-20-ACHT), degasser (DGU-20A5), and dual δ ultraviolet (UV) absorbance diode array detector (model no. SPD-M20A). The built in LCsolution software system was used for data processing. Chromatographic separation was achieved on a CAPCELL PAK C18 MG II S5 250–4.6 mm (5 μm) column. A mobile phase consisting of “A” (acetonitrile), “B” (water), and “C” (glacial acetic acid) were used for separation with 12:85:3 in an isocratic mode with injection volume of 10 μL. The flow rate was 0.7 mL/min and the detection wavelength of photodiode array was set 280 nm with 20 min run time for both standard and sample. The calibration curve for the standards with above analytical column was established to determine unknown concentration of GA, CAT, and EPI in the bark samples.
Statistical and multivariate analysis
Statistical analysis was performed using the statistical software GraphPad Prism Evaluation version. The data were reported as means and ± standard deviation. Significant differences between means were determined using repeated measure one-way ANOVA at P < 0.05. The chromatographic profiles of all extracts were analyzed using built in Shimadzu LC solution software (Version 1.25, Shimadzu Corporation, Kyoto, Japan). Multivariate analysis for correlations was analyzed using BioDiversity Pro, version 2: (Scottish Association for Marine Science and the Natural History Museum, London) eco-statistical software to understand the possible natural groupings and correlation in and among the samples collected. The hierarchical clustering and principal component analysis (PCA) was based on the relative peak area of the standard reference chemical constituents in all samples.
RESULTS AND DISCUSSION
Reversed phase high-performance liquid chromatography analysis of gallic acid, catechin, and epicatechin
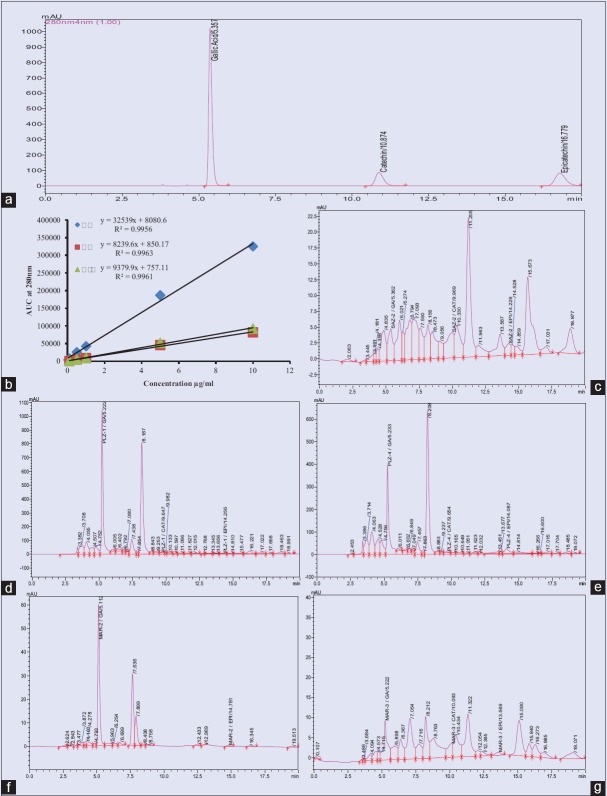
The chromatographic condition was optimized for simultaneous quantification of GA, CAT, and EPI with the best resolution in a short time of analysis. In the present study, an optimized detection wavelength (δ =280 nm) was used to monitor all compounds simultaneously in a single run to provide sufficient sensitivity for each analyte [Figure 1a]. Retention times and UV spectra of compounds from samples were used in comparison with those of the standards.
Figure 1.
High-performance liquid chromatography chromatograms of (a) Standard gallic acid, catechin and epicatechin (20 μg/mL each); (b) Five-point calibration curve; (c) SAZ-2, (d) PLZ-1; (e) PLZ-4; (f) MAR-2; (g) MAR-3
Simultaneous quantitative determination of GA, CAT, and EPI was achieved using RP-HPLC method. The content (g/100 g) of GA, CAT, and EPI was determined in extracts of five bark samples each of S. asoca and P. longifolia from two localities and five commercial market samples [Table 1]. Calibration curves were constructed against its respective areas under curve to obtain the regression equations for GA (y = 32539x + 8080.6), CAT (y = 8239.6x + 850.1), and EPI (y = 9379.9x + 757.11), with a coefficient of determination (R2) above 0.995 [Figure 1b]. These equations were used to estimate respective contents from all the bark samples obtained from different locations. The relative standard deviation values for analytes and samples were found to be < 2% indicating precision and reproducibility of the method. The limit of detection for GA, CAT, and EPI was 0.110, 0.116, and 0.119 μg/mL whereas limit of quantification was 0.332, 0.351, 0.359 μg/mL, respectively.
Table 1.
Content of gallic acid, catechin and epicatechin in Saraca asoca, Polyalthia longifolia, and commercial market samples as determined by high-performance liquid chromatography analysis
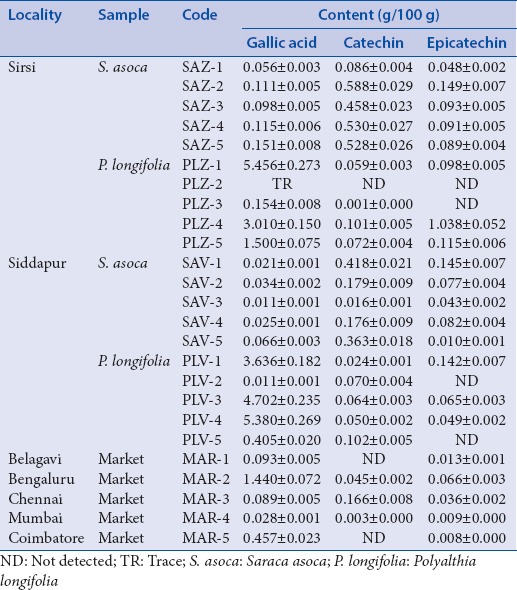
The sample profiles yielded well-separated, distinct, clear, and sharp peaks of GA, CAT, and EPI with retention times of 5.290 ± 0.079, 9.884 ± 0.200, and 14.108 ± 0.289 min, respectively [Figure 1c–g],. The GA content varied from 0.010 ± 0.001-5.455 ± 0.273%, CAT from 0.001 ± 0.000 to 0.588 ± 0.029%, and EPI content from 0.008 ± 0.000 to 1.038 ± 0.052%. Sample from Sirsi region (PLZ-1, SAZ-2, PLZ-4) showed highest content of GA, CAT and EPI respectively, whereas SAV-3 (GA), PLV-2 (GA) from Siddapur, and PLZ-3 (CAT) from Sirsi region along with MAR-5 (EPI) showed lowest contents [Table 1].
The sample PLZ-2 showed trace amount of GA whereas, CAT and EPI were not detected [Table 1]. EPI was not detected in samples PLZ-3, PLV-2, and PLV-5, whereas CAT was not detected in market samples of MAR-1 (Belagavi market) and MAR-5 (Chennai market). In market samples, out of the 3 compounds, GA and EPI were highest in MAR-2 (1.438 ± 0.072; 0.066 ± 0.003%) and CAT was highest in MAR-3 (0.166 ± 0.008%) [Table 1].
The amount of CAT in the present investigation was higher than reported in the earlier studies reported like in bark, 0.083 g/100 g[15] and 0.048 g/100 g.[29] The discrepancy in the content may be due to seasonal and/or geographical constraints as the genetic makeup of the plant is governed by these factors. It is reported that in S. asoca bark, EPI content (3.315%) is higher during January–March and GA (0.211%) during November–January.[30] Furthermore, it was also interesting to note that GA content in S. ascoa was lower whereas CAT and EPI were higher as compared to that in P. longifolia, signifying them as the major compounds. The observation is in accordance with earlier study.[15]
Earlier studies have shown presence of GA, CAT, EPI along with other compounds in different parts of S. asoca.[15,18,19] It is mentioned that these compounds showed unique pattern of metabolites in the plant parts. It has been reported that S. asoca is rich in CAT and its derivatives that accumulate in all the parts of the plant, especially in bark.[15,23]
Compound-based analysis
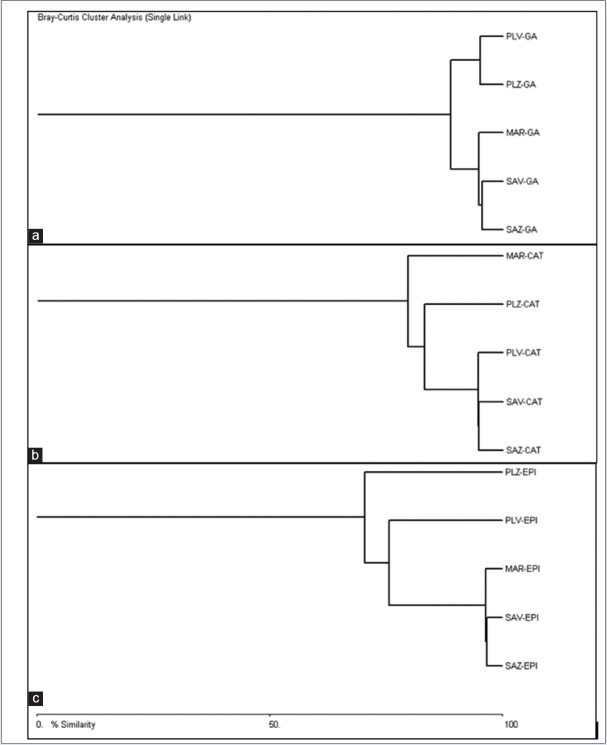
Figure 2 represents dendrograms generated using area obtained from HPLC analysis for GA, CAT and EPI for S. asoca (Sirsi, Siddapur), P. longifolia (Sirsi, Siddapur) and commercial market samples. Percent similarity up to 88.56% was observed in GA, followed by CAT (79.09%) and EPI (70.31%). Dendrogram for GA produced two clades one of P. longifolia with two leaves (PLV-GA and PLZ-GA) representing the two localities of their collection and another with again two leaves (SAV-GA and SAZ-GA) representing S. asoca populations [Figure 2a]. The commercial market sample was simplicifoliously connected with the later. Similarly, dendrogram for CAT showed a major clade for S. asoca (SAV-CAT, SAZ-CAT) connected with P. longifolia populations (PLV-CAT: 94.47%), (PLZ-CAT: 83.07%) and commercial market samples (MAR-CAT: 79.09%) simplicifoliously with decreased percent of similarity [Figure 2b]. Dendrogram for EPI was same as that of CAT with changes in position and percent similarity of PLZ, PLV and MAR leaves [Figure 2c]. The S. asoca clade (SAV-CAT, SAZ-CAT) herein was connected in a decreasing percent similarity to MAR-EPI (96.25%), PLV-EPI (75.52%), and PLZ-EPI (70.31%).
Figure 2.
Dendrogram generated for populations using area obtained from high-performance liquid chromatography run for all samples (a) gallic acid, (b) catechin, and (c) epicatechin
It is inferred from the results that the S. asoca populations had a compact clustering in all the compounds tested. P. longifolia samples showed similar clustering in GA whereas CAT and EPI dendrograms were simplicifoliously clustered. Commercial market samples made a linkage with S. ascoa clusters in GA and EPI dendrograms, it appeared simplicifoliously in CAT generated dendrogram [Figure 2]. Therefore, appealing use of CAT as important phytochemical marker in identification of adulterants and to help resolve quality issues is S. asoca also supported with the earlier study reports.[18] In another study, nontargeted identification of phenolic and other compounds from S. ascoa using HPLC-positive electrospray ionization and quadrupole time-of-flight mass spectrometry (MS) have shown CAT to be the important group of compounds from S. asoca.[23] They have also suggested the possibility of using this data in quality control and identification of the plant with its products.
Furthermore, if area under curve obtained from HPLC run for all the three compounds are considered to generate a single dendrogram, it can be observed that six arms made from S. asoca (SAZ-GA, SAZ-CAT, SAZ-EPI, SAV-GA, SAV-EPI, and SAV-CAT) and two arms from P. longifolia (PLV-CAT and PLV-EPI) appeared closely to form a cluster with similarity percent more than 80% (dendrogram not shown). A keen observation revealed that the upper clusters were mainly of P. longifolia and market samples whereas the lower half comprised more samples of S. asoca except PLV-CAT, PLV-EPI, and MAR-EPI. This is in accordance with the individually obtained dendrograms for each analyte, suggesting evidence toward presence of P. longifolia as a substitute/adulterant in commercial samples.
Sample-based analysis
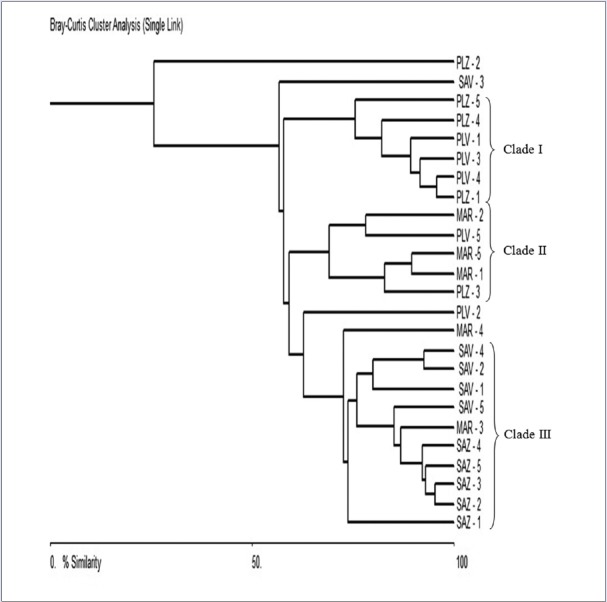
Multivariate analysis for individual samples using areas of phytochemical markers obtained by HPLC run as variable components yield dendrogram represented in Figure 3. On a first look the dendrogram may be differentiated into three main clades (I, II, and III) and five simplicifoliously arranged individuals (PLZ-2, SAV-3, PLV-2, MAR-4, and SAZ-1) positioned at different levels of percent similarity. Clade I comprised of all P. longifolia samples except 4 (PLZ-2, PLV-5, PLZ-3, and PLV-2), which were mainly connected to market samples. Clade II included market samples and P. longifolia samples whereas, Clade III exclusively comprised of S. asoca samples with only one market sample from Chennai in the cluster. These observations add to the inference from the previous dendrogram wherein, separate clustering of S. asoca and P. longifolia based on both compound and sample based dendrograms now can be conclusively inferred. Further, similarity linkages of P. longifolia towards market samples suggesting its use as substitute/adulterant may be justified. Although GA, CAT, EPI are common phenolic compounds present in most of the plant species, in case of S. asoca, they can be used as phytochemical markers to detect levels of adulteration. Commercial market samples used in analysis showed possibility of adulteration as it appeared close to P. longifolia samples. As suggested in many studies, it is important here to emphasize that phytochemical variations are influenced by genetic and environmental factors including different extraction methods.[31,32,33] Therefore, multiple sampling from different localities during a particular season is the most applicable and reliable method for such authentication studies.[25,33]
Figure 3.
Dendrogram generated for individuals using area obtained from high-performance liquid chromatography run of gallic acid, catechin, and epicatechin cumulatively
Principal component analysis
PCA was also used to distinguish adulterated samples during the study. It quantitatively evaluated the diversity among S. asoca, P. longifolia, and market samples. As a result, three principal components and the biplot of the samples were able to discriminate S. ascoa from P. longifolia with clear linkages of market samples to both [Figure 4]. Allied observations in American ginseng using similar methodology.[34] The results suggested authenticity of S. asoca could be determined through HPLC analysis with validation using PCA. Thus, the present study becomes important in the lights of the world market of the products from this botanical and also since there are no data in any of the pharmacopeias to regulate the adulteration in this plant.
Figure 4.
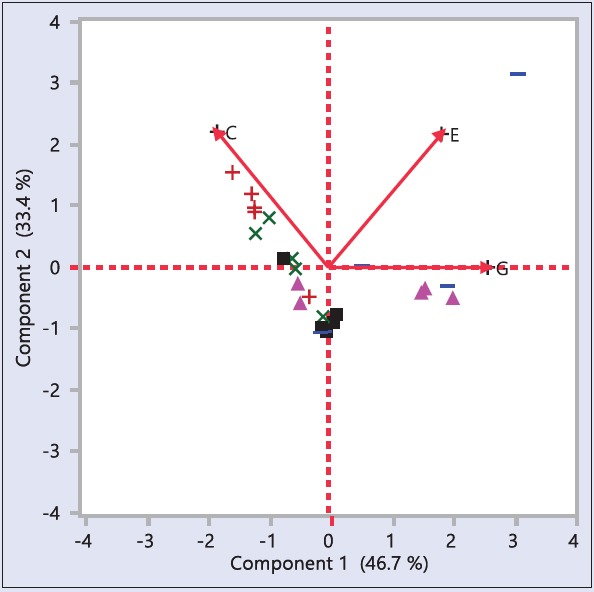
Principal component analysis for chemical variables (G: Gallic acid; E: Epicatechin; C: Catechin) of Saraca asoca (red + Sirsi; green ×: Siddapur) and Polyalthia longifolia (blue−: Sirsi; pink filled Δ: Siddapur) samples from two different localities with market (◼) samples
CONCLUSION
Our study showed differentiation of adulterant of P. longifolia samples with S. asoca samples using HPLC data coupled with multivariate analysis. The method used for simultaneous determination of these compounds was stable, reliable with good precision and repeatability. Unlike liquid chromatography with mass spectrometry (MS), the present method cost is lower, and there is no need of derivatization during detection. Multivariate analysis with PCA showed market samples to cluster randomly with similarity to the putative adulterant P. longifolia. Although S. asoca is rated endangered, its products are widely available in the market, which creates a doubt of substitution/adulteration with uncertainty on the quality. Thus, we further suggest pharmacological studies of the plant drugs versus products, to understand the implications of P. longifolia as an adulterant or a substitute.
Financial support and sponsorship
ICMR, New Delhi, and KLE University provided the financial support (45/53/2013/BMS/TRM; KLEU/Accs/12-13/D-1552).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are indebted to Director-in-Charge and Scientific Advisory Committee Members of Regional Medical Research Centre, ICMR, Belagavi, for providing necessary inputs and support. Authors are also thankful to Mr. Mohan Raj, MTS, and Mr. Prashant Agasar, Attendant, RMRC, Belagavi for sample collection and sample processing. SH is grateful to ICMR, New Delhi, and KLE University for providing financial support (45/53/2013/BMS/TRM; KLEU/Accs/12-13/D-1552).
REFERENCES
- 1.Santapau H, Henry AN. A Dictionary of the Flowering Plants in India. Reprint. New Delhi, India: National Institute of Science Publication, (CSIR), Dr. K. S. Krishnan Marg; 1998. p. 152. [Google Scholar]
- 2.Begum SN, Ravikumar K, Ved DK. ‘Asoka’ – An important medicinal plant, its market scenario and conservation measures in India. Curr Sci. 2014;107:26–8. [Google Scholar]
- 3.Warrior PK, Nambiar VP, Ramankutty C. Indian Medicinal Plants: A Compendium of 500 Species. Vol. 5. India: Orient Black Swan/Universities Press; 1996. [Google Scholar]
- 4.Madaleno IM. Traditional medicinal knowledge in India and Malaysia. Pharmacogn Commun. 2015;5:116–29. [Google Scholar]
- 5.Bhat P, Hegde GR, Hegde G, Mulgund GS. Ethnomedicinal plants to cure skin diseases-an account of the traditional knowledge in the coastal parts of Central Western Ghats, Karnataka, India. J Ethnopharmacol. 2014;151:493–502. doi: 10.1016/j.jep.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 6.Tandon N, Yadav SS. Contributions of Indian Council of Medical Research (ICMR) in the area of medicinal plants/traditional medicine. J Ethnopharmacol. 2016 doi: 10.1016/j.jep.2016.07.064. pii: S0378-874130492-5. [DOI] [PubMed] [Google Scholar]
- 7.Varghese CD, Nair SC, Panikkar B, Panikkar KR. Effect of Asoka on the intracellular glutathione levels and skin tumour promotion in mice. Cancer Lett. 1993;69:45–50. doi: 10.1016/0304-3835(93)90031-4. [DOI] [PubMed] [Google Scholar]
- 8.Chhetri DR, Parajuli P, Subba GC. Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J Ethnopharmacol. 2005;99:199–202. doi: 10.1016/j.jep.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Somani G, Sathaye S. Bioactive fraction of Saraca indica prevents diabetes induced cataractogenesis: An aldose reductase inhibitory activity. Pharmacogn Mag. 2015;11:102–10. doi: 10.4103/0973-1296.149722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satyavati GV, Prasad DN, Sen SP, Das PK. Oxytocic activity of a pure phenolic glycoside (P2) from Saraca indica linn (Ashoka): A short communication. Indian J Med Res. 1970;58:660–3. [PubMed] [Google Scholar]
- 11.Shahid AP, Salini S, Sasidharan N, Padikkala J, Raghavamenon AC, Babu TD. Effect of Saraca asoca (Asoka) on estradiol-induced keratinizing metaplasia in rat uterus. J Basic Clin Physiol Pharmacol. 2015;26:509–15. doi: 10.1515/jbcpp-2014-0124. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Krishna TH, Kamalraj S, Kuriakose GC, Valayil JM, Jayabaskaran C. Phytomedicinal importance of Saraca asoca (Ashoka): An exciting past, an emerging present and a promising future. Curr Sci. 2015;109:1790–801. [Google Scholar]
- 13.Ved DK, Goraya GS. Demand and Supply of Medicinal Plants in India. National Medicinal Plant Board (NMPB), New Delhi and FRLHT, Bengaluru, India. 2007. [Last accessed on 2016 Aug 22]. Available from: http://www.nmpb.nic.in/FRLHT/Contents.pdf .
- 14.Nayar MP, Sastry AR. Red Data Book of Indian Plants. III. Calcutta, India: Botanical Survey of India; 1990. [Google Scholar]
- 15.Senapati SK, Das GK, Aparajita S, Rout GR. Assessment of genetic variability in the Asoka tree of India. Biodiversity. 2012;13:16–23. [Google Scholar]
- 16.Gupta AK, Tandon N, Sharma M, editors. Quality Standards of Indian Medicinal Plants. Vol. 2. New Delhi: Indian Council of Medical Research; 2005. pp. 201–8. [Google Scholar]
- 17.Khatoon S, Singh N, Kumar S, Srivastava N, Rathi A, Mehrotra S. Authentication and quality evaluation of an important Ayurvedic drug – Ashoka bark. J Sci Ind Res. 2009;68:393–400. [Google Scholar]
- 18.Srivastava GN, Bagchi GD, Srivastava AK. Pharmacognosy of Ashoka stem bark and its adulterants. Int J Crude Drug Res. 1988;26:65–72. [Google Scholar]
- 19.Shirolkar A, Gahlaut A, Chhillar AK, Dabur R. Quality analysis of catechin in Saraca asoca and correlation with antimicrobial activity. J Pharm Anal. 2013;3:421–8. doi: 10.1016/j.jpha.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad F, Misra L, Tewari R, Gupta P, Gupta VK, Darokar MP. Isolation and HPLC profiling of chemical constituents of Saraca asoca stem bark. Indian J Chem. 2016;55B:353–61. [Google Scholar]
- 21.Zhao GX, Jung JH, Smith DL, Wood KV, McLaughlin JL. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med. 1991;57:380–3. doi: 10.1055/s-2006-960122. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarty M, Nath AC. A new clerodane-type butenolide diterpene from the bark of Polyalthia longifolia. J Nat Prod. 1992;55:256–8. [Google Scholar]
- 23.Katkar KV, Suthar AC, Chauhan VS. The chemistry, pharmacologic, and therapeutic applications of Polyalthia longifolia. Pharmacogn Rev. 2010;4:62–8. doi: 10.4103/0973-7847.65329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal A, Kadyan P, Gahlaut A, Dabur R. Nontargeted identification of the phenolic and other compounds of Saraca asoca by high performance liquid chromatography-positive electrospray ionization and quadrupole time-of-flight mass spectrometry. ISRN Pharm 2013. 2013:293935. doi: 10.1155/2013/293935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smillie TJ, Khan IA. A comprehensive approach to identifying and authenticating botanical products. Clin Pharmacol Ther. 2010;87:175–86. doi: 10.1038/clpt.2009.287. [DOI] [PubMed] [Google Scholar]
- 26.Pawar N, Pai S, Nimbalkar M, Dixit G. RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from different ginger cultivars. Food Chem. 2011;126:1330–6. [Google Scholar]
- 27.Urumarudappa SK, Gogna N, Newmaster SG, Venkatarangaiah K, Subramanyam R, Saroja SG, et al. DNA barcoding and NMR spectroscopy-based assessment of species adulteration in the raw herbal trade of Saraca asoca (Roxb.) Willd, an important medicinal plant. Int J Legal Med. 2016;130:1457–70. doi: 10.1007/s00414-016-1436-y. [DOI] [PubMed] [Google Scholar]
- 28.Wohlmuth H, Savage K, Dowell A, Mouatt P. Adulteration of Ginkgo biloba products and a simple method to improve its detection. Phytomedicine. 2014;21:912–8. doi: 10.1016/j.phymed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Villani TS, Reichert W, Ferruzzi MG, Pasinetti GM, Simon JE, Wu Q. Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration. Food Chem. 2015;170:271–80. doi: 10.1016/j.foodchem.2014.08.084. [DOI] [PubMed] [Google Scholar]
- 30.Rathee P, Rathee S, Rathee D, Rathee D. Quantitative analysis of (+)-catechin in stem bark of Saraca asoca linn using HPTLC. Pharma Chem. 2010;2:306–14. [Google Scholar]
- 31.Ketkar PM, Nayak SU, Pai SR, Joshi RK. Monitoring seasonal variation of epicatechin and gallic acid in the bark of Saraca asoca using reverse phase high performance liquid chromatography’ (RP-HPLC) method. J Ayurveda Integr Med. 2015;6:29–34. doi: 10.4103/0975-9476.146568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadi A, Ahmadzadeh Sani T, Ameri AA, Imani M, Golmakani E, Kamali H, et al. Seasonal variation in the chemical composition, antioxidant activity, and total phenolic content of Artemisia absinthium essential oils. Pharmacogn Res. 2015;7:329–34. doi: 10.4103/0974-8490.158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annan K, Dickson RA, Amponsah IK, Nooni IK. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacogn Res. 2013;5:103–8. doi: 10.4103/0974-8490.110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Wang CZ, Zhou CJ, Wang B, Han L, Zhang CF, et al. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J Pharm Biomed Anal. 2014;99:8–15. doi: 10.1016/j.jpba.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]