Abstract
Background:
Diabetes is a metabolic disease prevalent worldwide in all age group of people. The source of diabetes is due to an oxidation process that can produce free radicals. An increase in oxidative free radicals in the body is reported to be one of the several causes of diabetes. The best remedy to combat oxidative stress is the use of antioxidants, which inhibit and scavenge free radicals.
Aim:
This study has been undertaken to evaluate the antioxidant activity and antidiabetic effect of mulberry leaf extract in diabetic mice.
Materials and Methods:
Antioxidant activity of mulberry leaves was determined by 2,2-diphenyl-1-picryl-hydrazyl (DPPH) and ferric reducing/antioxidant power (FRAP) assay. Antidiabetic assay of mulberry leaf extract was analyzed by oral administration of leaf extract up to 3 weeks in diabetic mice induced by streptozotocin.
Results:
In vitro antioxidant activity in both DPPH and FRAP assays showed significantly (P < 0.05) higher inhibition of free radicals than that with ascorbic acid. Diabetic mice fed with mulberry leaf extract showed increment (+25.88%) in body weight and a significant reduction in blood glucose concentration (−71.58%). Further, glucose-6-phosphate dehydrogenase enzyme activity was significantly (P < 0.05) increased, whereas activities of other enzymes particularly catalase, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase were decreased in diabetic mice after oral administration of mulberry leaf extracts. Histology of liver revealed regeneration of hepatocytes, central vein, and nucleus.
Conclusion:
This study demonstrated that S-1708 mulberry variety has a potential therapeutic value in diabetes and related complications.
SUMMARY
Diabetes mellitus is a grave metabolic deviations and responsible for many complications affecting various organs in the human body. In spite of the known antidiabetic medicine available in the market, diabetes and the associated impediments sustained to be a major medical crisis. Medicinal plants have been proven to be useful in diabetes due to their rich therapeutic value. In the current study, S-1708 mulberry variety not only authenticated the earlier results obtained from other medicinal plants but also turn out to be known as a potential source for treating diabetes by demonstrating tremendous ant- diabetic properties.
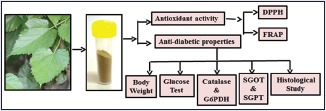
Abbreviations used: S-1708, DPPH, FRAP.
Keywords: 2, 2-diphenyl-1-picryl-hydrazyl, antioxidant, ferric reducing/antioxidant power, mulberry, streptozotocin
INTRODUCTION
Natural antioxidant compounds from plant origin are medicinally helpful for human health. Antioxidant compounds including ascorbic acid, carotenoids, flavonoids, and tannins are well known to play an important role in the prevention of several chronic diseases.[1] The capacity between the production and neutralization of reactive oxygen species (ROS) by antioxidants is very reasonable, and if this balance tends toward the overproduction of ROS, the cells start to suffer the penalties of oxidative stress.[2] Oxidative stress is linked with numerous pathologic conditions such as cardiovascular and neurodegenerative diseases. Dietary antioxidants including Vitamins E, C, and carotenoids are well known to be effective in the prevention of oxidative stress-related diseases.[3] Mulberry is rich in alkaloids, polyphenols, flavonoids, and anthocyanin which have been suggested to be accountable for health benefits.[4] Mulberry leaves contain a variety of essential micronutrients, for example, ascorbic acid, carotene, Vitamin B, Vitamin D and flavonol glycosides.[5] Traditionally, mulberry leaves have been used as medicinal agents to nourish the blood, benefit the and treat weakness, fatigue, anemia, and premature graying of hair. It is also used to treat urinary incontinence, tinnitus, dizziness, and constipation in the aged patient. Mulberry leaves have other pharmacological properties such as analgesic, antiasthmatic, anti-rheumatic, antitussive, and astringent.[6] The phytochemicals of mulberry vegetative parts are getting more attention nowadays due to their numerous applications in served industries such as food, pharmaceutical, nutraceuticals, and cosmetics.[7] Further, mulberry leaves have been used to treat hyperglycemia, inflammation, cough, hypertension, cancer, and fever.[8] Mulberry acts as a growing resource of combating stress-related diseases. Hence, this study was aimed to determine the antioxidant and antidiabetic properties of mulberry (variety-S-1708) in streptozotocin (STZ)-induced diabetic mice.
MATERIALS AND METHODS
Chemicals
2,2-diphenyl-1-picryl-hydrazyl (DPPH), 2,4,6-Tripridyl-s-triazine (TPTZ), were purchased from Sigma-Aldrich, USA. supplied by Mumbai, India. Ascorbic acid, sodium acetate buffer (pH-3.6), glacial acidic acid, sodium acetate, were procured from Loba Chemie Pvt. Ltd., Mumbai, India. Glucose-6-phoshtate were dehydrogenase (G6PDH), β-nicotinamide adenine dinucleotide phosphate (β-NADP) were purchased from Sisco Research Laboratory Pvt., Mumbai, India. Serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) were obtained from ARKRAY Healthcare Pvt. Ltd., India. STZ was bought from HiMedia, Mumbai, India. All other chemicals used were of analytical grade.
Collection of sample
A sample of mulberry leaves (variety-S-1708) was collected from the Central Sericultural Germplasm Resources Centre (CSGRC), Hosur, Tamil Nadu, India. The plant species was identified by Dr. P. Sharawati, an eminent scientist in the mulberry division of the CSGRC. The identification of the variety of mulberry was based on the different morphological characters present in different varieties of mulberry. A catalog, having all aspect of different characters of mulberry is also provided by CSGRC to scientists to make the identification easy and precise. The variety was further confirmed by another eminent scientist Dr. M. M. Borpuzari of CSGRC Hosur, Tamil Nadu, India, which is also serving as repository center for the identification of mulberry Germplasm.
Preparation of plant extracts
Leaves were washed with distilled water, left to dry naturally at room temperature and powdered with a grinder. The powdered leaves (50 mg) was extracted in both methanol and ethanol solvents, separately for 48 h. The extracts were filtered using Buchner funnel and Whatman No. 1 filter paper and freshly prepared extracts were used for the analysis of antioxidant activity through DPPH and ferric reducing/antioxidant power (FRAP) assay. The powder leaf material (100 mg) was dissolved in 10 ml of 20% ethanolic solvent for 2 h,[9] an extract was filtered and used for in vivo study of diabetic mice. Hence, composition of extracts was not analyzed due to the limitation of the facility.
Determination of free radical scavenging activity using 2,2-diphenyl-1-picryl-hydrazyl method
The free radical scavenging activity of mulberry leaf extracts was measured by the spectrophotometric method for the assay of hydrogen donating free radical.[10] Different concentrations (50, 100, 200, 300, 400 μl/ml) of mulberry leaf extracts and ascorbic acid as standard were prepared in both methanol and ethanol solvents separately. Thereafter, 3 ml of 0.004% DPPH reagent was added. The reaction mixture was mixed thoroughly and left for incubation at room temperature in dark. The absorbance was measured at 517 nm using a spectrophotometer and antioxidant activity was expressed as percentage inhibition.
Percentage inhibition I % = (Ablank − Asample/Ablank) × 100.
Where Ablank is the absorbance of the control (without test material) and Asample is the absorbance of the test material. The assay was carried out in triplicate.
Determination of ferric reducing/antioxidant power assay
FRAP assay was carried out with minor modifications following Szeto et al. 2002.[11] The FRAP reagent was prepared from 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride and 10 mM TPTZ in 40 mMHCl. All three solutions were mixed together in the ratio of 10:1:1 (v/v/v). The absorbance was measured at 595 nm using a spectrophotometer. The results were expressed in μmole/Fe [II] mg. The assay was carried out in triplicate.
Ethics clearance
All the experiments were conducted in the Department of Zoology, Banaras Hindu University, in accordance with the Institutional practice and within the framework of experiment of experimental animals (scientific procedure) Act of 2007, of the Committee for the Purpose of Supervision and Control of Experiments on Animals, Government of India.
Test animals and induction of diabetes
Male Park strain mice 4–6 week old (25 ± 5 g) were used for the antidiabetic test. The mice were housed in an individual cage in an air-habituated room with 12 h light/dark cycle at the temperature of 25°C ± 20°C with free access to food and water. All mice were acclimatized to the laboratory conditions for 7 days before the experiment. Inductions of diabetes in mine were carried out with minor modification of Hua et al.[12] Diabetes in 18 mice (overnight fasted) was induced by intraperitoneal injection of 1% STZ prepared in 0.1 M citrate buffer (pH 4.5) at a single dose of 125 mg/kg body weight. After 72 h, fasting blood glucose (FBG) levels of the mice was examined. Mice with FBG values >226 mg/dl were considered hyperglycemic. For further experiments, mice were divided into five groups according to their FBG and weight (six animals in each group) as following:
Group I: Control
Group II: Diabetic (FGB > 226 mg/dl)
Group III: Diabetic treated with Insulin (4 U/dl)
Group IV: Control treated with S-1708 mulberry leaf extract
Group V: Diabetic treated with S-1708 mulberry leaf extract.
Feeding schedule
Standard feed of laboratory diet was purchased from Paramount Techno Chem, Varanasi, Uttar Pradesh, India. Groups (IV and V) of experimental mice were orally fed by mulberry leaf extract (4 U/dl) two times (7 am and 7 pm) in 24 h.[13] Group III mice were given insulin at the same dose and time.[14]
Measurement of body weight and blood glucose level
The effect of mulberry leaf extract in different groups of diabetic mice was measured by changes in body weight of mice and FBG level at 7, 14, and 21 days.
Hepatic enzyme assay
After 21 days of the experiment, mice of all groups were anesthetized under diethyl ether; liver was excised, washed with phosphate buffer saline (PBS) at pH-7.4 and homogenized with cold PBS containing protease inhibitors. Homogenate was then centrifuged at 10,000 × g for 15 min, and supernatant was collected and stored at −80°C. The supernatant was used for the assay of catalase, glucose-6-phasphate dehydrogenase, SGOT, and SGPT.
Catalase (EC 1.11.1.6)
The activity of catalase was assayed by the method of Beers and Sizer.[15] The assay system contained 1.9 ml sodium phosphate buffer (0.05 M, pH 7.0), 0.1 ml liver supernatant and 1.0 ml H2O2 (0.059 M in buffer). The change in absorbance was read at 240 nm for 3 min at 30 s intervals against blank containing 0.1 ml distilled water instead of enzyme source. The specific activity was calculated by a molar absorbance index for H2O2 as 43.6 and expressed as moles of H2O2 decomposed/min/mg protein.
Glucose-6-phosphate dehydrogenase (EC1.1.1.49)
The enzyme activity was measured as per Worthington enzyme manual.[16] The assay system contained 2.7 ml of 0.055 M Tris–HCl buffer (pH 7.8 with 0.0033 M MgCl2), 0.1 ml liver supernatant, 100 μl ml of 0.006 mM NADP+ and 0.1 ml glucose-6-phosphate (0.1 M). The change in absorbance was recorded at 340 nm for 5 min against blank containing 0.1 ml of distilled water instead of enzyme source.
The specific activity was expressed as micromoles of NADP+ reduced/min/mg protein using extinction coefficient for NADPH as 6.22 cm2/μmol.
Serum glutamic oxaloacetic transaminase and serum glutamic pyruvate transaminase
The activities of SGOT and SGPT in liver tissue of each group of mice were assayed by commercial span kit obtained from ARKRAY Healthcare Pvt., Ltd., India.[17,18,19,20,21,22,23,24]
Histopathological study of liver tissue
To evaluate the histopathological alterations, after 21 days treatment of mulberry leaf extract, mice of each group were anesthetized with diethyl ether. Liver was excised and fixed in aqueous Bouin's fluid, following Bancroft and Gamble.[25] The fixed tissues were then dehydrated in an ethanol series of ascending concentration, cleared in cedar wood oil and embedded in paraffin wax (melting point 58°C–60°C) (E-Merck, Mumbai, India). Serial sections were cut at a thickness of 6 μm using a Leica Rotary Microtome (Model RM 2125RT; Leica Microsystems, Bensheim, Germany). The sections were mounted on ethanol cleaned glass slides and were kept in an oven at 37°C overnight to dry. Sections were deparaffinized and were stained with Ehrlich's hematoxylin and eosin (Ehrlich 1886).[26] The stained sections were dehydrated in an ascending ethanol series, cleared in xylene and mounted in distrenedibutylphthalate xylene.
Statistical analysis
Data were analyzed by applying one-way analysis of variance followed by Dunnett's post hoc test and results were analyzed as mean ± standard deviation. Levels of significance were tested at the level of P < 0.05 by using IBM SPSS (version 20, Armonk, New York, USA) package.
RESULTS
In vitro antioxidant activity assay
Antioxidant scavenging activity of 2,2-diphenyl-1-picryl-hydrazyl
In this study, mulberry leaf (variety S-1708) exhibited higher scavenging activity of DPPH as free radicals compared to the ascorbic acid used as a standard. The concentration dependent percent inhibition of mulberry leaf and ascorbic acid are summarized in Table 1. Maximum activity is observed in both methanol (71.58% ±1.71%) and ethanol (82.06% ±0.4%) solvents of the mulberry leaf at 400 μg/ml concentration. In contrast to mulberry leaf, standard compounds of ascorbic acid revealed less activity in both methanol (62.37% ±0.6%) and ethanol (72.89% ±0.9%) solvents at the same concentration. Further, IC50 value of methanolic and ethanolic extracts of mulberry leaf and ascorbic acid are calculated and summarized in Table 2. Mulberry leaf revealed lower IC50 value of methanolic and ethanolic solvents were 196.12 mg/ml and 143.56 mg/ml as compared to ascorbic acid 271.73 mg/ml and 218.319 mg/ml, respectively.
Table 1.
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of mulberry leaf extract and ascorbic acid
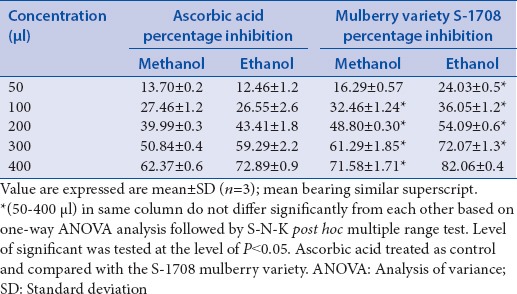
Table 2.
IC50 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of mulberry leaf extract and ascorbic acid

Antioxidant reducing activity of ferric reducing/antioxidant power
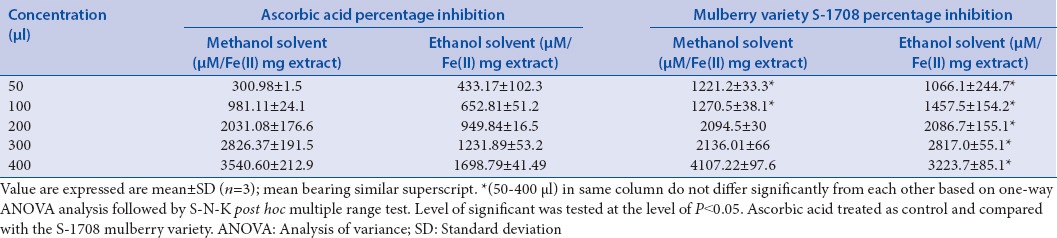
FRAP assay was used to determine the antioxidant activity of mulberry leaf (Variety S-1708) was in the methanolic and ethanolic solvents. The reducing activity of mulberry leaf and ascorbic acid are summarized in Table 3 and ethanolic extracts was found to be 4107.22 ± 97.6 μM/Fe(II) mg and 3223.7 ± 85.1 μM/Fe(II) mg, respectively, at the concentration of 400 μg/ml, whereas ascorbic acid recorded in methanolic solvent 3540.60 μM/Fe(II) mg and ethanolic 1698.7 μM/Fe(II) mg at the same concentration.
Table 3.
Ferric reducing/antioxidant power activity of mulberry leaf extract and ascorbic acid
In vivo streptozotocin drug exposures
Change in body weight of different group of mice
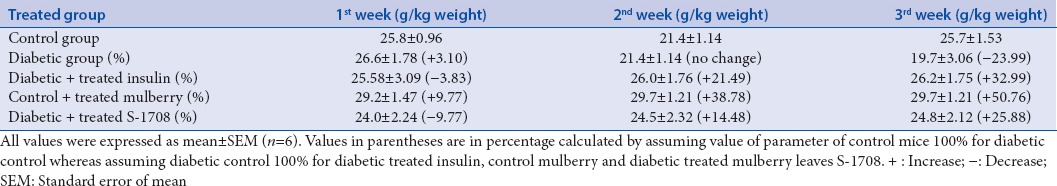
In the time growth of diabetic study, during in vivo exposure of mice to STZ, the body weight decreased significantly 23.99% from control Group I (25.7 ± 1.53 g) to experimental Group II (19.7 ± 3.06 g), after 21 days. Administration of mulberry leaf extracts to diabetic mice, however, results in significant gain in body weight 25.88% form (Group II 19.7 ± 3.06 g to Group IV 24.8 ± 2.21 g). Experimental changes in other groups of mice are expressed in Table 4.
Table 4.
Effect of mulberry leaf extract administration on weight (g/kg) in chronic diabetic mice
Change in glucose concentration of different group of mice
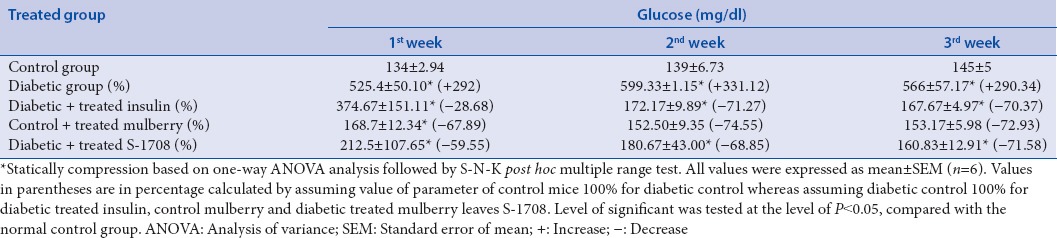
Glucose concentration levels in the STZ induced diabetic mice showed a significant (P < 0.05) (+322.39%) increment after induction of STZ at 21 days (Group I 134 mg/dl to Group II, 566 mg/dl. Diabetic mice treated with mulberry leaf extracts, however, showed a significant (P < 0.05) decline in the concentration of glucose level up to a value of 566–160.83 mg/dl (71.58%), (which was almost equivalent to those of normal control group) as compared to STZ diabetic mice. Consequentially, diabetic treated with mulberry leaf had been significant decline more a percentage (−71.58%) as compared to insulin diabetic mice (−70.37%). Glucose concentration changes in different groups of mice are summarized in Table 5.
Table 5.
Effect of mulberry leaf extract administration on fasting blood glucose inchronic diabetic mice
Change in enzyme activity of different groups of mice
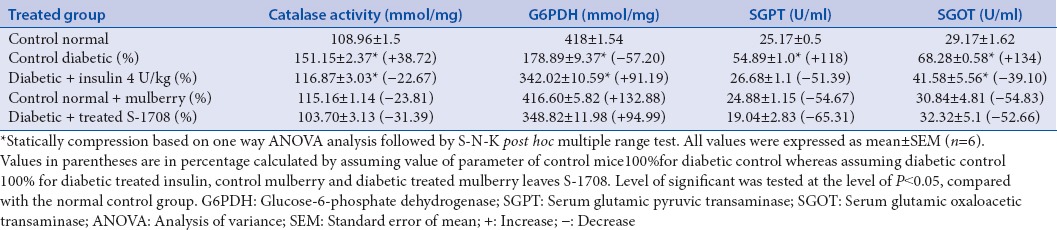
In STZ-induced diabetic mice the activity of catalase was significantly increased (P < 0.05) 38.72% (Group I, 108.96 ± 1.5 to Group IV, 151.15 ± 2.37 mmol/mg), SGOT activity was also increased 134% from (Group I, 29.17 ± 1.62 to Group IV, 68.28 ± 0.58 U/ml), respectively. Furthermore, SGPT activity was significant increases 118% (Group I 25.17 ± 0.5 to Group IV, 55.59 ± 0.61 U/ml), whereas the G6PDH activity decreased significantly 57.20% (Group I, 418 ± 1.54 to Group IV, 178.89 ± 9.31 mmol/mg). After 21 days treatment of mulberry leaf extracts in different groups of mice, significant positive changes were observed in the activity of catalase, SGOT, SGPT, were reduced to 31.39%, 52.66%, and 65.31%, respectively, whereas G6PDH activity was increased to 94.99%) with compared with diabetic mice (Group II). The alterations in the enzyme activity of different group mice are expressed in Table 6.
Table 6.
Effect of mulberry leaf extract administration on enzymes assay in chronic diabetic mice
Histopathological change in different group of mice
The liver of the control group [Group I], showed the normal architecture of the definitive hepatic cells such as the sheet of hepatocytes, nucleus, and central vein. The hepatocytes radiate from the central vein. In diabetic mice, the sheet of hepatocytes and central vein [Group II], show deformations and degenerative changes, however, significant changes are observed in Group III and Group V with compared to Group II. The hepatocytes, nucleus, and central vein show regeneration of cellular structure and appear similar to the Group 1.
DISCUSSION
2,2-diphenyl-1-picryl-hydrazyl radical scavenging activity
Natural antioxidants that are present in leaf and other parts of plant are responsible for inhibiting or preventing the harmful costs of oxidative stress. In the present study, mulberry leaf (S-1708) in both methanolic and ethanolic solvents exhibited significantly higher antioxidant activity than ascorbic acid. Scavenging free radical activity of mulberry leaf is increased significantly with the concentration of mulberry extracts increases [Figures 1 and 2]. This is because of the presences of phytochemicals constituents are more in the leaf of S-1708 mulberry variety, supported by my previous phytochemicals analysis and Khalaf et al., 2007[27] in some common plants such as Camellia sinensis Linn, Eugenia caryophyllus by DPPH assay. Similar works was also analyzed by Khan et al. 2013[28] to check antioxidant activity of three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves in different solvents such as methanol and ethanol extracts of Indian origins showed the highest antioxidant activities 65.1% and 66.8%, respectively, which is showing direct agreement with the present study. Further, results are compared with other researcher works of different mulberry varieties namely Morus alba and Morus rubra, Morus nigra and founds that S-1708 mulberry are showed higher potential activity among them.[29,30,31]
Figure 1.
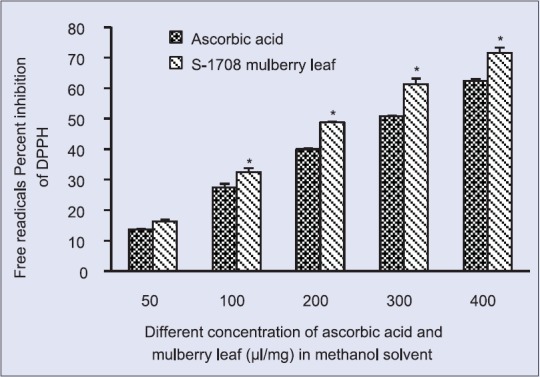
2,2-diphenyl-1-picryl-hydrazyl radical scavenging activity at different concentration of S-1708 mulberry leaf extract and ascorbic acid in methanol solvent. Values are mean ± standard deviation. *On bars indicates significant diffrenence from control. Mulberry leaf extracts compared with the ascorbic acid (control). Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis
Figure 2.
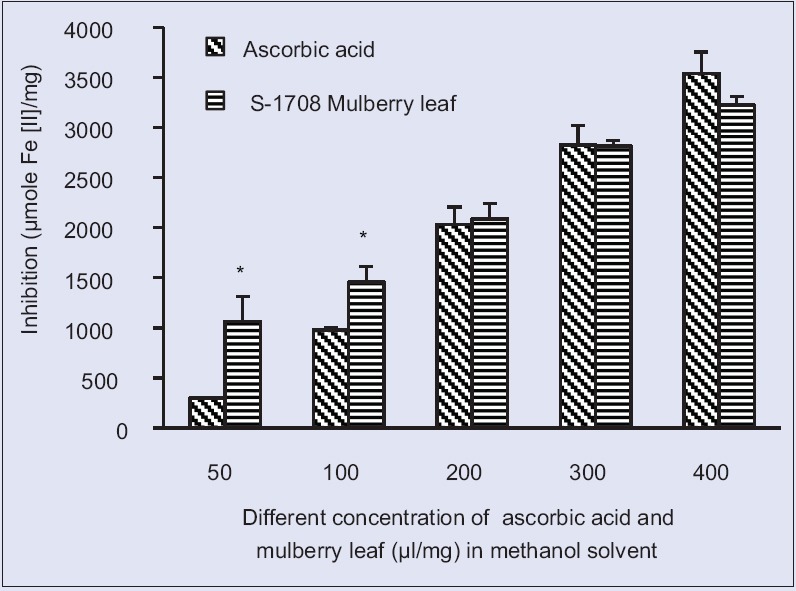
Ferric reducing/antioxidant power activity at different concentration S-1708 mulberry leaf extract and ascorbic acid in methanol solvent. Values are mean ± standard deviation. *On bars indicates significant diffrenence from control. Mulberry leaf extracts compared with the ascorbic acid (control). Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis
Ferric reducing/antioxidant power assay
The FRAP assay measures the antioxidant effect of any substance in the reaction medium as reducing ability. FRAP assay was used by several authors for the assessment of antioxidant activity of various samples. Halvorsen et al. 2006[32] suggested most of the secondary metabolites are redox-active compounds that will be selected by the FRAP assay. Therefore, the antioxidant potential of S-1708 mulberry leaf extract estimated and calculated in both methanolic and ethanolic solvents of the S-1708 mulberry leaf extract were showed significant (P < 0.05) higher activity as than ascorbic acid [Figures 3 and 4]. The result of reducing powers demonstrated the electron donor properties of S-1708 mulberry leaf extract thereby neutralizing free radicals by forming stable products. Comparable works in other plant and outcome of results is significantly supported[33] the assessment of antioxidant capacity of sedum (Sedum sarmentosum) as a valuable natural antioxidant source in different solvent and in methanolic solvent 2301.71 ± 248.92 μM/mL reduction was recorded, which is that mulberry leaves are also showed higher activity than S. sarmentosum plants.
Figure 3.
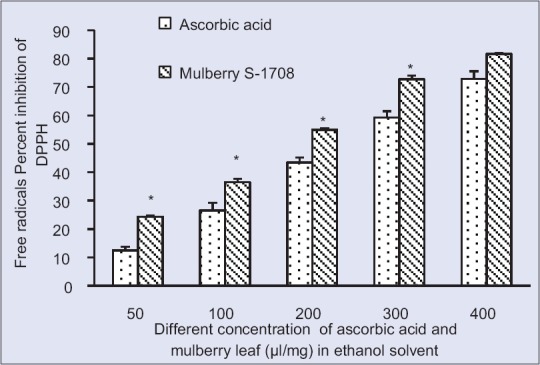
2,2-diphenyl-1-picryl-hydrazyl radical scavenging activity at different concentration of S-1708 mulberry leaf extract and ascorbic acid in ethanol Solvent. Values are mean ± standard deviation. *On bars indicates significant diffrenence from control. Mulberry leaf extracts compared with the ascorbic acid (control). Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis
Figure 4.
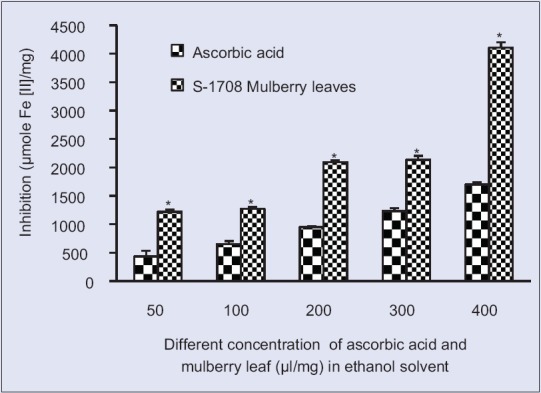
Ferric reducing/antioxidant power activity at different concentration S-1708 mulberry leaf extract and ascorbic acid in ethanol solventValues are mean ± standard deviation. *On bars indicates significant diffrenence from control. Mulberry leaf extracts compared with the ascorbic acid (control). Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis
Anti-hyperglycemic effect of mulberry leaves
Body weight
In the current study, observed reduction of body weights due to drugs effects besides that increment of body weight of diabetic mice after administration mulberry leaf clearly indicates mulberry leaf potentially involved in enhancing the weight of diabetic mice. This is because of mulberry leaves are effectively acting on the cellular mechanism until unless a mechanism is unknown. It might be due to potentially of bioactive compounds in the mulberry leaf of S-1708 variety which act as the potent antioxidant and provided protection against to stress, additionally helpful for gaining weight in diabetic mice [Figure 5]. The present study are supported Eun et al., 2011 by the study of the assessment of antioxidant capacity of sedum (S. sarmentosum) as a valuable natural antioxidant source and reported that diabetic mellitus is a chronic disease caused by overproduction of excessive hepatic glycogenolysis and gluconeogenesis, resultant of that decreased body weight and loosed utilization of glucose by tissues and gained body weight after administration of S. sarmentosum.[33]
Figure 5.
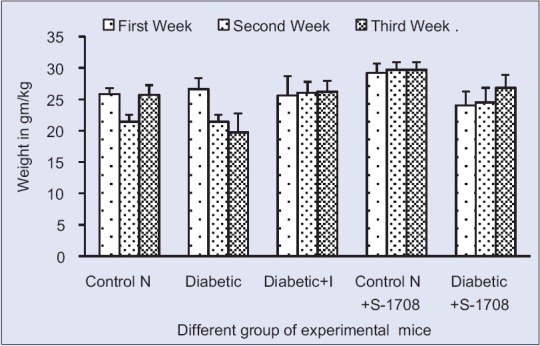
Effect of mulberry leaf extract on weight of different group of mice in of mice at 21 days. Symbols: U for Unit, Control N: Normal Group, Control D: Diabetic Group, Diabetic + I: Diabetic treated with insulin at 4 U/kg body weight, Control N+ S-1708: Control group treated with mulberry leaf, Diabetic +S-1708: Diabetic group treated with mulberry leaf
Fasting blood glucose
In the present study, clearly, indicates that STZ drug created a lot of stress inside the body and obstructs the gluconeogenesis pathway of liver, therefore, raised the glucose levels in blood serum. Diabetic treated with mulberry leaf extract (-71.58%) instantly decreased the FBG, controlled the glucose levels and significantly reduced the glucose level to become normalized after the 3rd week. Whereas diabetic group insulin drug treated with were also reduced significantly (P < 0.05) by (−70.37%) glucose level when compared to diabetic control, interesting results was observed that mulberry leaves S-1708 are more efficiently control than insulin drug. This study revealed that the supremacy of mulberry leaf extract to act on pancreas and liver cells to the removal of free radicals of tissue; therefore, it shows satisfactory effective to controlling of hyperglycemic [Figure 6]. Current studies are reinforced by Andallu and Vardacharyula, 2012[34] on mulberry leaves of Morus indica L. reported that after administration of mulberry leaf lipid peroxidation decreased and help to the reduction of hyperglycemia.
Figure 6.
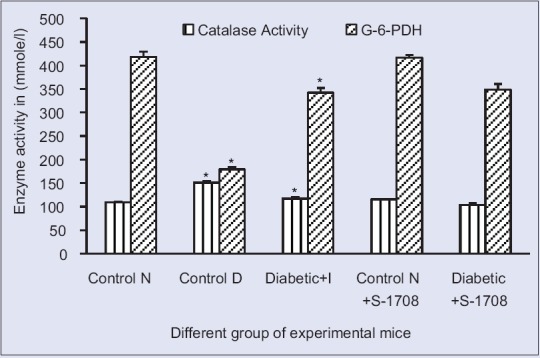
Effect of mulberry leaf extract on hepatic antioxidant enzyme of glucose-6-phosphate dehydrogenase (glucose 6 phosphate dehydrogenase and catalase activity in liver tissue of different group of mice at 21 days (x ± standard error of mean, n = 6 in each group), values are mean ± standared deviation. *On bars indicates significant diffrenence from control group. Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis. Symbols: Control N: Normal group, Control D: Diabetic group, Diabetic + I: Diabetic treated with insulin at 4 U/kg body weight, Control N+ S-1708: Control group treated with mulberry leaf, Diabetic +S-1708: Diabetic group treated with mulberry leaf
Enzyme in liver
Catalase and glucose-6-phosphate dehydrogenase
The glutathione (GSH) redox system are connected by G6PDH enzyme, which controlled GSH level and well known to play a key role in free radical and peroxide metabolism. This is relative plays a vital role in cellular protection including oxidative damage. Mulberry leaf of S-1708 variety effectively acting on enzyme level of diabetic mice and significantly (P < 0.05) increase the activity of G6PDH. This increment of G6PDH activity force is due to NADPH supply as a concern of decrease sorbitol synthesis/or from hexose monophosphate pathway (HMP), as showed by increased activity of G6PDH in might be leading to improving GSH level.[35] In the observation, catalase activity is increased in hepatic tissue of diabetic mice, and generated unnecessary production of peroxide free radicals in diabetic stress and storage of the enzyme. Oral administrative of S-1708 mulberry leaf extract to the diabetic group and notices the catalase enzyme level was tremendous (−31.39%) decreased and to become normalized after 3 weeks. This giving inference mulberry leaf powder is effective acting on controlling of catalase breakdown pathway; therefore, mulberry extract of S-1708 variety inhibited unnecessary production of free radicals inside the diabetic mice [Figure 7]. A similar test was performed and similar results were obtained by[36] to the analysis of M. indica L. leaves antioxidants activity and antioxidant enzymes in STZ-diabetic rats.
Figure 7.
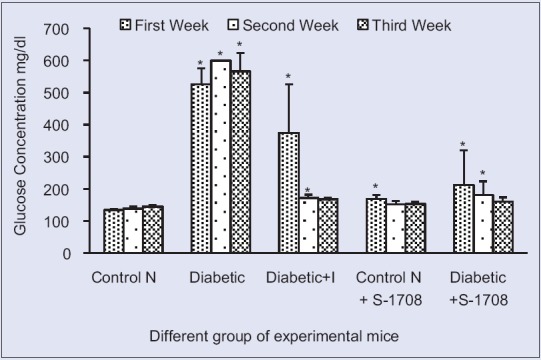
Effect of mulberry leaf extract on fasting blood glucose of different group of mice in different of mice at 21 days (x ± standard error of mean, n = 6 in each group), values are mean ± standard deviation. *On bars indicates significant diffrenence from control group. Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis. Symbols: Control N: Normal group, Control D: Diabetic group, diabetic + I: Diabetic treated with insulin at 4 U/kg body weight, Control N+ S-1708: Control group treated with mulberry leaf, Diabetic +S-1708: Diabetic group treated with mulberry leaf
Serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase
The activity of SGOT and SGPT is cytosolic marker enzyme refueling hepatocellular necrosis as they are released into the blood after cell membrane damage. Therefore, the tests are performed to check the activity of SGOT and SGPT as the indicator of hepatic damage. It is evident from Table 6 and Figure 8, the activity of SGOT and SGPT were significant (P < 0.05) increased in diabetic mice, respectively, as compared to control group. This increment in enzyme due to the action of STZ on liver cells which captured the liver cell membrane, sheet hepatocytes cells, and central vein. Therefore, SGOT and SGPT are released in the high amount from the liver into blood serum which caused the stress and damage of cell membrane. Compared the both results insulin treated and mulberry leaf treated diabetic mice, conclude that mulberry leaf is more efficient than insulin which efficiently reduced the SGOT and SGPT activity, respectively. This is due action S-1708 on pancreas and liver cells, therefore, they control the SGOT and SGPT level. This is might be possible due combine action of natural antioxidant compounds from the mulberry leaf. Based on the results suggested that mulberry leaves of S-1708 extract efficient extract which prevent hepatic injury associated with diabetes.
Figure 8.
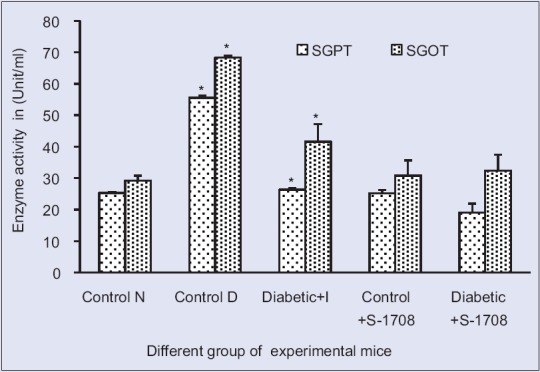
Effect of mulberry leaf extract on liver enzyme of serum glutamic oxaloacetic transaminase and serum glutamic-pyruvic transaminase activity in liver tissue of different group of mice at 21 days (x ± standard error of mean, n = 6 in each group), values are mean ± standard deviation. *On bars indicates significant diffrenence from control group. Level of significant was tested at the level of P < 0.05 by one-way analysis of variance analysis. Symbols: Control N: Normal group, Control D: Diabetic group, Diabetic + I: Diabetic treated with insulin at 4 U/kg body weight, Control N+ S-1708: Control group treated with mulberry leaf, Diabetic +S-1708: Diabetic group treated with mulberry leaf
Histopathological study
In the liver tissue of mice, injuries in the form of vacuolation and necrosis were mainly demonstrated in the peripheral zones of hepatic lobules. The pathological changes extended to involve the central zones and this might be explained by the type of blood circulation inside the hepatic lobule. Normally, the direction of blood flow proceeds from the periphery of the lobule toward the central vein, which is the flow of blood, is centripetal. Blood percolates within the sinusoids to the central vein and is exposed to the activities of the hepatocytes around the sinusoids. Plasma flows freely through the sinusoidal wall into the sinusoidal spaces where it is exposed to the various activities of the hepatocytes and then flows back into the bloodstream.[34] Most of the injected STZ drug reached the liver through the portal vein and end finally in the terminal portal venules in the portal tracts. Thus, the peripheral hepatocytes became exposed to a higher concentration of the STZ drug.
In the current study, Figure 9a shows normal cell morphology structure of the liver tissue-like sheet of hepatocytes, sinusoids, nuclei, and central vein are regularly arranged. Therefore, proper cellular synthesis mechanisms are takes places and control the glucose levels inside the body. Whereas, diabetic control group [Figure 9b] showed highly disruption of cell organization due to the effect of STZ drugs and its loss own identity of cell morphology like the sheet of hepatocytes, sinusoids, central vein, and forming large intercellular spaces appeared around central vein. This disruption of the cell triggers the process of inflammation and affects the normal functioning of the liver. Therefore, glycogenesis may be disturbed which leads to the rise of blood glucose (BG) level.
Figure 9.
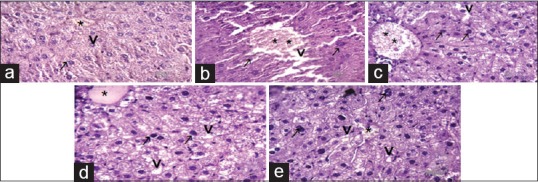
(a-e) Photomicrograph of liver section of mice showing the changes in cellular organization after three week of experiment (H and E), scale bar = 40 μm abbreviation marks- * (stick) central vein, ⇨ (open arrow) nucleus, ↗ (arrow) sheet of hepatocytes, ⇟(barred arrow) sinusoids. (a) Normal control group showed normal central vein, sheet of hepatocytes, and healthy nucleus are present, it is participated in normal glycogenolysis and gluconeogenesis synthesis therefore maintain glucose level and enzyme level- catalase, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, and glucose-6-phosphate dehydrogenase inside the body. (b) Diabetic group representing destruction of central vein, de-leafing of sheet of hepatocyte and sinusoids cells therefore its loosed controlling power of glucose liver synthesis and increased glucose level and increased serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase inside the liver. (c) Insulin treated diabetic group appears almost similar to normal control mice but in central vein showed regaining of normal structure therefore its indicate insulin help to control of glucose synthesis and normalizing glucose levels. (d) Normal control treated with mulberry S-1708 which similar to control groups in cellular organization and reporting that S-1708 has no any side effect. (e) S-1708 Mulberry leaf treated with diabetic group is showing regain of cells structure such as central vein, nucleus, sheet of hepatocytes, sinusoids, after treatment of mulberry and performing good controlling of glucose level in blood and help insynthesis of glycogenolysis and gluconeogenesis inside the liver of mice
In Figure 9c, the diabetic group treated with insulin appearing normal cells organization because the action of insulin in liver cells which might be controlling of glucose pathway synthesis. The observations of Figure 9d which are representing the normal group of mice treated with S-1708 mulberry leaves shown similar structure like to normal control mice. Therefore, proper secretion of insulin was released from the pancreas, and controlled manner gluconeogenesis occurs in liver cells which lead to controlling of glucose level inside the body system.
This study showed [Figure 9e] the diabetic group treated with S-1708 mulberry leaves extract to enhance the cellular structure alike in the sheet of hepatocytes, central vein, and the sinusoids. Therefore it is helpful to the reduction of glucose and maintains of proteins levels inside the body system. Findings of histopathological studies indicate that mulberry leaves of S-1708 are effectively acting and improving the health status of tissue and maintain the enzymatic level inside the body system along with repairing of the cellular structure due to the presence of antioxidant properties in the leaf to threating oxidative free radicals. Histological and Histochemistry analysis of glycogen was completed by Brijendra et al., 2013[37] in liver rat under stress of diabetic mice and suggested similar observation.
CONCLUSION
In summary, this study concludes that both in vitro and in vivo studies, S-1708 mulberry variety emerged as highly potential since it has the maximum capacity to inhibit free radicals and most effective extracts for reducing the BG level. Hence, S-1708 variety may exploit for further studies in the field of natural product research.
Financial support and sponsorship
UGC Research Fellowship by University Grant Commission, Government of India, New Delhi.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yunfeng L, Changjiang G, Jijun Y, Jingyu W, Jing X, Shuang C. Evaluation of antioxidant properties of pomegranate peel extract in composition with pomegranate pulp extract. Food Chem. 2006;96:254–60. [Google Scholar]
- 2.López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal Chim Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 3.Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH. Phytochemical profiles of different mulberry (Morus sp.) species from China. J Agric Food Chem. 2009;57:9133–40. doi: 10.1021/jf9022228. [DOI] [PubMed] [Google Scholar]
- 4.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Ameyaw Y, Barku VY, Ayivor J, Forson A. Phytochemical screening of some indigenous medicinal plant species used in the management of diabetes mellitus in Ghana. J Med Plants Res. 2012;6:4573–81. [Google Scholar]
- 6.Khattak KF, Rahman TR. Effect of geographical distributions on the nutrient composition, phytochemical profile and antioxidant activity of Morus nigra. Pak J Pharm Sci. 2015;28:1671–8. [PubMed] [Google Scholar]
- 7.Ramesh HL, Sivaram V, Yogananda VN. Murthy. Antioxidant and medicinal properties of mulberry (Morus sp.): A review. World J Pharm Res. 2014;3:320–43. [Google Scholar]
- 8.Aysel S, Munevver SK. Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.) Plant Growth Regul. 2004;44:251–6. [Google Scholar]
- 9.Wang Y, Xiang L, Wang C, Tang C, He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One. 2013;8:e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güllüce M, Sökmen M, Daferera D, Agar G, Ozkan H, Kartal N, et al. In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J Agric Food Chem. 2003;51:3958–65. doi: 10.1021/jf0340308. [DOI] [PubMed] [Google Scholar]
- 11.Szeto YT, Tomlinson B, Benzie IF. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br J Nutr. 2002;87:55–9. doi: 10.1079/BJN2001483. [DOI] [PubMed] [Google Scholar]
- 12.Hua YL, Meng F, Yu-Qing Z. In vivo hypoglycaemic effect and inhibitory mechanism of the branch bark extract of the mulberry on STZ-induced diabetic mice. ScientificWorldJournal. 2014;(2014):11. doi: 10.1155/2014/614265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmati AA, Jalali MT, Rashidi I, Kalantar HT. Impact of aqueous extract of black mulberry (Morus nigra) on liver and kidney function of diabetic mice. Jundishapur J Nat Pharm Prod. 2010;5:18–25. [Google Scholar]
- 14.Kasono K, Yasu T, Kakehashi A, Kinoshita N, Tamemoto H, Namai K, et al. Nicorandil improves diabetes and rat islet beta-cell damage induced by streptozotocin in vivo and in vitro. Eur J Endocrinol. 2004;151:277–85. doi: 10.1530/eje.0.1510277. [DOI] [PubMed] [Google Scholar]
- 15. [Last accessed on 2016 Aug 30]. Available from: http://www.guidelines.diabetes.ca/Browse/Appendices/Appendix3/
- 16. [Last accessed on 2016 Aug 30]. Available from: http://www.worthington-biochem.com/zf/
- 17.Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002;40:725–33. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- 18.Bergmeyer HU, Scheibe P, Wahlefeld A W. “Optimization of methods for aspartate aminotransferase and alanine aminotransferase.”. Clinical chemistry 24.1. 1978:58–73. [PubMed] [Google Scholar]
- 19.Moss DW, Henderson AK. Clinical enzymology. In: Burits CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: WB Saunders; 1994. pp. 617–721. [Google Scholar]
- 20.Murray RL. Enzymes. In: Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis and Correlation. Toronto: C.V. Mosby; 1994. pp. 1079–134. [Google Scholar]
- 21.Young D. Effect of Preanalytical Variable on Clinical Laboratory Tests. 2nd ed. Washington: AACC Press; 1997. pp. 4–489. [Google Scholar]
- 22.Bergmeyer HU, Scheibe P, Wahlefeld AW. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- 23.Rej R, Vanderlinde R E. Effects of temperature on the steady-state kinetics and measurement of aspartate aminotransferases. Clinical Chemistry. 1981;27:213–219. [PubMed] [Google Scholar]
- 24.Kaplan A, Lavelnel LS. In Clincical Chemistry. Interpretation and Technique. 2nd ed. Philadelphia: Lae and Febiger; 1983. pp. 219–96. [Google Scholar]
- 25.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 5th ed. Edinburg: Churchill Livingstone; 2002. [Google Scholar]
- 26.Ehrlich P. “Fragekasten”. Z Wiss Mikrosk. 1886;3:203–48. [Google Scholar]
- 27.Khalaf NA, Ashok K, Atif AL, Zaha EA, Husni F. Antoidant activity of some common plants. Turk J Biol. 2008;32:51–5. [Google Scholar]
- 28.Khan MA, Rahman AA, Islam S, Khandokhar P, Parvin S, Islam MB, et al. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae) BMC Res Notes. 2013;6:24. doi: 10.1186/1756-0500-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal S, Younas U, Chan K W, Sarfraz RA, Uddin MK. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. International Journal of Molecular Sciences. 2012;13:6651–64. doi: 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suraphan N, Borompichaichartkul C, Duangmal K. Production of Antioxidation Powder from Mulberry Morus alba L. leaf extract. International Symposium on Agri-Foods for Health and Wealth; 5-8 August. 2013:227–35. [Google Scholar]
- 31.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–55. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 32.Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR, Jr, et al. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Eun KM, Seung MK, Sun AY, Chang JO, Chang KS. Assessment of antioxidant capacity of sedum (Sedum sarmentosum) as a valuable natural antioxidant source. Food Sci Biotechnol. 2011;20:1061–7. [Google Scholar]
- 34.Andallu B, Vardacharyulu NC. Effect of mulberry leaves on diabetes. Int J Diab Dev Countries. 2001;21:147–151. [Google Scholar]
- 35.Andallu B, Vinay AK, Varadacharyulu N. Oxidative stress in streptozocin-diabetic rats: Amelioration by mulberry (Moru sindica L) leaves. Chin J Integr Med. 2012;22:1–6. doi: 10.1007/s11655-012-1234-4. [DOI] [PubMed] [Google Scholar]
- 36.Andallu B, Vinay AK, Allagadda V, Varadacharyulu N. Influence of mulberry (Moru sindica L.) leaves on antioxidants and antioxidant enzymes in STZ-diabetic rats. Int J Diabetes Dev Ctries. 2014;34:69–76. [Google Scholar]
- 37.Brijendra B, Sexena PN, Nishi S. Comparative evaluation of hitochemical change in rat liver under stress of type II pyrethroids. Int J Adv Res Technol. 2013;1:22–9. [Google Scholar]


