Abstract
Background:
The escalating dominance of resistant Pseudomonas aeruginosa strains as infectious pathogen had urged the researchers to look for alternative and complementary drugs.
Objective:
The objective of this study is to address the biological targets and probable mechanisms of action underlying the potent antibacterial effect of the isolated compounds from Euphorbia hirta (L.) against P. aeruginosa.
Materials and Methods:
The action mechanisms of caffeic acid (CA) and epicatechin 3-gallate (ECG) on P. aeruginosa cells were investigated by several bacterial physiological manifestations involving outer membrane permeabilization, intracellular potassium ion efflux, and nucleotide leakage.
Results:
The findings revealed that ECG and CA targeted both cell wall and cytoplasmic membrane of P. aeruginosa. The cellular membrane destruction and ensuing membrane permeability perturbation of P. aeruginosa had led to the ascending access of hydrophobic antibiotics, release of potassium ions, and leakages of nucleotides.
Conclusion:
The overall study concludes that ECG and CA isolated from E. hirta possess remarkable anti-infective potentials which can be exploited as drug template for the development of new antibacterial agent against resistant P. aeruginosa pathogen.
SUMMARY
Epicatechin 3-gallate (ECG) and caffeic acid (CA) exhibited remarkable bactericidal abilities by increasing the outer membrane and plasma membrane permeability of Pseudomonas aeruginosa pathogen
ECG and CA had facilitated the entry of hydrophobic antibiotics into P. aeruginosa by disintegrating the lipopolysaccharides layer of the outer membrane
ECG-induced potassium efflux with efficiency close to that obtained with cefepime suggesting mode of action through membrane disruption
Both ECG and CA had caused consistent leakage of intracellular nucleotide content with the increase in time.
Abbreviations used: ECG: Epicatechin 3-gallate; CA: Caffeic acid; E. hirta: Euphoria hirta.
Keywords: Erythromycin, Euphorbia hirta (L.), nucleotide leakage, potassium ion efflux, rifampicin
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen that instigates severe illness in immunosuppressed hosts, especially patients in hardship of cancer, AIDS, cystic fibrosis, severe burns, and individuals in Intensive Care Unit.[1,2] P. aeruginosa infections are accounted responsible for the increasing percentage of morbidity and mortality rate ranging from 40% to 60% among immunocompromised patients.[3] Not long ago, this nosocomial pathogen has become part of a “superbug” group as it is often resistant to various antibiotics, host defenses, and possessed vast competence to provoke resistance. Most antibiotics failed to eradicate P. aeruginosa infection owing to low outer membrane permeability which linked to adaptive mechanisms and ultimately attained clinical resistance.[4]
Prospecting for new therapeutic agent to combat P. aeruginosa resistance, therefore, has encouraged research into natural products. Antibacterial compounds isolated from plants are receiving immense attention as alternatives to antibiotics. The reason for this is due to the very limited number of cases documented for the development of bacterial resistance toward natural plant products.[5] Even though the mechanism of action for the antibacterial activity of natural plant product is uncertain, cell membrane disruption by phenolics, flavonoids and terpenoids, inhibition of protein synthesis by phenols and coumarin, and interference with DNA/RNA function are thought to inhibit the growth of the bacteria.[6] The antibacterial action of plant secondary metabolites includes the following sequence of cell membrane contact, diffusion through the membrane to enter the interior of the cell, and finally interaction with intracellular processes.[7] Earlier, we have reported the isolation of caffeic acid (CA) and epicatechin 3-gallate (ECG) from Euphorbia hirta (L.) using bioactivity-guided fractionation.[8] These antibacterial compounds exhibited remarkable bioactivity against the resistant clinical isolate of P. aeruginosa. Prompted by the above findings, the current study was embarked on in the effort to address the biological targets and mechanisms of action of the isolated antibacterial compounds from E. hirta against P. aeruginosa. The action mechanisms of CA and ECG were investigated by several bacterial physiological manifestations involving outer membrane permeabilization, intracellular potassium ion efflux, and nucleotide leakage. Moreover, the present study is assumed the initial to investigate the mechanism of action underlying the antibacterial effect of isolated compounds from E. hirta against P. aeruginosa.
MATERIALS AND METHODS
Antibacterial agent
Laboratory-grade standard powder of cefepime (lot no: 341037; International Laboratory, San Francisco, USA) was obtained and reconstituted according to the manufacturer's instruction. Stock solution of cefepime was freshly prepared on the day of the experiments. The stock solutions were stored under refrigeration until the time of use and discarded after 24 h as recommended by the manufacturer. Cefepime was required to pass quality control standard (P. aeruginosa; ATCC 27853) on the day of the experiment.[9]
Bacterial strain
Isolates of P. aeruginosa were obtained from clinical specimens submitted to the Department of Medical Microbiology and Parasitology (JTMP), School of Medical Sciences, Universiti Sains Malaysia. Species identification was confirmed by biochemical test using analytical profile index system. Isolates were stored in tryptic soy broth containing 20% glycerol and frozen at −80°C. Isolates were subcultured a minimum of two times before mechanism studies.
Inoculum preparation
P. aeruginosa culture was recovered on a fresh tryptic soy agar (Difco, USA) plate, 24 h before action mechanism tests. To prepare the standardized inoculum, four to five well-isolated colonies of P. aeruginosa with similar morphological type were selected from an agar plate culture. Each colony was transferred with sterile swab into a tube containing 5 mL of sterile cation-adjusted Mueller-Hinton broth (Hi-Media, India) to obtain a liquid suspension that matches to McFarland 0.5 turbidity standards. The resulting suspension in the tube contained about 1 × 108 CFU/mL of microorganism. Subsequent proper dilution will result in final inoculum or test concentration of bacteria to 5 × 105 CFU/mL.
Effects of the isolated antibacterial compounds on outer membrane permeability
The hydrophobic antibiotics erythromycin and rifampicin were tested in association with the isolated antibacterial compounds.[10] An overnight culture of P. aeruginosa in logarithmic-phase was washed and resuspended in fresh nutrient broth medium (1 × 106 CFU/mL) containing isolated antibacterial compounds at the concentration of half minimum inhibitory concentration (MIC) and hydrophobic antibiotics of various concentration ranging from 0.5 μg/mL to 50.0 μg/mL (erythromycin) and 0.5–40.0 μg/mL (rifampicin) at 37°C for 12 h. Decrease of absorbance was monitored at 630 nm.
Effects of the isolated antibacterial compounds on inner membrane permeability
Measurement of potassium ion efflux
The inner membrane permeability of P. aeruginosa was determined by measuring the amount of potassium ion effluxed from the treated cells.[11] P. aeruginosa cells were cultured overnight at 37°C. Cells of P. aeruginosa were resuspended in 30 mL of deionized water to obtain cell density of 1 × 106 CFU/mL. The bacteria were treated with isolated antibacterial compounds at the concentration of 2 × MIC and incubated at 37°C. Samples (5 mL) of cell suspension were removed at various times intervals of 30, 60, 90, 120, and 150 min and were centrifuged at 10,000 rpm for 10 min. The resulting supernatant was diluted at 100-fold and filtered through a 0.2 μm pore size membrane (Sartorius, Germany) to remove bacteria. Cefepime-treated cells were used as positive control, whereas untreated cells as negative control. The amounts of released K+ were measured by atomic absorption spectrometer (AAS, Varian, USA). A series of potassium standards (2–10 ppm) were prepared to generate a calibration curve (y = 0.0287x; R2 = 0.9957) which was used to quantitatively measure the amount of potassium ion released from the treated cells.
Measurement of nucleotide leakage
The leakages of nucleotide from P. aeruginosa cells were determined.[12] The cells of P. aeruginosa in logarithmic-phase (1 × 106 CFU/mL) were washed and resuspended in 10 mM PBS (pH 7.4). The suspension was incubated with isolated antibacterial compounds at twice the MICs for various times (0, 60, 120, 180, 240, and 600 min). The untreated bacterial suspension was used as negative control, and bacterial cells treated with cefepime was included as positive control. The mixture was filtered through 0.22 μm to remove the bacterial cells. The filtrate was then diluted appropriately, and the optical density (OD) at 260 nm was recorded (Perkin-Elmer 45, USA) at room temperature (25°C).
Statistical analysis
All the experiments were conducted in triplicate. The values are presented as mean ± standard deviation. The statistical analyses were done using Statistical Package for the Social Sciences software version 17.0 (United States). Statistical procedures were performed at a significance level of 95%.
RESULTS AND DISCUSSIONS
Outer membrane permeability
The antibacterial activity of hydrophobic antibiotics was significantly improved after adding CA and ECG. Rifampicin and erythromycin are distinctive embodies of hydrophobic antibacterial agents. The outer membrane of Gram-negative cell wall is well acknowledged as an effective permeability barrier against rifampicin and erythromycin. These antibiotics are incapable to effectively permeate the intact outer membrane of Gram-negative bacteria composed entirely of lipopolysaccharide and protein.[10] Rifampicin and erythromycin are usually used as probe to evaluate the outer membrane permeability flow generated by other antibacterial compounds. The previous results from the time-kill study had confirmed that half MIC of the isolated antibacterial compounds could not inhibit the growth of P. aeruginosa.[8] Nevertheless, in the presence of half MIC of isolated antibacterial compounds, particularly ECG and CA had triggered P. aeruginosa to become more susceptible to the probe antibiotics at each concentration. The growth inhibition of P. aeruginosa even occurred at below MIC of rifampicin (10 μg/mL) and erythromycin (1 μg/mL) [Figure 1]. In addition, both hydrophobic antibiotics displayed more potent growth inhibition in association with ECG than CA. The growth inhibition induced by ECG was greater in the presence of erythromycin. In the absence of ECG and CA, bacterial cells of P. aeruginosa were healthily proliferating as erythromycin and rifampicin have no effect on Gram-negative bacteria (data not shown).
Figure 1.
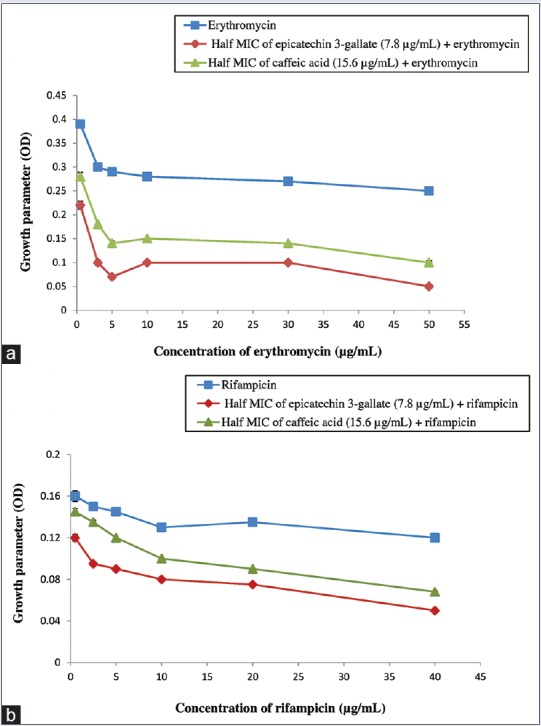
Outer membrane permeabilization of Pseudomonas aeruginosa induced by epicatechin 3-gallate and caffeic acid. Growth inhibition assay in association with antibiotics erythromycin (a) and rifampin (b). Antibiotic (■), half minimum inhibitory concentration of epicatechin 3-gallate (7.8 μg/mL) + antibiotic (♦), half minimum inhibitory concentration of caffeic acid (15.6 μg/mL) + antibiotic (▲). Each point represents the mean values of three experiments (n = 3). No statistically significant difference was found between data points (P < 0.05)
The findings revealed that ECG and CA possessed remarkable ability to facilitate the entry of hydrophobic antibiotics into P. aeruginosa cells by enhancing the permeability of intact OM. These compounds probably operated as permeabilizers by disintegrating the lipopolysaccharides layer of the OM.[13] These results are consistent with earlier work that described the bacterial OM disruption which causes barrier function detriment contributes to the mode of action of phytochemical compounds such as phenolics and flavonoids.[14] It is noteworthy that ECG is a type of flavonoid and has been previously reported to possess antibacterial activity against methicillin-resistant Staphylococcus aureus.[15] Meanwhile, some phenolics such as CA, syringic acid, and p-coumaric have been depicted as OM disintegrators and thereby increasing the permeability of cytoplasmic component.[16]
Potassium ion efflux from Pseudomonas aeruginosa
Figure 2 had illustrated the amount of potassium ion that has effluxed from P. aeruginosa cells at different incubation time. The treated P. aeruginosa cells released potassium at a time-dependent manner on treatment. The permeability of the treated P. aeruginosa membrane to intracellular potassium was compared with that from untreated cells and cefepime-treated cells. According to the results obtained, cefepime-treated cells induced immense effluxes of potassium from the membrane compared to the isolated antibacterial compounds treated cells. The relative amount of extracellular potassium reached the maximum at 13.1 ± 0.45 μg/mL after 150 min of contact with cefepime. ECG showed more marked effect on potassium leakage contrasted to CA. This compound induced a distinct leakage of intracellular potassium from P. aeruginosa attaining 10.8 ± 0.55 μg/mL after 150 min of treatment with 2 × MIC. CA released moderate amount of potassium ion from the bacterial cell achieving 6.1 ± 0.42 μg/mL at 150 min.
Figure 2.
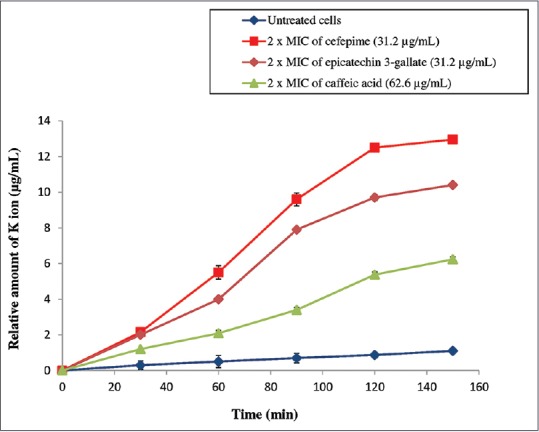
Amount of potassium ions (K+) released from Pseudomonas aeruginosa after being treated with epicatechin 3-gallate and caffeic acid. (■) Cefepime (31.2 μg/mL), (♦) 2 × minimum inhibitory concentration of epicatechin 3-gallate (31.2 μg/mL), (▲) 2 × minimum inhibitory concentration of caffeic acid (62.6 μg/mL) and (●) untreated cells. Cefepime works as a positive control. Each point represents the mean values of three experiments (n = 3). No statistically significant difference was found between data points (P < 0.05)
The bacterial cytoplasmic membrane serves as a selective permeable barrier, allowing only particular ions and molecules to diffuse into and out of the cell. Compromised cytoplasmic membrane of bacteria will emancipate intracellular components such as potassium ion which can be experimentally quantified. Potassium ion is essential for numerous important cellular functions and the most abundant intracellular cation presents in bacteria.[17] The leak of potassium ion gradient is a consequence of breakdown of the permeability barrier across cell membrane. Potassium leakage is known to be the first indication of membrane damage in microorganisms.[18]
The bacterial cell membrane is accountable for many vital functions such as transport, respiration processes, and synthesis of lipids. Any direct or indirect disruption on the membrane integrity can cause massive metabolic dysfunction and cell death.[19] To ascertain whether the inhibitory activity of isolated antibacterial compounds from E. hirta causes cell membrane injury, potassium cation depletion from the treated P. aeruginosa cells were measured first. In this study, cefepime, a drug inducing membrane permeabilization was used as a positive control. ECG had demonstrated approximately a similar profile of potassium release with cefepime [Figure 2]. This suggests that ECG isolated from E. hirta may induce potassium release with efficiency close to that obtained with cefepime through membrane disruption. This observation is in agreement with the previous study reported on the mechanism of antibacterial activity of polyphenols and its function on the plasma membrane permeabilization of P. aeruginosa.[20] Phenolic acids are partially lipophilic in character and able to enter the bacterial cell by passive diffusion. They are capable to instigate disruption to the cell membrane structure and generate immense intracellular acidification in the bacteria.[21] Intracellular acidification alters bacterial membrane permeabilization and causes severe membrane damages, leading to cell death. This acidification effect could be the possible potential mechanism to explain the K+ efflux and bactericidal effect observed in this study. Another previous study also had testified that a flavonoid known as sophoraflavanone had induced membrane permeability to cations such as K+ thus caused destruction to the cytoplasmic membrane of bacteria.[22] Other authors similarly verified that epigallocatechin gallate, a flavonoid-induced pore formation on the external monolayer of the lipid membranes resulting in leakage of the intracellular components.[23,24] Therefore, it can be hypothesized that the antibacterial activity of phenolic acid and flavonoid are associated with the affinity for the lipid layers in the cell membrane. CA and ECG being phenolic acid and flavonoid certainly had used this mode of action to permeabilize the cytoplasmic membrane.
Effect of isolated antibacterial compounds on nucleotide leakage
The impact of isolated antibacterial compounds on cell membrane integrity can be assessed by measuring the leakages of intracellular components such as nucleotides from the damaged bacterial cell membrane. Leaked cellular components which absorb light at 260 nm primarily represent nucleotide group. The results of total nucleotide leakage from the cells of P. aeruginosa after being treated with isolated antibacterial compounds from E. hirta are presented in Figure 3. The results obtained were referred to two controls, one of untreated cells signifying intact membrane and the other of cefepime-treated cells representing permeabilized membrane. According to the results observed in Figure 3, the treatment with 2 × MIC of ECG and CA had caused consistent leakage of intracellular nucleotide content with time. Both the compounds showed substantial release of cellular material (OD value in the range of 0.181 nm to 0.380 nm at 600 min) compared to untreated cells. Nevertheless, cefepime exhibited the maximum nucleotide leakage with OD value of 0.450 nm at 600 min from the P. aeruginosa cells.
Figure 3.
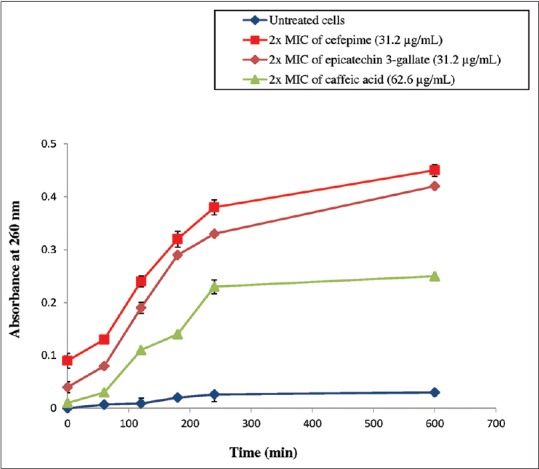
Nucleotide leakage from Pseudomonas aeruginosa after being treated with epicatechin 3-gallate and caffeic acid. (■) cefepime (31.2 μg/mL), (♦) 2 × minimum inhibitory concentration of epicatechin 3-gallate (31.2 μg/mL), (▲) 2 × minimum inhibitory concentration of caffeic acid (62.6 μg/mL) and (●) untreated cells. Cefepime works as a positive control. Each point represents the mean values of three experiments (n = 3). No statistically significant difference was found between data points (P < 0.05)
The results obtained exhibited that ECG and CA compromised the integrity of the cytoplasmic membrane. These isolated antibacterial compounds were capable of eliciting apparent nucleotide leakage by enhancing cell membrane permeability of P. aeruginosa. The destabilization of bacterial plasma membrane had instigated the outflow of low molecular weight compounds from the bacterial cells.[25] The results from this study are in accordance with previous works that reported the capacity of flavonoid and phenolic compounds to induce alteration to the bacterial cytoplasmic membrane.[26,27] This alteration eventually, led to the loss of membrane permeability thereby leading to the loss of essential cytoplasmic components such as nucleotides. These existing changes ultimately directed to the cell death.
CONCLUSION
The findings revealed that ECG and CA targeted both cell wall and cytoplasmic membrane of P. aeruginosa. These phytocompounds showed remarkable bactericidal abilities by increasing the outer membrane and plasma membrane permeability of P. aeruginosa pathogen. The cellular membrane destruction and ensuing membrane permeability perturbation of P. aeruginosa had led to the ascending access of hydrophobic antibiotics, rapid release of potassium ions, and leakages of nucleotides. These antibacterial molecules of clinical value await further research and development as a new chemotherapeutic agent.
Financial support and sponsorship
This work was supported by E-Science Fund (305/PFARMASI/613223), Ministry of Science, Technology and Innovation and Short-term Research Grant (304/PFARMASI/6312024), Universiti Sains Malaysia.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The first author would like to acknowledge MyBrain15 (Ministry of Education, Malaysia) for providing scholarships.
REFERENCES
- 1.Botzenhardt K, Doring G. Ecology and epidemiology of Pseudomonas aeruginosa.Pseudomonas aeruginosa as an Opportunistic Pathogen. New York: Plenum Press; 1993. pp. 1–18. [Google Scholar]
- 2.Balcht T, Aldona P, Smith R, Raymond K. Pseudomonas aeruginosa: Infections and Treatment. England: CRC Press; 1994. pp. 83–4. [Google Scholar]
- 3.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 4.Breidenstein EB, de la Fuente-Núñez C, Hancock RE. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011;19:419–26. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem. 2013;20:932–52. [PubMed] [Google Scholar]
- 6.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–22. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perumal S, Mahmud R, Ramanathan S. Anti-infective potential of caffeic acid and epicatechin 3-gallate isolated from methanol extract of Euphorbia hirta (L.) against Pseudomonas aeruginosa. Nat Prod Res. 2015;29:1766–9. doi: 10.1080/14786419.2014.999242. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. CLSI document M100-S22. Wayne: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 10.Viljanen P, Matsunaga H, Kimura Y, Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot (Tokyo) 1991;44:517–23. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- 11.Hao G, Shi YH, Tang YL, Le GW. The membrane action mechanism of analogs of the antimicrobial peptide buforin 2. Peptides. 2009;30:1421–7. doi: 10.1016/j.peptides.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Tang YL, Shi YH, Zhao W, Hao G, Le GW. Insertion mode of a novel anionic antimicrobial peptide MDpep5 (Val-Glu-Ser-Trp-Val) from Chinese traditional edible larvae of housefly and its effect on surface potential of bacterial membrane. J Pharm Biomed Anal. 2008;48:1187–94. doi: 10.1016/j.jpba.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou Z, Wang H, Zhu S, Ma C, Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci. 2011;76:M398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 15.Shiota S, Shimizu M, Mizushima T, Ito H, Hatano T, Yoshida T, et al. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis) Biol Pharm Bull. 1999;22:1388–90. doi: 10.1248/bpb.22.1388. [DOI] [PubMed] [Google Scholar]
- 16.Nohynek LJ, Alakomi HL, Kähkönen MP, Heinonen M, Helander IM, Oksman-Caldentey KM, et al. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer. 2006;54:18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 17.Orlov DS, Nguyen T, Lehrer RI. Potassium release, a useful tool for studying antimicrobial peptides. J Microbiol Methods. 2002;49:325–8. doi: 10.1016/s0167-7012(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 18.Lambert PA, Hammond SM. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem Biophys Res Commun. 1973;54:796–9. doi: 10.1016/0006-291x(73)91494-0. [DOI] [PubMed] [Google Scholar]
- 19.Pag U, Oedenkoven M, Sass V, Shai Y, Shamova O, Antcheva N, et al. Analysis of in vitro activities and modes of action of synthetic antimicrobial peptides derived from an alpha-helical ‘sequence template’. J Antimicrob Chemother. 2008;61:341–52. doi: 10.1093/jac/dkm479. [DOI] [PubMed] [Google Scholar]
- 20.Yi SM, Zhu JL, Fu LL, Li JR. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int J Food Microbiol. 2010;144:111–7. doi: 10.1016/j.ijfoodmicro.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Campos FM, Couto JA, Figueiredo AR, Tóth IV, Rangel AO, Hogg TA. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int J Food Microbiol. 2009;135:144–51. doi: 10.1016/j.ijfoodmicro.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya H, Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine. 2000;7:161–5. doi: 10.1016/S0944-7113(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 23.Tamba Y, Ohba S, Kubota M, Yoshioka H, Yoshioka H, Yamazaki M. Single GUV method reveals interaction of tea catechin (-)-epigallocatechin gallate with lipid membranes. Biophys J. 2007;92:3178–94. doi: 10.1529/biophysj.106.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Hung WC, Chen FY, Lee CC, Huang HW. Interaction of tea catechin (-)-epigallocatechin gallate with lipid bilayers. Biophys J. 2009;96:1026–35. doi: 10.1016/j.bpj.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston MD, Hanlon GW, Denyer SP, Lambert RJ. Membrane damage to bacteria caused by single and combined biocides. J Appl Microbiol. 2003;94:1015–23. doi: 10.1046/j.1365-2672.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- 26.Mirzoeva OK, Grishanin RN, Calder PC. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol Res. 1997;152:239–46. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 27.Hugo WB, Bloomfield SF. Studies on the mode of action of the phenolic antibacterial agent fentichlor against Staphylococcus aureus and Escherichia coli 3. The effect of fentichlor on the metabolic activities of Staphylococcus aureus and Escherichia coli. J Appl Bacteriol. 1971;34:579–91. doi: 10.1111/j.1365-2672.1971.tb02320.x. [DOI] [PubMed] [Google Scholar]


