Abstract
Background
Chronic hepatitis B virus (HBV) infection occurs in 90% of infants infected perinatally but is prevented when a hepatitis B vaccine is given within 24 hours of birth (HepB-BD), followed by 2-3 additional doses.
Methods
Using Spearman's rho correlation coefficients (rho), we analyzed global and regional data to assess correlations between HepB-BD coverage, institutional delivery rates (IDR), skilled birth attendance (SBA) rates, and other potential co-variates.
Results
Significant correlations were observed worldwide between HepB-BD and SBA rates (rho=0.44, p < 0.001), IDR (rho=0.42, p < 0.001), adult literacy rate (rho=0.37, p=0.003), total health expenditure per capita (rho=0.24, p=0.03) and live births (rho= -0.27, p=0.014). HepB-BD, IDR, and SBA rates were significantly correlated in the World Health Organization African, South-East Asia and Western Pacific Regions.
Conclusions
Increasing IDR and SBA rates, training and supervising staff, increasing community awareness, and using HepB-BD outside the cold chain where needed would increase HepB-BD coverage and prevent chronic infections.
Keywords: hepatitis B virus, hepatitis B vaccine, vaccination, birth dose, institutional delivery rate, skilled birth attendance
1. Introduction
Two billion persons have been infected with hepatitis B virus (HBV) worldwide, and about 250 million are chronically infected, which increases their risk of liver cirrhosis and cancer [1,2]. Progression to chronic infection is age-related, occurring in 90%, 30%, and 6% of those infected perinatally, in early childhood, and after age five years, respectively [1]. Administration of a hepatitis B vaccine dose within 24 hours of birth (HepB-BD) followed by 2-3 additional hepatitis B vaccine (HepB) doses can prevent up to 95% of perinatal transmission of HBV [1].
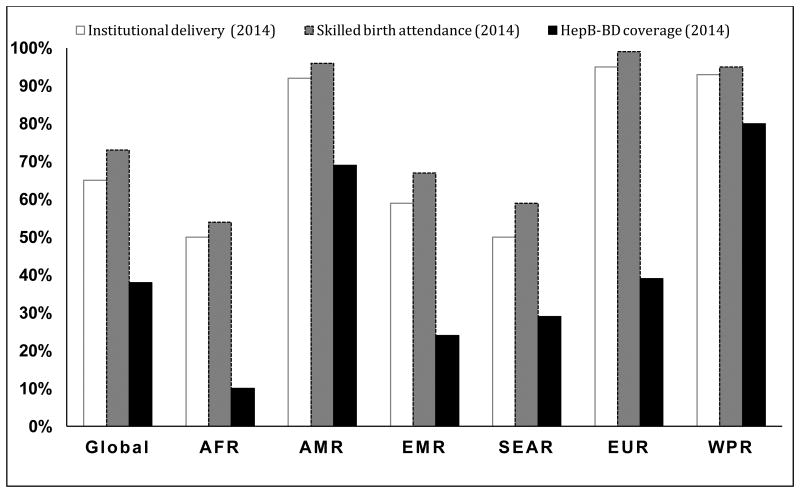
Despite the availability of an effective vaccine, overall coverage with HepB-BD in 2014 was 38%; the lowest coverage was reported in the World Health Organization (WHO) African Region (10%) and the highest coverage in the WHO Western Pacific Region (80%). In 2014, up to 30 million newborns were still unvaccinated in the 96 countries that provided universal HepB-BD in the national immunization schedule [3]. This low coverage is related to challenges unique to the HepB-BD, which must be given within 24 hours of birth to be most effective at preventing perinatal infections. Logistical barriers to timely HepB-BD administration can include lack of vaccine in the delivery ward, lack of cold chain for vaccine storage, and absence of skilled personnel to vaccinate children born at home [4]. Home births have been reported as important reasons for low coverage with timely HepB-BD in Cambodia and Indonesia [5,6], while timely HepB-BD vaccination was significantly associated with institutional delivery in China [7,8]. In 2014, global IDR and SBA rates were 65% and 73%, respectively [9]. The African and SouthEast Asia regions had the lowest IDR and SBA rates while the Americas, European, and Western Pacific regions had the highest rates (Figure 1).
Figure 1. Institutional delivery, skilled birth attendance and Hepatitis B birth dose vaccine coverage by WHO region, 2014.
AFR: African Region; AMR: Region of the Americas; EMR: Eastern Mediterranean Region; SEAR: South-East Asia Region EUR; European Region; WPR: Western Pacific Region
Sources:
WHO. Global routine vaccination coverage, 2014. Weekly epidemiological record 2015; 46 (90): 617–632 World Health Organization. Health Service data by region. Available at: http://apps.who.int/gho/data/view.main.1610?lang=en [Accessed 2 December, 2016].
UNICEF. Monitoring the Situation of Children and Women. Available at: http://data.unicef.org/maternal-health/delivery-care.html. [Accessed 2 December, 2016].
Nevertheless, the correlation between HepB-BD coverage and IDR or SBA rates have not been investigated globally or by WHO region. We analyzed global and regional data to assess the correlation between HepB-BD coverage, IDR, SBA rates, and other potential co-variates for all countries worldwide that include universal HepB-BD in national immunization schedules.
2. Methods
We compiled available individual country data on the latest IDR and SBA rates reported by each country [9,10]. Since the latest IDR and SBA rates for several countries were from 2014 or earlier, we used the 2014 WHO/UNICEF (WUENIC) estimates of HepB-BD vaccination coverage for comparison purposes [9]. We also compiled country data on total number of live births, hospital density per 100,000 population [9], total health expenditure per capita, adult literacy rates, and land mass [11].
To evaluate correlation between HepB-BD coverage and IDR and SBA rates, we calculated Spearman's rho correlation coefficients (rho) globally and for each WHO region. We also analyzed the number of live births in 2014, hospital density per 100,000 population, total health expenditure per capita in US dollars converted using 2013 purchasing power parity (THE-PPP) rates, adult literacy rates, and country land mass (in square kilometers), to explore correlation with HepB-BD coverage, IDR, and SBA rates. We used Spearman's rho because the distribution of each variable was statistically different from a normal distribution as indicated by the Shapiro-Wilk test performed on each variable. We excluded countries from the correlational analysis if HepB-BD coverage data were not available, if HepB-BD was not provided universally (e.g. maternal screening with targeted immunization or in health facilities only), if HepB-BD was introduced universally during 2014 or later, or if both IDR and SBA data were unavailable for a country. We conducted analyses using IBM SPSS for Windows version 22.0 (IBM Corp., Armonk, NY) and considered a two-tailed p-value of <0.05 statistically significant.
3. Results
Eighty-three (86%) of 96 countries that provided universal HepB-BD throughout 2014 were included in the analysis. Twelve (12%) countries (Australia, El Salvador, Estonia, Greece, Indonesia, Israel, Laos, Mauritania, Russian Federation, Saint Lucia, Spain and Vanuatu) were excluded because HepB-BD WUENIC coverage data were not available. Andorra was excluded because IDR and SBA data were not available. Of 83 countries analyzed, 2 (2%) were missing THE-PPP data, 8 (10%) were missing IDR, 21 (25%) were missing adult literacy data, and 29 (35%) were missing data on hospital density per 100,000 population.
Globally, significant positive correlations were observed between HepB-BD coverage and SBA rate (rho=0.44, p < 0.001), IDR (rho=0.42, p < 0.001), adult literacy rate (rho=0.37, p<0.001), hospital density (rho=0.33, p=0.02) and total health expenditure per capita (rho=0.24, p=0.03) (Figure 1). In contrast, a significant negative correlation was found between HepB-BD coverage and number of live births (rho= -0.27, p=0.01). IDR and SBA rates were highly correlated (rho=0.94, p < 0.001). In addition, adult literacy rates were positively correlated with SBA rates (rho=0.63, p < 0.001) and IDR (rho=0.52, p < 0.001) (Figure 2).
Figure 2. Correlations (rho) between hepatitis B vaccine birth dose coverage, institutional delivery, skilled birth attendance and other potential co-variates for 83 of 96 (86%) countries that provided universal HepB-BD in 2014.
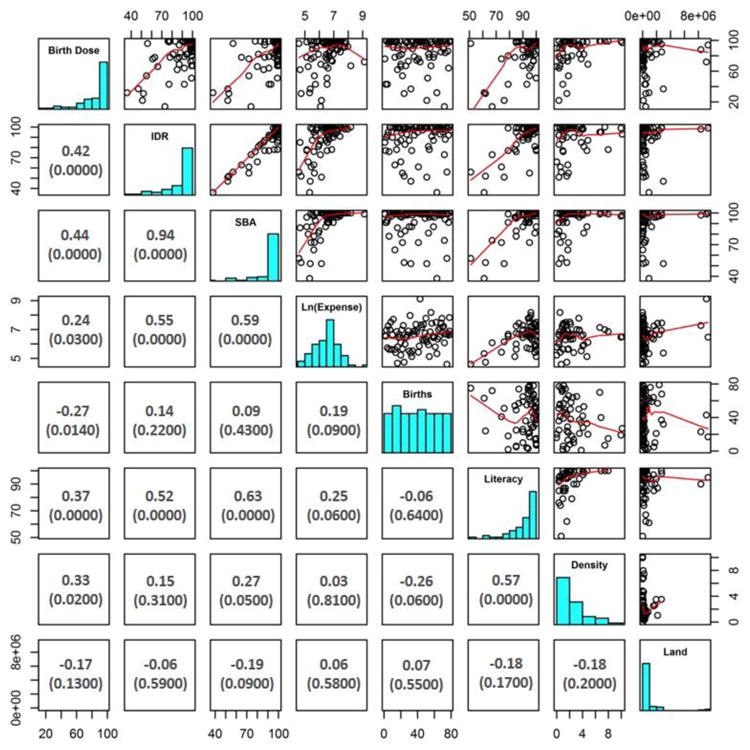
X and Y axes are percentages, except for Density (hospital density per 100,000 population) and Land (country land mass in square kilometers); Boxes to the left include Spearman's rho correlation between two variables (p-values are in parentheses); Boxes to the right include graphical representations of the correlations between two variables. Birth Dose, hepatitis B vaccine birth dose; IDR, institutional delivery rate; SBA, skilled birth attendance rate; Ln(Expense), natural log of total health expenditure per capita; Births, number of live births in 2014; Literacy, adult literacy rate
Significant positive correlations were observed between HepB-BD, IDR and SBA rates in the WHO African Region (IDR rho=0.89, p=0.04; SBA rho=0.89, p=0.04), the South-East Asia Region (IDR rho=0.92, p=0.03; SBA rho=0.92, p=0.03) and in the Western Pacific Region (IDR rho=0.75, p<0.001; SBA rho=0.61, p=0.002). Significant correlations were not observed in the Americas Region (IDR rho=0.41, p=0.15; SBA rho=0.37, p=0.18), the Eastern Mediterranean Region (IDR rho=0.35, p=0.24; SBA rho=0.42, p=0.13), or the European Region (IDR rho= -0.35, p=0.15; SBA rho= -0.21, p=0.34).
4. Discussion
In this paper we report significant positive correlations between HepB-BD coverage, institutional delivery rates and skilled birth attendance rates worldwide and in the WHO African, South-East Asia, and Western Pacific Regions. High rates of home births have been linked to low HepB-BD coverage in Cambodia, Indonesia, and China [5,6,7], and prevalence of chronic HBV infection was higher among children born at home compared to those born at large hospitals in China and Vietnam, indicating the importance of timely delivery of HepB-BD [12,13]. Since all WHO regions currently have hepatitis B control goals, many countries need either to introduce HepB-BD into the national immunization schedule or increase timely HepB-BD coverage to decrease the burden of chronic infection and meet their international commitments [14]. The experience of the Western Pacific Region, in particular, could be used to increase timely HepB-BD coverage in countries of the African and South-East Asia Regions that have low rates of institutional deliveries and/or of skilled birth attendance [15].
Increasing the IDR can contribute to an increase in HepB-BD coverage while simultaneously improving other maternal and neonatal health outcomes. Promoting institutional deliveries through provision of financial incentives and encouraging parents to deliver in health facilities in Cambodia led to an increase in timely HepB-BD coverage [15]. However, when countries encourage health facility births, they should also ensure that those facilities are staffed with skilled birth attendants to ensure adequate care and timely HepB-BD administration. Promotion of health facility birth in India, for example, did not lead to better care because SBA rates were low [16]. Training and supervision of hospital health care workers is important, as demonstrated in China and the Philippines [17,18]. Further, having standing orders can simplify the vaccination process and avoid confusion as to who is responsible for HepB-BD administration [15,17,18]. Provision of HepB-BD should be the responsibility of the person who delivers the baby to ensure timely administration [15,17]. Finally, vaccines should be readily available at the delivery wards to ensure timely administration [17,18].
To increase HepB-BD coverage among home births, a combination of strategies needs to be used depending on the context. First, educating parents on the importance of timely HepB-BD is important to ensure the infant receives the vaccine as soon as possible after birth [17]. Mothers can be educated during antenatal care visits and by community volunteers. In areas without skilled attendants for home births, training community volunteers to inform health care workers (HCW) about recent births can help increase timely HepB-BD [19,20]. In addition, having joint maternal and child health and Expanded Program on Immunization microplans for HepB-BD was shown to contribute to an increase in HepB-BD coverage in China [17].
In some settings, lack of a cold chain to store the HepB-BD can limit access to and provision of the vaccine, especially for home births. Hepatitis B vaccine from several manufacturers has been shown to be very heat stable and able to withstand exposure to temperatures as high as 37°C for up to one month without losing potency. The WHO Strategic Advisory Group of Experts (SAGE) supports use of monovalent hepatitis B vaccine outside the cold chain (OCC) [21]. Using HepB-BD OCC significantly increased timely HepB-BD coverage in China, Indonesia, Kiribati, Laos and Vietnam [6,15,22,23,24,25]. In addition, use of hepatitis B vaccine OCC in compact pre-filled auto-disabled injection devices (CPAD) in China, Indonesia, and Papua New Guinea improved timely HepB-BD coverage among home births and was widely accepted [15,23,24]. CPAD require minimal training, do not necessitate skilled HCW, and therefore the vaccine can be delivered by trained community volunteers.
Significant correlations between HepB-BD and IDR or SBA were not observed in the WHO Americas Region, Eastern Mediterranean Region and European Region. In these three regions, some countries had relatively low HepB-BD coverage despite nearly 100% IDR and SBA rates. This suggests alternative factors not captured in our analyses were adversely affecting HepB-BD coverage, such as vaccine hesitancy.
In addition to IDR and SBA rates, we found that adult literacy rate, hospital density, and total health expenditures per capita significantly correlated with HepB-BD coverage worldwide. Though not specific to HepB-BD coverage, prior studies have shown an association between immunization rates and these variables. A positive association between parental education level and vaccination has been previously reported [26]. Increased access to immunization services was shown to be associated with higher vaccination rates and might be related to the density of healthcare centers and hospitals [26]. Government spending on health per capita was previously correlated with three-dose diphtheria-pertussis-tetanus vaccine coverage in the WHO Eastern Mediterranean Region [27]. In our analysis, the number of live births negatively correlated with HepB-BD coverage; it is likely that higher birth rates overwhelm the capacity to provide timely HepB-BD in resource-poor settings. Family composition, including family size, have been linked to under-vaccination in previous studies [26].
There are several limitations to the analyses. Over 25% of countries lacked data on adult literacy rates and hospital density which might limit the interpretation of results. Missing data limited our ability to perform regional correlative analysis on variables other than HepB-BD, IDR and SBA rates. Also, more recent vaccine coverage data from 2016 or 2015 could not be included since the majority of country data on IDR and SBA were from 2014 or earlier. Further, the non-parametric nature of the data required use of non-parametric statistical testing, which cannot be used to explore partial correlations.
In conclusion, IDR and SBA rates have a significant positive correlation with HepB-BD coverage and should be targeted as part of national and regional strategies to meet hepatitis B control goals in settings that have low IDR and/or low SBA rates. Evidence-based strategies [4] that incorporate promoting institutional deliveries, providing proper training and supervision of HCW, increasing community awareness of the need for HepB-BD and using innovative vaccine delivery approaches in areas that lack a cold chain and skilled birthing attendants could increase timely HepB-BD and significantly reduce HBV-related morbidity and mortality worldwide.
Acknowledgments
Kathleen Wannemuehler (Statistician) and Sarah Pallas (Health Economist), Centers for Disease Control and Prevention
Funding: The work was supported by the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: All authors declare that they have no conflicts of interest.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the position of the Centers for Disease Control and Prevention
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–19. [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global routine vaccination coverage, 2014. Wkly Epidemiol Rec. 2015;46(90):617–32. [PubMed] [Google Scholar]
- 4.World Health Organization. Preventing perinatal hepatitis b virus transmission: a guide for introducing and strengthening hepatitis B birth dose vaccination. WHO; 2015. [Accessed 13 April 2017]. available at: http://apps.who.int/iris/bitstream/10665/208278/1/9789241509831_eng.pdf. [Google Scholar]
- 5.Mao B, Patel MK, Hennessey K, Duncan RJW, Wannemuehler K, Soeung SC. Prevalence of chronic hepatitis B virus infection after implementation of a hepatitis B vaccination program among children in three provinces in Cambodia. Vaccine. 2013;31:4459–64. doi: 10.1016/j.vaccine.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creati M, Saleh A, Ruff TA, Stewart T, Otto B, Sutanto A, Clements CJ. Implementing the birth dose of hepatitis B vaccine in rural Indonesia. Vaccine. 2007;25:5985–93. doi: 10.1016/j.vaccine.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Wang H, Zheng J, Zhu X, Xia W, Hipgrave DB. Coverage of and influences on timely administration of hepatitis B vaccine birth dose in remote rural areas of the People's Republic of China. Am J Trop Med Hyg. 2009;81:869–74. doi: 10.4269/ajtmh.2009.09-0238. [DOI] [PubMed] [Google Scholar]
- 8.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Health Service data by region. [Accessed 2 December 2016]; Available at: http://apps.who.int/gho/data/view.main.1610?lang=en.
- 10.UNICEF. Monitoring the Situation of Children and Women. [Accessed on 2 December, 2016]; Available at: http://data.unicef.org/maternal-health/delivery-care.html.
- 11.The World Bank. [Accessed on 4 December 2016]; Available at: http://data.worldbank.org/indicator.
- 12.Cui F, Li L, Hadler S, Wang F, Zheng H, Chen Y, et al. Factors associated with effectiveness of the first dose of hepatitis B vaccine in China: 1992–2005. Vaccine. 2010;28:5973–78. doi: 10.1016/j.vaccine.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TH, Vu MH, Nguyen VC, Nguyen LH, Toda K, Nguyen TN, et al. a reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32:217–22. doi: 10.1016/j.vaccine.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison RD, Teleb N, Al Awaidy S, Ashmony H, Alexander JP, Patel MK. Hepatitis B control among children in the Eastern Mediterranean Region of the World Health Organization. Vaccine. 2016;34(21):2403–9. doi: 10.1016/j.vaccine.2016.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzano-Mariano KM, Diostista S. Hepatitis B control in the World Health Organization's Western Pacific Region: targets, strategies, status. Vaccine. 2013;31S:J85–92. doi: 10.1016/j.vaccine.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi S, De Costa A, Raven J. Does the Janani Suraksha Yojana cash transfer programme to promote facility births in India ensure skilled birth attendance? A qualitative study of intrapartum care in Madhya Pradesh. Glob Health Action. 2015;8:27427. doi: 10.3402/gha.v8.27427. http://dx.doi.org/10.3402/gha.v8.27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutin Y, Hennessey K, Cairns L, Zhang Y, Li H, Zhao L, et al. Improving hepatitis B vaccine timely birth dose coverage: Lessons from five demonstration projects in China, 2005-2009. Vaccine. 2013;31S:J49–55. doi: 10.1016/j.vaccine.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Sobel HL, Mantaring JB, 3rd, Cuevas F, Ducusin JV, Thorley M, Hennessey KA, et al. Implementing a national policy for hepatitis B birth dose vaccination in Philippines: lessons for improved delivery. Vaccine. 2011;29:941–5. doi: 10.1016/j.vaccine.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Xeuatvongsa A, Datta SS, Moturi E, Wannemuehler K, Philakong P, Vongxay V, et al. Improving hepatitis B birth dose in rural Lao People's Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016;34:5777–84. doi: 10.1016/j.vaccine.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami H, Cuong NV, Huynh L, Hipgrave DB. Implementation of and costs associated with providing a birth dose of hepatitis B vaccine in Viet Nam. Vaccine. 2008;26:1411–19. doi: 10.1016/j.vaccine.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016 – conclusions and recommendations. Wkly Epidemiol Rec. 2016;91(48):561–82. [PubMed] [Google Scholar]
- 22.Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74:255–60. [PubMed] [Google Scholar]
- 23.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out of cold chain delivery strategy. Bull World Health Organ. 2007;85:688–694. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77:119–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, Wannemuehler K, Vongxay V, Vilayvone V, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine. 2016;34:3324–30. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29(46):8215–21. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 27.de Figueiredo A, Johnston IG, Smith DM, Agarwal S, Larson HJ, Jones NS. Forecasted trends in vaccination coverage and correlations with socioeconomic factors: a global time-series analysis over 30 years. Lancet Glob Health. 2016;4(10):e726–35. doi: 10.1016/S2214-109X(16)30167-X. [DOI] [PubMed] [Google Scholar]