Key Clinical Message
Mosaicism, an important cause for recurrent T21, should be suspected in families with more than one affected child wishing to receive prenatal counseling. Fluorescence in‐situ hybridization analysis in a large number of cells and in different tissue samples is critical for detecting low‐level mosaicism and is a key prognostic factor.
Keywords: Aneuploidy, Down Syndrome, gonadal, mosaicism, Trisomy 21
As Trisomy 21 (T21) is the most common genetic disorder in the human population, it has been intensively studied. Although the recurrence risk for Down Syndrome (DS) in phenotypically normal young parents is estimated to be 1–2% 1, multiple cases of T21 may be observed. Several hypotheses have been proposed to explain this recurrence risk: (1) parental gonadal mosaicism; (2) chance alone as a consequence of maternal age‐associated risk; (3) genetic predisposition to nondisjunction 2, 3. Parental mosaicism, the most frequently reported mechanism, and gonadal mosaicism, specifically, is the most likely one associated with recurrent homotrisomy in the same couple. The majority of the remaining cases are thought to be due to chance 2.
We report a case of a healthy 27‐year‐old woman, gravida 5, para 2, who was referred to our Prenatal Diagnosis Unit during her third pregnancy, at 13 weeks of gestation (WG). Her first two children were from another partner, a consanguineous relationship: The first child is a healthy girl and the second one is a male child with postnatal diagnosis of DS with a free T21. The current partner is healthy and unrelated, and the family history was unremarkable.
In her third pregnancy, the first trimester screen was positive (free beta‐hCG levels increased), with an increased risk of one in 33 of having a child with DS. Chromosome analysis of chorionic villus sample (CVS) revealed a male fetus with T21. The pregnancy was medically interrupted and free T21 was confirmed in fetal cells. A few months later she got pregnant again and CVS was performed at 12 WG due to the obstetrical antecedents, as there were no anomalies detected in the first trimester scan. The pregnancy ended in a miscarriage at 16 WG, sonographically 13 WG. The cytogenetic analysis of CVS was scored in 30 metaphases with G‐banding pattern and the karyotype was normal (46,XX). However, minor phenotypic features of DS were described in anatomopathological examination of this fetus, including bilateral 5th digit clinodactyly and right clubfoot, raising the possibility of a T21 mosaic.
In her fifth pregnancy, the ultrasound examination showed a fetal nuchal translucency thickness above the 99th centile (3.7 mm) and the karyotype on CVS confirmed the diagnosis of another male fetus with T21. Conventional karyotype analysis of peripheral lymphocytes, by G‐banding, was normal for the couple. However, fluorescence in‐situ hybridization (FISH) analysis in interphase and metaphase maternal blood cells showed only one cell with T21 in the 230 cells analyzed (Fig. 1). To confirm the suspicion of maternal T21 mosaicism, another a sample from the oral mucosa was studied by FISH, revealing 97 normal cells and three T21 cells (Fig. 2). FISH analysis in paternal cells was normal. Genetic counseling was performed; due to a high risk of DS recurrence this couple is now coming for preimplantation genetic diagnosis 4.
Figure 1.
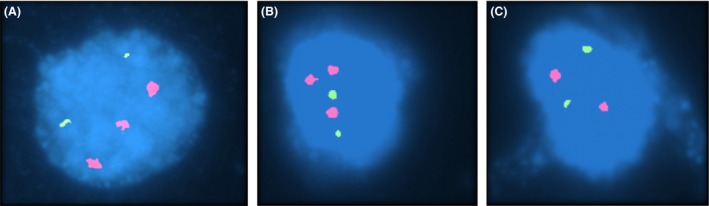
FISH in oral mucosa cells with probes for chromosome 13 (Vysis LSI 13) in green and for chromosome 21 (Vysis LSI 21) in red from Aneuvysion (Vysis), showing two interphases with trisomy 21 (A and B) and one normal interphase with only two chromosomes 21 (C).
Figure 2.
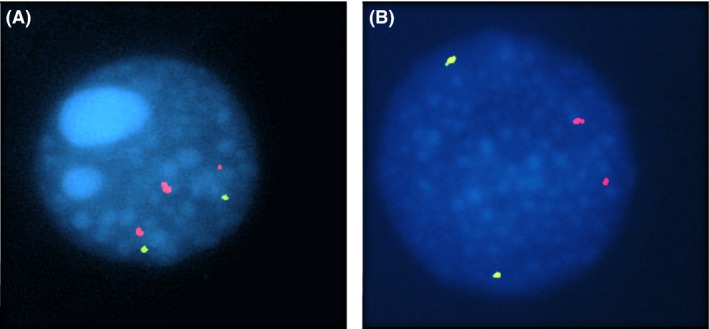
FISH in peripheral blood lymphocytes with probes for chromosome 13 (Vysis LSI 13) in green and for chromosome 21 (Vysis LSI 21) in red from Aneuvysion (Vysis), showing one interphase with trisomy 21 (A) and one normal interphase with only two chromosomes 21 (B).
This woman, with an unexpected high recurrence risk, experienced three T21 pregnancies and a miscarriage. Understanding the mechanism that gives rise to more than one child being affected with DS is necessary to estimate the risk of recurrence. In our case, with three free T21, the major cytogenetic variants related are parental mosaicism (errors occurring during the first divisions of the zygote), genetic predisposition for meiotic nondisjunction or by chance, including postzygotic errors during the first division of the zygote 5. Other possible reasons for recurrence are parental carriers of Robertsonian translocations and other structural rearrangements of the chromosomes (isochromosomes of the long arm, partial trisomy of the region 21q22.3) 5.
Some studies have emphasized the importance of T21 mosaicism involving germinal and somatic lines in asymptomatic carriers 6. While the incidence of parental T21 mosaicism is only discovered in around 2.7–4.3% of families with only one child with DS, the incidence is higher in families with recurrent T21 as evaluated by DNA polymorphism analysis 7, 8. More recent studies indicate that gonadal mosaicism (germinal line mosaicism) may be more common than previously believed, possibly a general constitutional characteristic of our species, and may be the main predisposing factor for the maternal origin of T21 6, 9.
Gonadal T21 mosaicism is rarely documented directly, bearing in mind the need for ovarian biopsies or germ cells 6. The detection of somatic mosaicism would depend on the proportion of mosaic cells, the tissues studied and the number of cells counted 10. With a suspicion of mosaicism, it is recommended to search for the trisomic line in at least two different tissue samples 5. Oral mucosa cells are a noninvasive alternative and deliver a higher diagnostic yield compared with blood‐derived DNA 11. Fetal oogonial/oocyte T21 mosaicism is regarded as the most probable explanation for the increased recurrence risk in younger women 9. It has been documented that such women may, although not necessarily, show somatic T21 mosaicism as well 9.
In our description, all cases of T21 were male fetuses, the only living normal child is a girl and the miscarriage with normal karyotype was also a female. Although well known, the male prevalence in DS patients with nonmosaic T21 is currently a poorly explained phenomenon 1. The literature suggests that females may have gonadal mosaicism more frequently than males, which may be explained by a sex‐specific chromosome loss in early embryogenesis, with a female‐specific trisomy rescue 12.
The DS phenotype varies as a function of the proportion of trisomic cells present in different tissues. It is well‐defined that some people with minimal or even without any obvious signs of DS are low‐level T21 mosaics (<3–5% of cells affected) 6. Nowadays, there is only one available approach for the detection of low‐level/cryptic mosaicism, as exemplified in our case, involving FISH technology with chromosome‐specific probes on large cell populations from different tissue samples 3. The conventional G‐banding analysis that was used for the evaluation of the karyotype of miscarried fetus, with minor phenotypic features of DS, may not have detected low‐level mosaicism and it may be a case of underdiagnosis.
In conclusion, a thorough cytogenetic study of both parents is recommended after at least two pregnancies with free T21. Genetic counseling should reflect the increased recurrence risk caused by the possible existence of undetected parental mosaicism for T21. As an important cause of recurrent T21, gonadal mosaicism, typically with maternal origin, should be strongly suspected in families with more than one affected child. Although we are in the molecular era, with high‐resolution diagnosis devices, care should be taken not to overlook the fact that the level of mosaicism influences the interpretation of data and can lead to misdiagnosis. Therefore, FISH analysis in a large number of cells in different tissue samples, like blood and oral mucosa cell, is critical for detecting low‐level mosaicism, which may be missed by conventional cytogenetic alone, and is a key prognostic factor.
Authorship
MM: summarized case information and drafted the manuscript; review of the literature. CM: reviewed the literature and drafted the manuscript. FR: was responsible for the genetic evaluation of the cases described and genetic counseling of the couple. AJ: was coresponsible for the laboratory evaluation of the cases described. SF: was responsible for the ultrasounds performed. FC: was coresponsible for the obstetric counseling of the couple and participated in the follow‐up of the patient. IC: was coresponsible for the laboratory evaluation of the cases described. PM: coordinated the case and was coresponsible for the obstetric counseling of the couple. All the authors participated in the manuscript revision process, read, and approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
The research was carried out at the Centro Hospitalar e Universitário de Coimbra.
References
- 1. Kovaleva, N. V. 2010. Germ‐line transmission of trisomy 21: data from 80 families suggest an implication of grandmaternal age and a high frequency of female‐specific trisomy rescue. Mol. Cytogenet. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruyere, H. , Rupps R., Kuchinka B. D., Friedman J. M., and Robinson W. P.. 2000. Recurrent trisomy 21 in a couple with a child presenting trisomy 21 mosaicism and maternal uniparental disomy for chromosome 21 in the euploid cell line. Am. J. Med. Genet. 94:35–41. [DOI] [PubMed] [Google Scholar]
- 3. Warburton, D. , Dallaire L., Thangavelu M., Ross L., Levin B., and Kline J.. 2004. Trisomy recurrence: a reconsideration based on North American data. Am. J. Hum. Genet. 75:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iourov, I. Y. , Vorsanova S. G., and Yurov Y. B.. 2006. Intercellular genomic (chromosomal) variations resulting in somatic mosaicism: mechanisms and consequences. Curr. Genomics 7:435–446. [Google Scholar]
- 5. Garduño‐Zarazúa, L. M. , Alois L. G., Kofman‐Epstein S., and Peredo A. B. C.. 2013. Prevalence of mosaicism for trisomy 21 and cytogenetic variant analysis in patients with clinical diagnosis of Down syndrome: a 24‐year review (1986–2010) at the Servicio de Genética, Hospital General de México “Dr. Eduardo Liceaga”. Bol. Med. Hosp. Infant. Mex. 70:29–34. [Google Scholar]
- 6. Hulten, M. A. , Jonasson J., Nordgren A., and Iwarsson E.. 2010. Germinal and somatic trisomy 21 mosaicism: how common is it, what are the implications for individual carriers and how does it come about? Curr. Genomics 11:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachs, E. S. , Jahoda M. G., Los F. J., Pijpers L., and Wladimiroff J. W.. 1990. Trisomy 21 mosaicism in gonads with unexpectedly high recurrence risks. Am. J. Med. Genet. Suppl. 7:186–188. [DOI] [PubMed] [Google Scholar]
- 8. Tseng, L. H. , Chuang S. M., Lee T. Y., and Ko T. M.. 1994. Recurrent Down's syndrome due to maternal ovarian trisomy 21 mosaicism. Arch. Gynecol. Obstet. 255:213–216. [DOI] [PubMed] [Google Scholar]
- 9. Hulten, M. A. , Oijerstedt L., Iwarsson E., and Jonasson J.. 2014. Maternal germinal trisomy 21 in Down syndrome. J. Clin. Med. 3:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris, D. J. , Begleiter M. L., Chamberlin J., Hankins L., and Magenis R. E.. 1982. Parental trisomy 21 mosaicism. Am. J. Hum. Genet. 34:125–133. [PMC free article] [PubMed] [Google Scholar]
- 11. Sdano, M. R. , Vanzo R. J., Martin M. M., Baldwin E. E., South S. T., Rope A. F., et al. 2014. Clinical utility of chromosomal microarray analysis of DNA from buccal cells: detection of mosaicism in three patients. J. Genet. Couns. 23:922–927. [DOI] [PubMed] [Google Scholar]
- 12. Kovaleva, N. V. 2005. Sex‐specific chromosome instability in early human development. Am. J. Med. Genet. A 136A:401–413. [DOI] [PubMed] [Google Scholar]


