Abstract
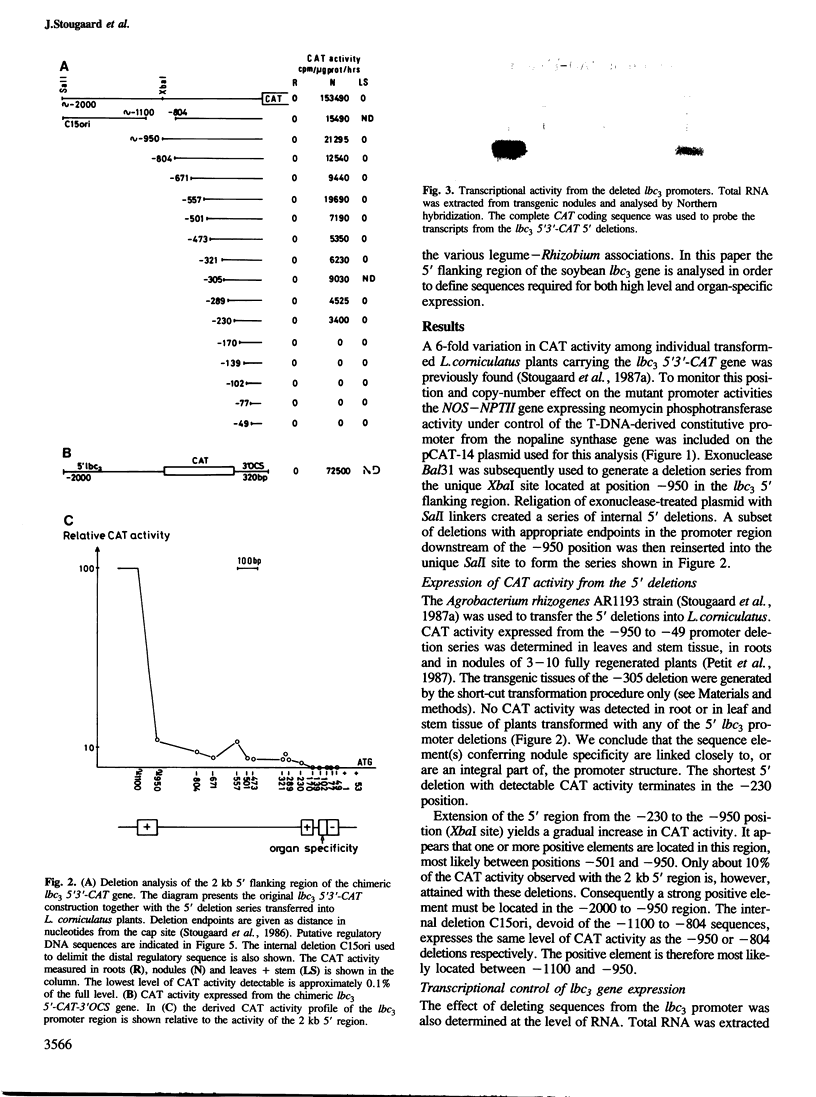
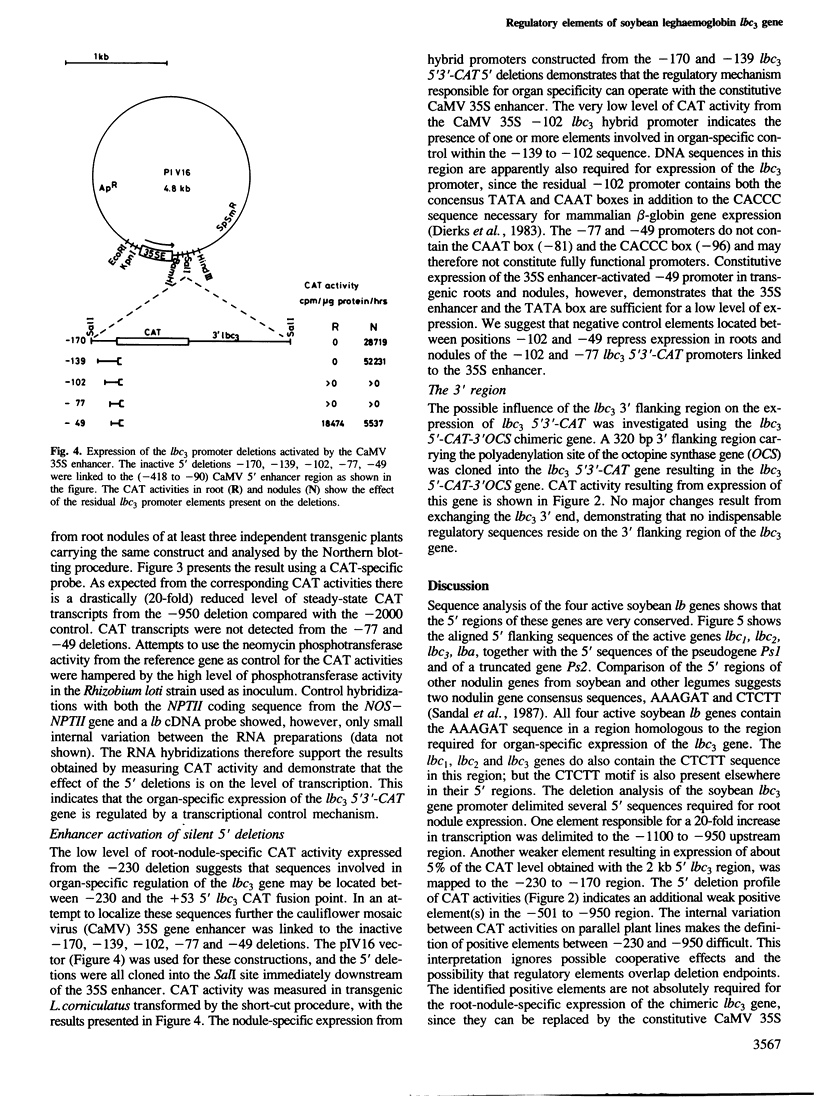
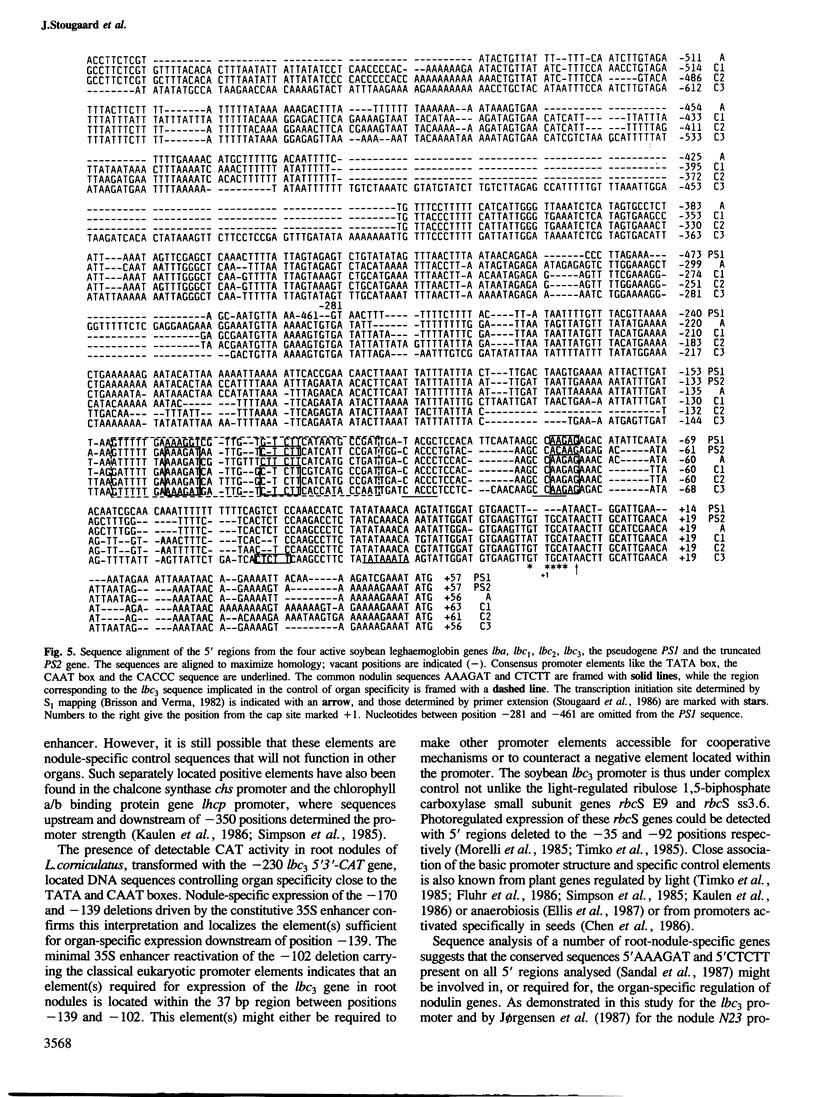
The soybean leghaemoglobin lbc3 gene promoter was analysed in transgenic Lotus corniculatus plants. Hybrid-promoter constructions and 5' deletions were studied using chimeric genes composed of the various promoters, the chloramphenicol acetyltransferase (CAT) coding sequence and the lbc3 3' flanking region. A 5' Bal31 deletion series mapped a strong positive regulatory element between −1100 and −950. A weaker element located between −230 and −170 defined the minimum 5' region required for detectable promoter activity. Reactivation of inactive promoters with deletion endpoints between −230 and the transcription initiation site was obtained employing the constitutive cauliflower mosaic virus (CaMV) 35S enhancer. The position of cis regulatory element(s) required for nodule-specific expression was defined to 37 bp between −139 and −102. This region contains sequences conserved in other leghaemoglobin and nodulin genes. No indispensable control elements were found on the lbc3 3' flanking region.
Keywords: promoter elements, Bal31 deletions, hybrid promoters, root nodule expression, transgenic legumes
Full text
PDF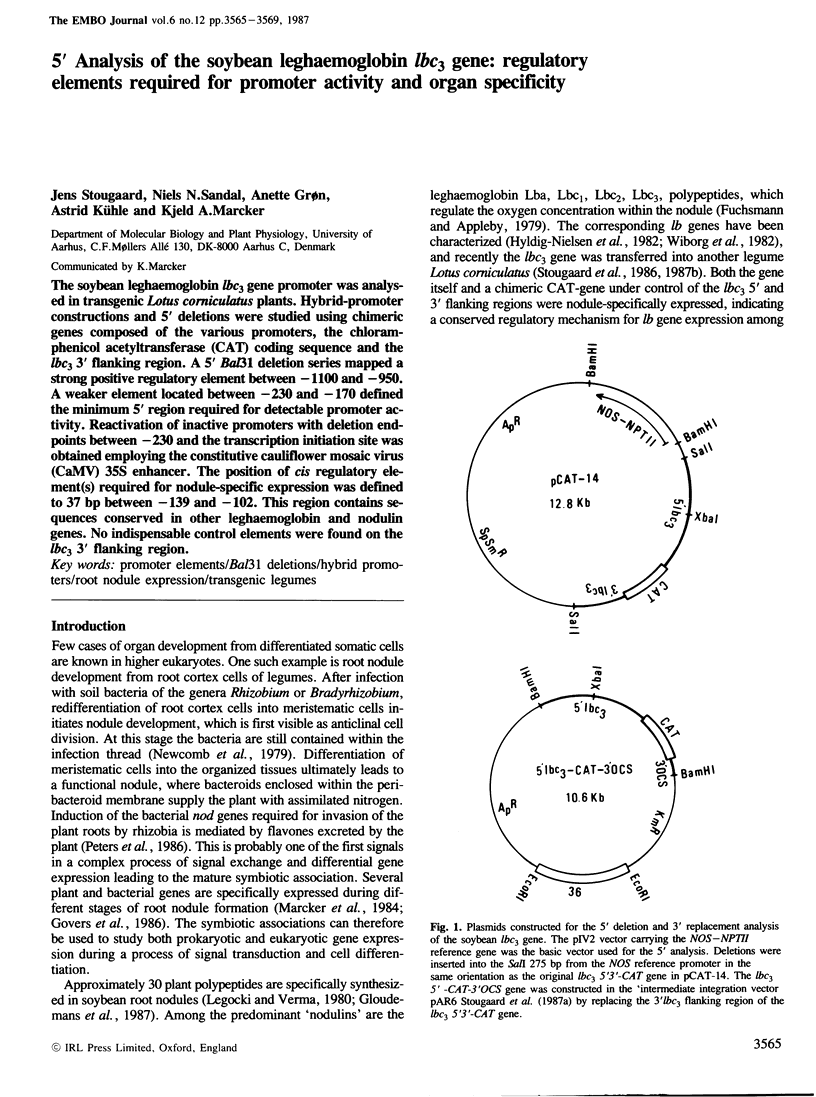

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987 Jan;6(1):11–16. doi: 10.1002/j.1460-2075.1987.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979 Aug 28;579(2):314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Gielen J., De Beuckeleer M., Seurinck J., Deboeck F., De Greve H., Lemmers M., Van Montagu M., Schell J. The complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J. 1984 Apr;3(4):835–846. doi: 10.1002/j.1460-2075.1984.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella L., Block M. D., Messens E., Hernalsteens J. P., Montagu M. V., Schell J. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 1983;2(6):987–995. doi: 10.1002/j.1460-2075.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Wiborg O., Garrett R., Jørgensen P., Marcker K. A. The primary structures of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jan 22;10(2):689–701. doi: 10.1093/nar/10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. O., Marcker K. A., Villadsen I. S. Heme regulates the expression in Saccharomyces cerevisiae of chimaeric genes containing 5'-flanking soybean leghemoglobin sequences. EMBO J. 1986 May;5(5):843–847. doi: 10.1002/j.1460-2075.1986.tb04293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Marcker A., Lund M., Jensen E. Ø, Marcker K. A. Transcription of the soybean leghemoglobin genes during nodule development. EMBO J. 1984 Aug;3(8):1691–1695. doi: 10.1002/j.1460-2075.1984.tb02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Sandal N. N., Bojsen K., Marcker K. A. A small family of nodule specific genes from soybean. Nucleic Acids Res. 1987 Feb 25;15(4):1507–1519. doi: 10.1093/nar/15.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., Timko M. P., Cashmore A. R., Schell J., Montagu M. V., Herrera-Estrella L. Light-inducible and tissue-specific expression of a chimaeric gene under control of the 5'-flanking sequence of a pea chlorophyll a/b-binding protein gene. EMBO J. 1985 Nov;4(11):2723–2729. doi: 10.1002/j.1460-2075.1985.tb03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stougaard J., Petersen T. E., Marcker K. A. Expression of a complete soybean leghemoglobin gene in root nodules of transgenic Lotus corniculatus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5754–5757. doi: 10.1073/pnas.84.16.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]




