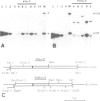
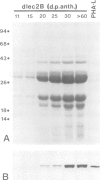
Abstract
Using Agrobacterium-mediated transformation, two genes for phytohemagglutinin-L (PHA-L), the lectin seed protein of the common bean Phaseolus vulgaris, were stably integrated into the tobacco genome. The two alleles for PHA-L, dlec2 and pdlec2, were obtained from a normal cultivar (Greensleeves) and a lectin-deficient cultivar (Pinto) respectively. In the bean embryos, the expression of dlec2 is 30 times greater than the expression of pdlec2. In the dlec2-transformed tobacco, PHA-L accumulated specifically in the seeds at the same stages as the tobacco seed storage proteins and was degraded after germination. PHA-L was found in the embryo, and at a 5–7 times lower concentration in the endosperm tissue of the mature tobacco seeds. No PHA could be detected in other parts of the plants. We conclude that the signals for temporal and spatial regulation of the dlec2 gene are present in the DNA fragment used for transformation. Transformation with the second PHA-L allele pdlec2 from the cultivar Pinto caused the accumulation of about 50 times less PHA-L in tobacco seeds when compared to dlec2. We conclude from analyzing the 5' sequences of dlec2 and Pdlec2 that the low expression phenotype of the Pdlec2 allele could be due to the absence or mutation of a cis-acting element carried by the dlec2 fragment.
Keywords: phytohemagglutinin genes, Phaseolus vulgaris, transgenic tobacco, promoter analysis
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J., Chrispeels M. J. Transcriptional and Posttranscriptional Control of Phaseolin and Phytohemagglutinin Gene Expression in Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1986 May;81(1):50–54. doi: 10.1104/pp.81.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Vitale A., Staswick P. Gene Expression and Synthesis of Phytohemagglutinin in the Embryonic Axes of Developing Phaseolus vulgaris Seeds. Plant Physiol. 1984 Nov;76(3):791–796. doi: 10.1104/pp.76.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Hain R., Herrera-Estrella L., Lörz H., Goyvaerts E., Baker B. J., Schell J. Fate of selectable marker DNA integrated into the genome of Nicotiana tabacum. DNA. 1986 Apr;5(2):101–113. doi: 10.1089/dna.1986.5.101. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Leavitt R. D., Bachur N. R. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim Biophys Acta. 1975 Sep 9;405(1):72–81. doi: 10.1016/0005-2795(75)90316-5. [DOI] [PubMed] [Google Scholar]
- Greenwood J. S., Chrispeels M. J. Correct targeting of the bean storage protein phaseolin in the seeds of transformed tobacco. Plant Physiol. 1985 Sep;79(1):65–71. doi: 10.1104/pp.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leavitt R. D., Felsted R. L., Bachur N. R. Biological and biochemical properties of Phaseolus vulgaris isolectins. J Biol Chem. 1977 May 10;252(9):2961–2966. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro J. K., Jofuku K. D., Goldberg R. B. Soybean seed lectin gene and flanking nonseed protein genes are developmentally regulated in transformed tobacco plants. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8240–8244. doi: 10.1073/pnas.83.21.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Hurrell J. G., Leach S. J. Elimination of nonspecific adsorption of serum proteins by Sepharose-bound antigens. Anal Biochem. 1978 Jul 1;87(2):299–305. doi: 10.1016/0003-2697(78)90679-6. [DOI] [PubMed] [Google Scholar]
- Staswick P., Chrispeels M. J. Expression of lectin genes during seed development in normal and phytohemagglutinin-deficient cultivars of Phaseolus vulgaris. J Mol Appl Genet. 1984;2(6):525–535. [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Florkiewicz R. Z., Chrispeels M. J. Secretion of phytohemagglutinin by monkey COS cells. Eur J Cell Biol. 1986 Dec;42(2):218–223. [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]