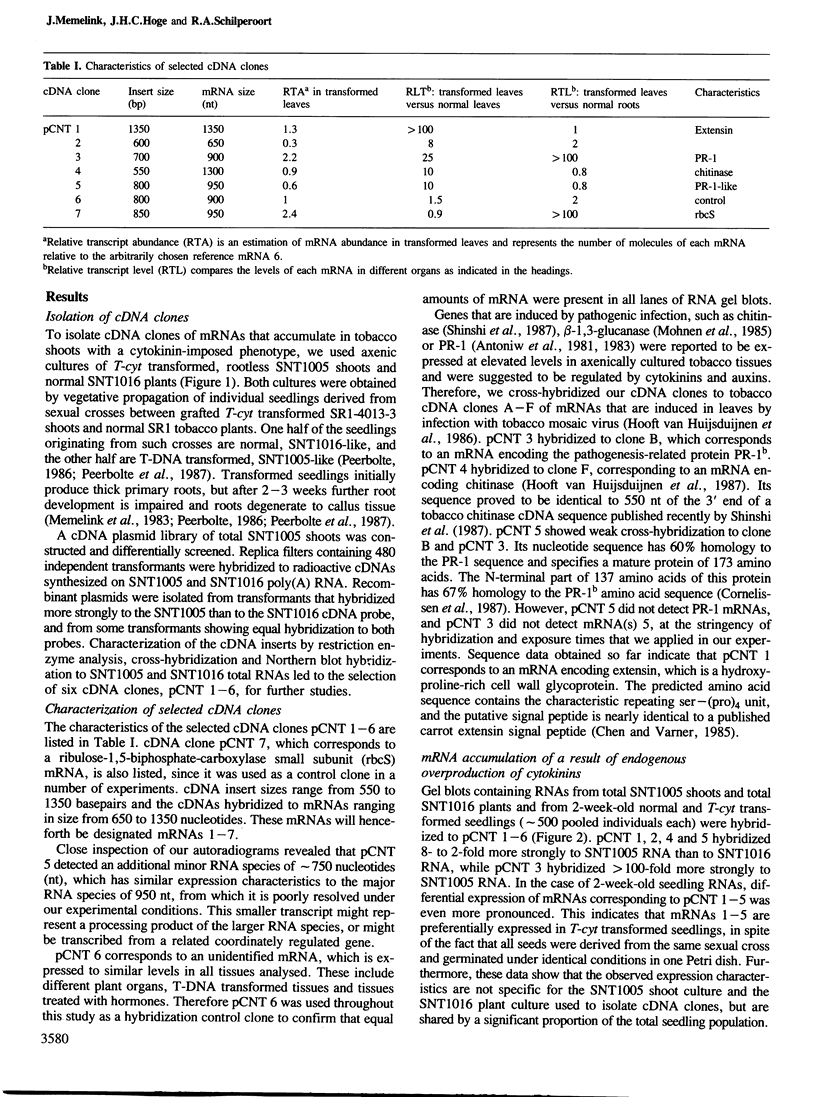
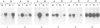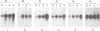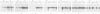Abstract
Tobacco shoots exposed to elevated endogenous or exogenous cytokinin levels are unable to develop roots and lack apical dominance. We have isolated cDNA copies of five mRNA species that accumulate to elevated levels in such cytokinin-stressed shoots via differential screening of a cDNA library of transgenic shoots which contain an active T-DNA cytokinin gene (T-cyt gene) from Agrobacterium tumefaciens. Four of the cDNA clones were found to correspond to plant defence-related mRNAs, encoding extensin, chitinase, PR-1 and a PR-1-like protein, respectively. In normal tobacco plants PR-1 mRNA is relatively rare in all organs. The other four mRNAs occur at relatively low levels in shoots, especially in leaves, but are very prevalent in roots. Extensin mRNA, for example, is not detectable in leaves, while it is an abundant mRNA in roots and stems. In normal shoots cultured on cytokinin-containing medium all five mRNAs accumulate to elevated levels, similar to those found in transgenic T-cyt shoots. We conclude that the imposed cytokinin stress causes changes in the tissue-specific control of the levels of several defence-related mRNA species in tobacco.
Keywords: cytokinins, defence-related mRNAs, gene regulation, T-DNA, tobacco
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aké S., Péaud-Lenoël C. 5,6-Dichlorobenzimidazole-1-beta-D-riboside, specific inhibitor of cytokinin activity in tobacco cell suspension cultures. Biochimie. 1985 Jan;67(1):155–160. doi: 10.1016/s0300-9084(85)80243-1. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Horowitz J., van Kan J. A., Goldberg R. B., Bol J. F. Structure of tobacco genes encoding pathogenesis-related proteins from the PR-1 group. Nucleic Acids Res. 1987 Sep 11;15(17):6799–6811. doi: 10.1093/nar/15.17.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., van Loon L. C., Bol J. F. cDNA cloning of six mRNAs induced by TMV infection of tobacco and a characterization of their translation products. EMBO J. 1986 Sep;5(9):2057–2061. doi: 10.1002/j.1460-2075.1986.tb04466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Uhlén M., Josephson S., Gatenbeck S., Philipson L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983 Nov 25;11(22):8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]