Abstract
Objectives
The authors describe associations between dental fluorosis and fluoride intakes, with an emphasis on intake from fluoride in infant formula.
Methods
The authors administered periodic questionnaires to parents to assess early fluoride intake sources from beverages, selected foods, dentifrice and supplements. They assessed relationships between fluorosis of the permanent maxillary incisors and fluoride intake from beverages and other sources, both for individual time points and cumulatively using area-under-the-curve (AUC) estimates. The authors determined effects associated with fluoride in reconstituted powdered infant formulas, along with risks associated with intake of fluoride from dentifrice and other sources.
Results
Considering only fluoride intake from age 3 to 9 months, the authors found that participants with fluorosis (97 percent of which was mild) had significantly greater cumulative fluoride intake (AUC) from reconstituted powdered infant formula, other beverages with added water or a combination of these than did those without fluorosis. For participants aged 16 to 36 months, participants with fluorosis had significantly higher fluoride intake from water by itself, dentifrice or a combination of these than did those without fluorosis. In a model combining both the 3- to 9-month and 16- to 36-months age groups, the significant variables were fluoride intake from reconstituted powder concentrate formula (by participants aged 3–9 months), other beverages with added water (also by participants aged 3–9 months) and dentifrice (by participants aged 16–36 months).
Conclusions
Greater fluoride intakes from reconstituted powdered formulas (when participants were aged 3–9 months) and other water-added beverages (when participants were aged 3–9 months) increased fluorosis risk as did higher dentifrice intake by participants when aged 16 to 36 months.
Clinical Implications
Results suggest that prevalence of mild dental fluorosis could be reduced by avoiding ingestion of large quantities of fluoride from reconstituted powdered concentrate infant formula and fluoridated dentifrice.
Keywords: Dental fluorosis, fluoride, incisors, infant formulas, beverages, dentifrice
Dental fluorosis mostly involves visual changes in enamel opacity caused by hypomineralization associated with fluoride ingested during tooth development.1 Fluorosis occurs in both primary and permanent teeth; effects typically are esthetic, but severe fluorosis can affect tooth structure. The prevalence of mild fluorosis in the permanent dentition among North American children in areas with fluoridated water increased from about 10 to 15 percent in the early 1940s (prior to the introduction of community water fluoridation, fluoridated dentifrice and other fluoridated products) to as high as 50 to 60 percent in the 1980s, and was reported to be about 40 to 48 percent among U.S. schoolchildren in the 1990s and early 2000s.2–6 The mechanisms by which fluoride modifies tooth development are not fully understood, but it appears that alterations in protein metabolism disrupt crystal organization in the developing tooth.7–8 Timing is critical; excess fluoride must be present during tooth development to affect enamel mineralization. The critical period for development of fluorosis in permanent maxillary central incisors, the most prominent teeth esthetically, is during the period from birth through age 4 years, in particular the first 24 to 30 months of life.9–13
Fluoride can be ingested from both dietary and nondietary sources. The primary source of dietary fluoride is water.2 Fluoride is naturally present in variable concentrations in ground water and is added to municipal waters for the purpose of dental caries prevention. Fluoridated water used to prepare foods and beverages provides fluoride in addition to that already present in the food or beverage. Most foods and beverages without added water provide minimal fluoride. The primary sources of nondietary fluoride are oral health products aimed at caries prevention, such as dentifrices, mouthrinses and gels. Dietary fluoride supplements are an additional source of intake for infants and young children. Investigators in previous studies have noted that intake of fluoride from fluoridated water, infant formulas, dietary fluoride supplements and fluoridated dentifrices contribute to fluorosis.14–23 However, some of these studies were conducted either prior to the infant formula industry’s voluntary reduction of fluoride in its products in 1979 or before secular dietary changes that resulted in increased consumption of alternative beverages (such as 100 percent juice and soda pop) by infants and young children.24
A 2006 National Research Council report25 restated concern that some U.S. infants could receive too much fluoride from infant formula reconstituted with fluoridated water. Soon thereafter, the American Dental Association26 (ADA) and U.S. Centers for Disease Control and Prevention27 (CDC) made interim statements suggesting that concerned parents of infants receiving substantial quantities of infant formula reconstituted with fluoridated water might want to be cautious about a possible increase in risk of fluorosis, but both called for additional research. Authors of a systematic review published in The Journal of the American Dental Association in 200928 concluded that, despite substantial heterogeneity and methodological limitations among the studies included in their review, consumption of infant formula was associated with increased risk, on average, of at least some detectable level of enamel fluorosis. However, the authors acknowledged many weaknesses in the data, and thus their conclusion should not be considered definitive.
We previously have reported that beverages consumed during infancy, in particular infant formulas prepared with fluoridated water, increase the risk of fluorosis in primary teeth.29 On the basis of these findings and those reported in the published literature, we hypothesized that higher beverage fluoride intake increases the risk of dental fluorosis in permanent teeth. Thus, our objectives in this article are to describe associations between fluorosis of the permanent maxillary incisors and intakes of fluoride from beverages consumed during infancy and early childhood and dentifrice ingested during early childhood, and to estimate risks associated with using substantial amounts of powdered infant formula reconstituted with fluoridated water.
METHODS
Participants
We enrolled participants in the Iowa Fluoride Study (IFS), a longitudinal investigation of dietary and nondietary fluoride exposures, dental fluorosis and dental caries.6,12–14,29–43 Research staff recruited mothers of newborn infants from eight Iowa hospital postpartum wards between 1992 and 1995 for their children’s participation. The convenience sample generally was representative of Iowa newborns. The institutional review board at the University of Iowa, Iowa City, approved all components of the IFS. We obtained written informed consent from mothers at recruitment and at the time of the dental examination portion of the IFS; we obtained assent from children at the time of examination (n = 630). We included in these analyses only the participants who had had dental examinations.
Data collection
We mailed IFS questionnaires to parents at regular intervals. Children underwent dental examinations in the General Clinical Research Center at the University of Iowa, Iowa City, or at a community site. Dental fluorosis examinations were visual and were conducted by one of two trained examiners (one of whom was J.J.W.) using calibrated techniques and portable equipment.12
Dental fluorosis
The examiners completed examinations of mixed dentitions when the participants were about 9 years of age (range, 7.7–12.0 years).6,12,13 The examiners used the Fluorosis Risk Index44 (FRI) to assess dental fluorosis on the various zones of early-erupting permanent teeth. Examiners distinguished fluorosis from other opaque lesions by using Russell’s45 criteria, which are based on color, texture and location. In this study, we defined a tooth with fluorosis as one having an FRI score of 2 (white striations) or 3 (staining, pitting, deformity or a combination of these) on the incisal edge or cusp tip, the incisal or occlusal one-third or the middle one-third; we excluded cervical zones because of variable incomplete eruption. Using the FRI scores, we categorized participants as case participants if they had fluorosis on two or more permanent maxillary incisors and control participants if they had no fluorosis on maxillary incisors. We excluded from the analyses those who had one maxillary incisor with fluorosis to reduce misclassification bias. Person-level interexaminer reliability showed 82 percent agreement (κ = .64) for permanent maxillary incisor fluorosis. Figure 1 shows examples of typical fluorosis cases seen in the IFS.
Figure 1.
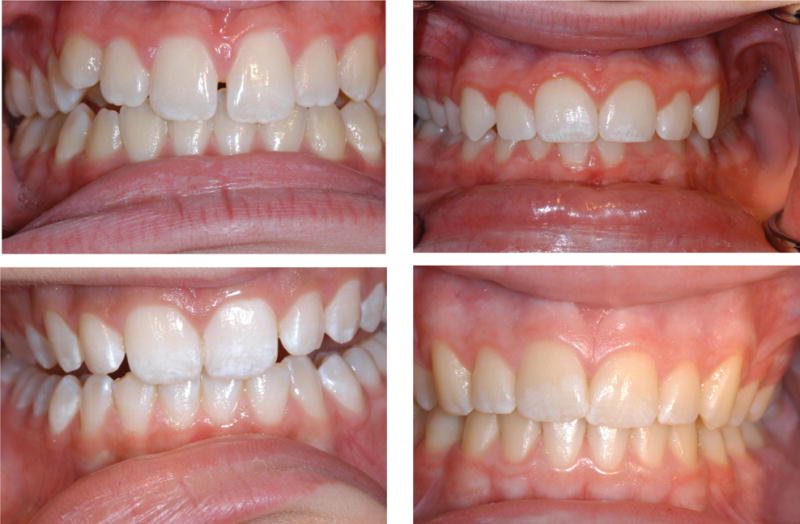
Four typical cases of mild fluorosis, seen in children participating in the Iowa Fluoride Study.
Diet analyses
We obtained data regarding participants’ intake of beverages and selected foods with substantial amounts of water added (such as infant cereal, cooked cereal, soup, rice, pasta, gelatin) from parents’ responses to questions on the food frequency questionnaires sent when children were 1.5, 3, 6, 9, 12, 16, 20, 24, 28, 32 and 36 months of age and about every six months thereafter.28,29,32 For these analyses, we considered data obtained through 36 months.
We asked parents to record types and amounts of selected foods and beverages consumed by children in the study during the preceding week. They were to include foods with substantial amounts of added water and all commonly consumed categories of beverages. We developed a fluoride concentration table with weighted average fluoride concentrations for categories of beverages, commercially prepared waters and the selected foods by using IFS assays of products commonly consumed by study participants. We analyzed nonmunicipal water supplies in homes, child-care settings and schools and filtered municipal waters used by our participants for fluoride as part of the IFS.36–40 We obtained fluoride concentrations of nonfiltered municipal water systems from the Iowa Department of Public Health.33,37 We calculated participants’ composite water fluoride levels as weighted averages of their water sources’ fluoride levels. We estimated dietary fluoride intake as daily intake amount multiplied by fluoride level.
Dentifrice and dietary fluoride supplement intake
IFS questionnaires completed at the same time as food frequency questionnaires queried about children’s brushing habits, use of fluoridated dentifrice and use of fluoride supplements during the preceding 1.5- to 4-month period.12,29–36,41–43 We used responses to estimate daily fluoride ingestion from dentifrices and fluoride supplements during each period. We estimated daily fluoride ingested from dentifrice as the daily brushing frequency multiplied by the estimated quantity of dentifrice used per brushing (which parents indicated by selecting from among seven pictured amounts) multiplied by the fluoride concentration of the dentifrice multiplied by a parent’s approximation of the proportion that was swallowed.34 We estimated daily dietary fluoride supplement consumption as the fluoride concentration of the supplement multiplied by the quantity of supplement consumed multiplied by the frequency of supplement use.42 No direct validation of the estimates was possible.
Statistical analyses
We conducted analyses by using statistical software (SAS 9.1.2 for Microsoft Windows, SAS Institute, Cary, N.C.). We categorized participants’ characteristics, which we present as percentages. Estimated daily fluoride intakes from selected foods, beverages and subcategories of beverages, dentifrice, supplements and fluoride concentrations of home water supplies are presented as medians (25th and 75th percentiles) because of the skewed nature of dietary intake data. We determined fluoride intakes for multiple periods using the area-under-the-curve (AUC) technique (the trapezoidal approach) to estimate average daily intakes across longer periods. We used the period from ages 3 to 9 months for cumulative assessment of the intakes during infancy; we excluded the period from ages 9 to 12 months because children are making dietary transitions at that age; and for early childhood, we used the ages from 16 to 36 months, the period during which incisors are developing. We used the Wilcoxon rank sum test to compare fluoride concentrations of children’s composite water (Table 1) and fluoride intakes (see Appendix 1 for major sources and Appendix 2 for major beverage categories in the supplemental data to the online version of this article at “http:jada.ada.org”) at different time points three to four months apart between participants with and without fluorosis. Owing to the number of bivariate tests involving individual periods, we considered a conservative P value of < .01 statistically significant in these bivariate analyses. We also used Wilcoxon rank sum tests to compare cumulative 3- to 9-month and 16- to 36-month AUC estimates of fluoride intakes determined using the trapezoidal approach (Table 2). We considered AUC fluoride intakes related to maxillary incisor fluorosis (shown in Table 2) (P < .05) for inclusion in multivariable logistic regression analyses (Table 3), which accomplished variable reduction with the best subset (using the score statistic). We then conducted Mantel-Haenszel stratified analyses of the main explanatory variables (upper quartile versus lower three quartiles combined) (Table 4). For these regressions and stratified analyses that use AUC intakes, we considered P values < .05 statistically significant.
TABLE 1.
Fluoride concentrations (parts per million) of water used by participants (composite of home and child care setting water sources and bottled water) at each age, according to fluorosis status of maxillary incisors.*
| AGE (MONTHS) | CASE PARTICIPANTS | CONTROL PARTICIPANTS | P VALUE† | ||||
|---|---|---|---|---|---|---|---|
| No. | Median | (25%, 75%) | No. | Median | (25%, 75%) | ||
| 1.5 | 163 | 1.00 | (0.23, 1.10) | 359 | 0.90 | (0.18, 1.10) | .15 |
| 3 | 168 | 1.00 | (0.24, 1.10) | 354 | 0.90 | (0.15, 1.10) | .22 |
| 6 | 154 | 1.00 | (0.28, 1.10) | 346 | 0.82 | (0.14, 1.10) | .02 |
| 9 | 153 | 1.00 | (0.42, 1.10) | 349 | 0.90 | (0.18, 1.10) | .02 |
| 12 | 139 | 1.00 | (0.60, 1.10) | 320 | 0.90 | (0.22, 1.07) | < .001 |
| 16 | 137 | 1.00 | (0.74, 1.10) | 301 | 0.90 | (0.26, 1.04) | < .001 |
| 20 | 126 | 1.00 | (0.70, 1.10) | 289 | 0.90 | (0.32, 1.10) | .02 |
| 24 | 128 | 1.00 | (0.74, 1.10) | 278 | 0.90 | (0.35, 1.00) | .005 |
| 28 | 128 | 1.00 | (0.59, 1.10) | 276 | 0.90 | (0.35, 1.03) | .01 |
| 32 | 116 | 1.00 | (0.60, 1.10) | 283 | 0.90 | (0.39, 1.10) | .04 |
| 36 | 118 | 1.00 | (0.73, 1.10) | 282 | 0.90 | (0.42, 1.10) | .004 |
Fluorosis assessed at approximately age 9 years. Case participants defined as those with two or more maxillary incisors exhibiting definitive fluorosis (Fluorosis Risk Index score = 2 or 3). Control participants were those with no definitive fluorosis on maxillary incisors. Participants who had only one maxillary incisor with fluorosis have been excluded from analyses.
P value obtained by means of two-tailed Wilcoxon rank sum test; P < .01 considered statistically significant.
TABLE 2.
AUC* estimates of children’s fluoride intakes† (milligrams per day) according to fluorosis status of maxillary incisors.‡
| FLUORIDE SOURCE, ACCORDING TO AGE | INTAKE BY CASE PARTICIPANTS (N = 161)
|
INTAKE BY CONTROL PARTICIPANTS (N = 354)
|
P VALUE§ | ||||
|---|---|---|---|---|---|---|---|
| % Ingesting/Using | Median | (25%,75%) | % Ingesting/Using | Median | (25%,75%) | ||
|
| |||||||
| 3–9 Month AUC | |||||||
|
| |||||||
| Formula | 96 | 0.334 | (0.105, 0.609) | 91 | 0.186 | (0.046, 0.505) | .002 |
| Powdered concentrate | 84 | 0.166 | (0.013, 0.581) | 73 | 0.066 | (0, 0.352) | .002 |
| Liquid concentrate | 34 | 0 | (0, 0.035) | 32 | 0 | (0, 0.027) | .69 |
| Ready-to-feed | 22 | 0 | (0, 0) | 24 | 0 | (0, 0) | .48 |
|
| |||||||
| Cow’s milk | 32 | 0 | (0, 0) | 42 | 0 | (0, 0) | .02 |
|
| |||||||
| Other beverages¶ | 85 | 0.029 | (0.009, 0.066) | 82 | 0.022 | (0.004, 0.051) | .06 |
|
| |||||||
| Water added | 61 | 0.011 | (0, 0.040) | 58 | 0.003 | (0, 0.024) | .045 |
|
| |||||||
| Ready-to-feed | 55 | 0.001 | (0, 0.021) | 58 | 0.005 | (0, 0.021) | .48 |
|
| |||||||
| Water by itself | 78 | 0.007 | (0, 0.033) | 71 | 0.003 | (0, 0.018) | .04 |
|
| |||||||
| All beverages | 100 | 0.409 | (0.167, 0.661) | 99 | 0.244 | (0.085, 0.584) | < .001 |
|
| |||||||
| Selected foods# | 98 | 0.003 | (0.002, 0.006) | 97 | 0.003 | (0.002, 0.005) | .32 |
|
| |||||||
| Supplements | 24 | 0 | (0, 0) | 25 | 0 | (0, 0) | .79 |
|
| |||||||
| Dentifrice | 9 | 0 | (0, 0) | 12 | 0 | (0, 0) | .44 |
|
| |||||||
| TOTAL | 100 | 0.440 | (0.185, 0.694) | 100 | 0.276 | (0.135, 0.600) | .002 |
|
| |||||||
|
CASE PARTICIPANTS (N = 163)
|
CONTROL PARTICIPANTS (N = 367)
|
P VALUE§ | |||||
| % Ingesting/Using | Median | (25%,75%) | % Ingesting/Using | Median | (25%,75%) | ||
|
| |||||||
| 16–36 Month AUC | |||||||
|
| |||||||
| Cow’s milk | 100 | 0.006 | (0.005, 0.008) | 100 | 0.006 | (0.005, 0.008) | .34 |
|
| |||||||
| Other beverages¶ | 100 | 0.224 | (0.169, 0.323) | 100 | 0.201 | (0.145, 0.297) | .03 |
| Water added | 99 | 0.102 | (0.040, 0.170) | 98 | 0.076 | (0.028, 0.153) | .02 |
| Ready-to-feed | 99 | 0.115 | (0.060, 0.181) | 99 | 0.104 | (0.059, 0.171) | .68 |
|
| |||||||
| Water by itself | 99 | 0.090 | (0.045, 0.172) | 99 | 0.074 | (0.037, 0.141) | .03 |
|
| |||||||
| All beverages | 100 | 0.366 | (0.265, 0.475) | 100 | 0.319 | (0.220, 0.434) | .003 |
|
| |||||||
| Selected foods# | 100 | 0.076 | (0.044, 0.110) | 100 | 0.062 | (0.033, 0.097) | .004 |
|
| |||||||
| Supplements | 15 | 0 | (0, 0) | 19 | 0 | (0, 0) | .37 |
|
| |||||||
| Dentifrice | 98 | 0.196 | (0.099, 0.381) | 99 | 0.158 | (0.076, 0.288) | .02 |
|
| |||||||
| TOTAL | 100 | 0.705 | (0.539, 0.898) | 100 | 0.600 | (0.449, 0.779) | < .001 |
AUC: Area under the curve.
25th and 75th percentiles.
Fluorosis assessed at approximately age 9 years. Case participants had two or more maxillary incisors exhibiting definitive fluorosis (Fluorosis Risk Index score = 2, 3). Control participants had no definitive fluorosis on maxillary incisors. Participants who had only one incisor with fluorosis have been excluded.
P value from two-tailed Wilcoxon rank sum test; P < .05 considered statistically significant.
Other beverages include juices, juice drinks, powder-based beverages, sport beverages and soda pop.
Selected foods include infant foods and foods with substantial amounts of added water (such as infant cereal, cooked cereal, soup, rice, pasta, gelatin).
TABLE 3.
Multivariable logistic regression models for fluorosis* of permanent maxillary incisors using AUC† fluoride intakes (0.1 milligram per day), according to exposure age.
| EXPOSURE VARIABLE, ACCORDING TO PARTICIPANT’S AGE IN MONTHS | ODDS RATIO (95% CI‡) | P VALUE |
|---|---|---|
|
| ||
| 3- to 9-Month Model | ||
| Formula reconstituted from powder | 1.09 (1.02, 1.15) | .008 |
| Other beverages with added water§ | 1.75 (1.08, 2.82) | .03 |
| Eliminated (P > .05): cow’s milk, water alone | ||
|
| ||
| 16- to 36-Month Model | ||
| Water by itself | 1.23 (1.04, 1.45) | .02 |
| Dentifrice | 1.11 (1.02, 1.21) | .03 |
| Eliminated (P > .05): other beverages with added water,§ selected foods¶ | ||
|
| ||
| Combined 3- to 9-Month and 16- to 36-Month Model | ||
| Formula reconstituted from powder (3–9 months) | 1.10 (1.03, 1.17) | .005 |
| Other beverages with added water (3–9 months)§ | 1.68 (1.02, 2.78) | .05 |
| Dentifrice (16–36 months) | 1.13 (1.03, 1.24) | .02 |
| Eliminated (P > .05): water by itself (16–36 months) | ||
|
| ||
| Combined Categorical Model# | ||
| Formula reconstituted from powder (3–9 months) | 1.62 (1.05, 2.51) | .03 |
| Other beverages with added water (3–9 months)† | 1.56 (1.01, 2.42) | .045 |
| Dentifrice (16–36 months) | 1.66 (1.07, 2.57) | .03 |
Fluorosis was defined as having definitive fluorosis on two or more maxillary incisors, and no flurorosis as having no definitive fluorosis on any of the maxillary incisors. Variable reduction was achieved using the best subset (using the Score statistic) with all retained variables being statistically significant (P < .05).
AUC: Area-under-the-curve estimates.
CI: Confidence interval.
Includes nonformula beverages made from frozen concentrate or powder.
Selected foods include infant foods with substantial amounts of added water (such as infant cereal, cooked cereal, soup, rice, pasta, gelatin).
Indicator variables for categorical model are upper quartiles of each fluoride intake component. The reference group contains the lower three quartiles (combined). There were no significant two-way interactions.
TABLE 4.
Prevalence of fluorosis on maxillary incisors (two or more teeth of four versus none) according to fluoride source: upper quartiles versus lower three quartiles.
| INTAKE GROUP* | FLUORIDE INTAKE SOURCE†
|
NO. OF PARTICIPANTS | PERCENTAGE PREVALENCE OF FLUOROSIS | RELATIVE RISK (95% CI§) | COMMON RELATIVE RISK¶ (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Ingested dentifrice (16–36 months) | Other beverages with added water‡ (3–9 months) | Powdered formula (3–9 months) | |||||
|
| |||||||
| 1A | Low/moderate | Low/moderate | Low/moderate | 203 | 20.7 | 1.68 (1.11, 2.54) | 1.40 (1.06,1.84) |
| 1B | Low/moderate | Low/moderate | High | 72 | 34.7 | ||
|
| |||||||
| 2A | Low/moderate | High | Low/moderate | 67 | 38.8 | 1.01 (0.58, 1.75) | |
| 2B | Low/moderate | High | High | 28 | 39.3 | ||
|
| |||||||
| 3A | High | Low/moderate | Low/moderate | 82 | 35.4 | 1.62 (0.94, 2.77) | |
| 3B | High | Low/moderate | High | 14 | 57.1 | ||
|
| |||||||
| 4A | High | High | Low/moderate | 17 | 35.3 | 0.94 (0.31, 2.91) | |
| 4B | High | High | High | 9 | 33.3 | ||
Stratified according to intake of fluoride from powdered formula, dentifrice and other beverages with added water.
All fluoride intake sources are grouped by area-under-the-curve intake quartiles.
Includes nonformula beverages made from frozen concentrate or powder.
CI: Confidence interval.
Mantel-Haenszel relative risk. Result of the Breslow-Day test for homogeneity of odds ratios was not significant (P = .48). General association P value was .02.
RESULTS
Demographic characteristics for the 630 participants and their families at enrollment have been presented elsewhere12,29,32 and did not differ according to the participants’ fluorosis status. Mothers were primarily white (98 percent) and participants were 51 percent female. The families were of relatively high socioeconomic status. At the time of recruitment (1992–1995), 13 percent of annual household incomes were less than $20,000, 38 percent ranged from $20,000 to $39,999, 30 percent ranged from $40,000 to $59,999 and 19 percent were $60,000 or greater. Twenty percent and 28 percent, respectively, of mothers and fathers had a high school–equivalent education or less; 35 percent and 31 percent, respectively, had up to two years of college education; and 46 percent and 42 percent, respectively, had four or more years of college education.
We excluded 30 participants from analyses owing to unerupted maxillary incisors. Of the remaining 600, 178 (29.7 percent) had two or more affected maxillary incisors, 382 (63.7 percent) had no maxillary incisor fluorosis and 40 (6.7 percent) had only one affected incisor and were excluded. The majority of fluorosis detected was mild (FRI score = 2, n = 173, 97 percent); only five participants had more involved fluorosis (FRI score = 3).
Figure 2 presents a summary of the participants’ mean fluoride intake from dietary and nondietary sources. Fluoride intake from formula (both reconstituted and ready-to-feed) was dominant from age 3 to 9 months, but declined quickly thereafter. Starting at 12 months, fluoride intake from other beverages became the dominant source of fluoride intake. Fluoride intake from formula (participants aged 3–9 months) and all other beverages (participants aged 16–36 months) consistently was higher among participants who later had fluorosis on permanent maxillary incisors. Fluoride intake from dentifrice (participants aged 20–36 months) also was higher for participants who developed fluorosis on permanent maxillary incisors.
Figure 2.
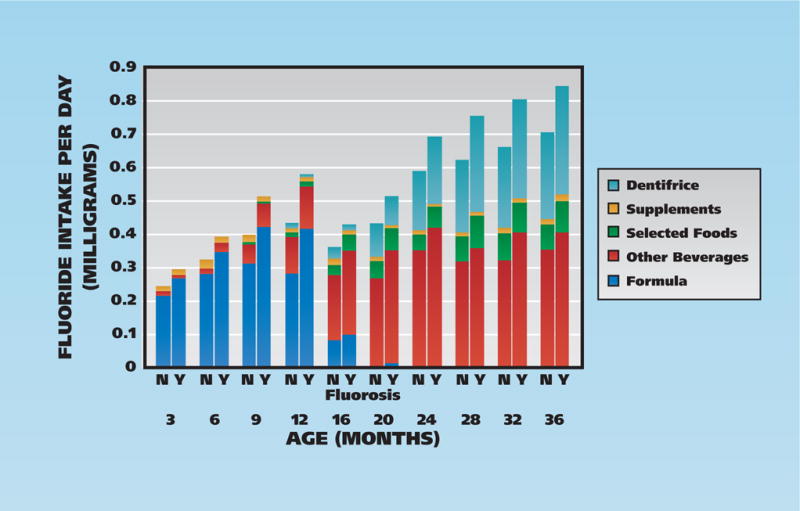
Mean fluoride intake (in milligrams) by age and fluorosis status. N: No maxillary incisor fluorosis. Y: Two or more maxillary incisors with fluorosis. Selected foods: Foods with substantial amounts of added water. Other beverages: All beverages other than formula.
Estimated total fluoride intake (that is, the sum of intakes from beverages, selected foods, dentifrice and supplements) for each assessment point from age 6 months to age 36 months was significantly (P < .01) higher in participants with permanent maxillary incisor fluorosis than in participants without fluorosis (data not shown; see Appendix 1 in the supplemental data to the online version of this article at “http:jada.ada.org”). Total fluoride intakes from all beverages combined were significantly (P < .01) higher in case participants at all ages except 1.5, 20, 24 and 32 months; fluoride intakes from selected solid foods with water added differed significantly (P < .01) between case and control participants only at 24, 32 and 36 months. Fluoride intakes from ingested dentifrice and supplements did not differ significantly (P < .01) between case and control participants at any individual age, except for intake from dentifrice at 36 months.
The fluoride concentrations of participants’ composite water sources at 12, 16, 24, 28 and 36 months were significantly higher (P < .01) in case participants than in control participants (Table 1), with a consistent, nonsignificant trend at the other ages.
The majority of formula used was reconstituted powdered concentrate infant formula (65 percent) versus 26 percent liquid concentrate and 9 percent ready-to-feed. Approximately 80 percent was milk-based and 20 percent soy-based (data not shown).
We compared participants’ daily estimated median fluoride intakes according to beverage category and according to fluorosis status of the permanent maxillary incisors (data not shown; see Appendix 2 in the supplemental data to the online version of this article at “http:jada.ada.org”). Fluoride intakes from infant formulas overall at ages 3, 6 and 9 months, and from water by itself at ages 16 and 28 months, were significantly higher (P < .01) in participants with fluorosis than in those without fluorosis. Fluoride intake from powdered concentrate formulas prepared with water was substantially higher in case participants than in control participants at 6 and 9 months, with a nonsignificant trend at age 3 months (P = .02). Fluoride intakes from other beverages categorized as prepared with water were higher at age 16 months in case participants than in control participants. Fluoride intake from cow’s milk at ages 9 and 36 months were lower for those with fluorosis than for those without fluorosis.
Table 2 summarizes cumulative patterns of fluoride intake AUCs according to category of fluoride source and in total for early (3- to 9-month) and later (16- to 36-month) age periods according to fluorosis status. The strongest positive bivariate associations with fluorosis prevalence were for 3- to 9-month AUCs with fluoride intakes from reconstituted powdered concentrate formula, cow’s milk, beverages with added water and water by itself and from 16- to 36-month AUCs with greater fluoride intakes from water-added beverages, water by itself, selected foods and dentifrice. We considered AUC fluoride intakes associated with maxillary incisor fluorosis (P < .05) in the subsequent multivariable logistic regressions.
Table 3 summarizes multivariable logistic regression models developed separately for fluoride intake AUCs for participants aged 3 to 9 months and 16 to 36 months and for both age groups combined. For those aged 3 to 9 months, fluoride AUC from formula reconstituted from powder was significantly related (P < .05) to maxillary incisor fluorosis, as was fluoride AUC intake from other beverages with added water. For participants aged 16 to 36 months, fluoride intake AUC from dentifrice and water by itself were related significantly to fluorosis. The significant variables in the continuous model combining these periods were 3- to 9-month-old participants’ ingestion of fluoride from reconstituted powdered concentrate formula and of fluoride from other beverages with added water, as well as 16- to 36-month-olds’ ingestion of fluoride from dentifrice. We conducted multivariable logistic regression analyses combining the age periods, but by using categorical AUC fluoride variables (upper quartile versus other three quartiles), to assess possible interactions. The same three variables were statistically significant, with no significant two-way interactions. The effects on fluorosis prevalence of being in the upper quartile of fluoride intake from other beverages with added water for 3 to 9 months and from dentifrice for 16–36 months were of similar magnitude (odds ratio ~ 1.6).
Table 4 summarizes results of the Mantel-Haenszel analyses considering the effects of 3- to 9-month-olds’ fluoride intake AUC from powdered formula stratified by 3- to 9-month-olds’ fluoride intake AUC from other beverages with added water, as well as 16- to 36-month-olds’ fluoride intake AUC from dentifrice. The overall relative risk (RR) associated with being in the upper quartile of 3- to 9-month-olds’ AUC fluoride intake from powdered formula was 1.40 (P = .02, 95 percent CI = 1.06, 1.84). The first stratum shows that, with lower fluoride intake among the other two significant variables, high fluoride intake from powdered concentrate is statistically significantly and clinically meaningfully related to incisor fluorosis (RR = 1.68, 95 percent CI = 1.11, 2.54). With the higher quartile of fluoride intake from these other sources, there are no statistically significant associations; however, the smaller sample sizes must be considered.
There were no significant differences in dental caries experience at ages 5 or 9 years on the basis of fluorosis case status or formula fluoride intake (AUC for participants aged 3–9 months) (data not shown).
DISCUSSION
Our data support the hypothesis that high fluoride intake from beverages is a primary contributor to dental fluorosis of permanent maxillary incisors. As a group, children in the IFS who had fluorosis of the maxillary incisors, albeit mostly mild fluorosis, had higher combined fluoride intakes throughout early childhood than did children without fluorosis. Fluoride from beverages (including infant formula) contributed the most to the total estimated fluoride consumed during the first 36 months, whereas the intake from foods and supplements was substantially less. Fluoride intake from dentifrice also was a major component in participants aged from 16 to 36 months. Furthermore, children with fluorosis generally had significantly higher fluoride intake from beverages alone, beginning at 3 months of age.
However, participants with fluorosis had only slightly higher median total beverage intakes (about 1–2 ounces per day) at most ages from 1.5 to 36 months (data not shown). This suggests that children with fluorosis do not have excessive beverage intakes, but rather higher fluoride intakes from beverages they consumed. Similarly, median total formula intakes were about 8 percent higher in children with fluorosis versus children without (about 2 oz per day more for the 3- to 9-month-olds’ AUC), but median fluoride intakes from infant formulas were 80 percent higher. Thus, fluorosis was not specifically associated with the quantity of formula consumed by case participants versus control participants, but rather with the amount of fluoride in the formula—a result of case participants’ having both higher consumption of powdered concentrate formula (median 3-to 9-month-olds’ AUC 14.7 oz versus 8.6 oz) and higher fluoride levels in the water used to reconstitute the formula (Table 1).
Fluoride intake from infant formula was significantly higher for case participants than in control participants. These data are consistent with our previous findings in the same cohort: that higher intakes of fluoride from water used to prepare infant formulas and from water as a beverage increased the risk of primary tooth fluorosis29 and reports by other investigators that infant formulas and fluoridated water are associated with dental fluorosis.15–18,28,46 Also, although total fluoride intake from infant formula was significantly higher for case participants than for control participants, virtually all of the difference was attributable to reconstituted powdered concentrate, with about two-thirds using fluoridated water. Reconstituting powder concentrated formula with low-fluoride–content water would result in much less fluoride ingestion and, presumably, substantially less or milder dental fluorosis.
Although these analyses focused on beverages and selected foods prepared with water at home, other food products have potential substantial fluoride content. Fluoride concentration typically is low in plants and animal flesh; that found in solid foods is largely a by-product of previous agricultural practices (for instance, use of organic pesticide, which contains high levels of fluoride, no longer is current practice for grapes), food processing (such as mechanical deboning, which results in the inclusion of small pieces of high-fluoride bone in foods) or commercial food preparation (as a component of water used in food preparation).37,40,47,48 Therefore, the consumer has little knowledge of or control over the fluoride content of purchased ready-to-feed commercially prepared foods.
Fluoride intake from selected food sources prepared with water at home was slightly higher in our participants with fluorosis than in those without fluorosis. Median intakes were only .02 to .032 milligram higher but achieved statistical significance only at 24, 32 and 36 months, and for the AUC of participants at ages 16 to 36 months; however, this AUC was not retained in the multivariable model. This suggests that the fluoride intake associated with food preparation had less effect on fluorosis risk than did the intake from beverages.
Fluoride is added to preventive oral health products and fluoride supplements recommended in certain instances to reduce risk of caries development. In our study, estimated fluoride intakes from supplements at individual time points were modest and generally similar in participants with and without fluorosis. Fluoride dentifrice intakes tended to be slightly higher for fluorosis case participants at individual time points from ages 16 to 36 months, with a significant relationship with 16–36 month AUC fluoride intake from dentifrice.
Thus, it appears that substantial fluoride intake from both reconstituted powdered infant formula and other beverages with added water during the ages from 3 to 9 months, from dentifrice during the ages from 16 to 36 months or a combination of these has the effect of elevating a child’s risk of developing fluorosis.
Although our data suggested that tap water—consumed by itself or used in preparation of powdered infant formulas and other beverages—is associated strongly with dental fluorosis, we must recognize that total fluoride intake is the true risk factor. Absolute differences in estimated total fluoride intake between participants with and without fluorosis were relatively small: true differences in median AUC intakes were 0.160 to 0.105 mg, respectively, at younger and older ages. At younger ages, the differences can be attributed largely to the fluoride in formula reconstituted with water and partially attributed to the fluoride in tap water added to beverages; at older ages, it was attributed to dentifrice ingestion.
Nearly all of the fluorosis in our study participants was mild. A recent review of the effect of mild dental fluorosis on oral health-related quality of life concluded that the effect of mild fluorosis was not adverse and could even be favorable.49 This suggests that concerns about mild dental fluorosis may be exaggerated. Therefore, no general recommendations to avoid use of fluoridated water in reconstituting infant formula are warranted. However, for those trying to avoid mild dental fluorosis, data suggest that following interim recommendations to avoid ingestion of large quantities of powdered concentrate infant formulas reconstituted with fluoridated water, such as those by the ADA26 and CDC,27 would be useful. Also, fluoride dentifrice ingestion should be kept modest by means of using a smear or a small, pea-sized amount and of ensuring appropriate parental supervision.
The IFS has many strengths, including its longitudinal nature and detailed assessment of fluoride intake from multiple sources. However, it also has limitations, such as parental or caregiver self-reporting of dietary data, which may or may not reflect actual consumption. Also, distribution of ready-to-feed products to east central Iowa from multiple production sites resulted in a range of fluoride concentrations’ being available for some products, and these concentrations may not be representative of those in products available elsewhere in the United States. Additionally, we applied the mean assayed fluoride concentration to beverage categories to estimate fluoride intake, and we could have overestimated or underestimated true intake. Relatively few children received dietary fluoride supplements, thus probably reducing our power to assess these as an important source of fluoride intake and a fluorosis risk factor. Because most of the children with fluorosis in the study had mild fluorosis of the incisors, we were unable to investigate moderate or severe fluorosis. Results are most relevant for young children who live in areas with water fluoride levels similar to those in the study; the majority of participants had composite water fluoride levels in the optimal range as defined by CDC. Lastly, IFS participants are a self-selected sample with a relatively high socioeconomic status and therefore are not fully representative of a more geographically or socioeconomically diverse population.
CONCLUSIONS
The primary source of fluoride for most young infants in areas with fluoridated water is reconstituted infant formula. Fluoride intakes from ready-to-feed beverages and supplements were fairly similar in participants with and without fluorosis of permanent maxillary incisors. Fluorosis, mostly mild, of maxillary incisors was associated significantly with fluoride intakes among participants when aged 3 to 9 months from reconstituted powdered concentrate infant formulas and other beverages with added water and among participants when aged 16 to 36 months from dentifrice. However, because mild dental fluorosis is not associated negatively with oral health-related quality of life, general recommendations to avoid reconstituting concentrated infant formula with fluoridated water are not warranted. However, for those concerned about reducing risk of developing mild fluorosis who are using substantial quantities of powdered concentrate infant formula reconstituted with fluoridated water, the family dentist or physician should provide recommendations to use water with lower fluoride levels. Parents also should be encouraged to follow recommendations for use of small (smear or pea-sized) amounts of fluoridated dentifrice and ensure proper supervision of the child’s tooth-brushing. Finally, given the study limitations, we recommend additional investigation with more diverse populations to confirm our findings and help identify the best fluoride concentration of water for use in formula reconstitution.
Supplementary Material
Acknowledgments
This study was supported by National Institute for Dental and Craniofacial Research grants RO1-DE09551 and RO1-DE12101 and National Center for Research Resources grant M01-RR00059. The contents are the responsibility of the authors and do not reflect the official views of the granting organizations.
ABBREVIATION KEY
- ADA
AMERICAN DENTAL ASSOCIATION
- AUC
Area under the curve
- CDC
Centers for Disease Control and Prevention
- FRI
Fluorosis Risk Index
- IFS
Iowa Fluoride Study
Footnotes
Disclosures. None of the authors reported any disclosures.
Portions of the results of this study were presented at the General Session of the American Association for Dental Research in Orlando, Fla., on March 9, 2006.
Contributor Information
Dr. Steven M. Levy, Wright-Bush-Shreves Endowed Professor of Research, Department of Preventive and Community Dentistry, College of Dentistry, and a profesor, Department of Epidemiology, College of Public Health, University of Iowa, N-328 Dental Science Building, Iowa City, Iowa 52242-1010.
Barbara Broffitt, Research assistant and statistician, Department of Preventive and Community Dentistry, College of Dentistry, University of Iowa, Iowa City.
Dr. Teresa A. Marshall, Associate professor, Department of Preventive and Community Dentistry, College of Dentistry, University of Iowa, Iowa City.
Dr. Julie M. Eichenberger-Gilmore, Adjunct assistant professor, Department of Preventive and Community Dentistry, College of Dentistry, University of Iowa, Iowa City.
Dr. John J. Warren, Professor, Department of Preventive and Community Dentistry, College of Dentistry, University of Iowa, Iowa City.
References
- 1.Centers for Disease Control. Achievements in public health, 1900–1999: fluoridation of drinking water to prevent dental caries. MMWR. 1999;48(41):933–940. doi: 10.1001/jama.283.10.1283. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR Recomm Rep. 2001;50(RR-14):1–42. [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, Md.: U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. pp. 160–161. [Google Scholar]
- 4.Clark DC. Trends in prevalence of dental fluorosis in North America. Community Dent Oral Epidemiol. 1994;22(3):148–152. doi: 10.1111/j.1600-0528.1994.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 5.Rozier RG. The prevalence and severity of enamel fluorosis in North American children. J Public Health Dent. 1999;59(4):239–246. doi: 10.1111/j.1752-7325.1999.tb03276.x. [DOI] [PubMed] [Google Scholar]
- 6.Beltrán-Aguilar ED, Barker LK, Canto MT, et al. Centers for Disease Control and Prevention Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis: United States, 1988–1994 and 1999–2002. MMWR Surveill Summ. 2005;54(3):1–43. [PubMed] [Google Scholar]
- 7.Wright JT, Chen SC, Hall KI, Yamauchi M, Bawden JW. Protein characterization of fluorosed human enamel. J Dent Res. 1996;75(12):1936–1941. doi: 10.1177/00220345960750120401. [DOI] [PubMed] [Google Scholar]
- 8.Limeback H. Enamel formation and the effects of fluoride. Community Dent Oral Epidemiol. 1994;22(3):144–147. doi: 10.1111/j.1600-0528.1994.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 9.El Nesr NM, Avery KJ. Tooth eruption and shedding. In: Avery KJ, Steele PF, editors. Oral Development and Histology. 2nd. New York: Thieme Medical Publishers; 1994. pp. 110–122. [Google Scholar]
- 10.Bårdsen A. “Risk periods” associated with the development of dental fluorosis in maxillary permanent central incisors: a meta-analysis. Acta Odontol Scand. 1999;57(5):247–256. doi: 10.1080/000163599428652. [DOI] [PubMed] [Google Scholar]
- 11.Evans RW, Darvell BW. Refining the estimate of the critical period for susceptibility to enamel fluorosis in human maxillary central incisors. J Public Health Dent. 1995;55(4):238–249. doi: 10.1111/j.1752-7325.1995.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 12.Hong L, Levy SM, Broffitt B, Warren JJ, Kanellis MJ, Wefel JS, Dawson DV. Timing of fluoride intake in relation to development of fluorosis on maxillary central incisors. Community Dent Oral Epidemiol. 2006;34(4):299–309. doi: 10.1111/j.1600-0528.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy SM, Warren JJ, Broffitt B, Kanellis MJ. Associations between dental fluorosis of the permanent and primary dentitions. J Public Health Dent. 2006;66(3):180–185. doi: 10.1111/j.1752-7325.2006.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 14.Levy SM, Hillis SL, Warren JJ, et al. Primary tooth fluorosis and fluoride intake during the first year of life. Community Dent Oral Epidemiol. 2002;30(4):286–295. doi: 10.1034/j.1600-0528.2002.00053.x. [DOI] [PubMed] [Google Scholar]
- 15.Pendrys DG, Katz RV, Morse DE. Risk factors for enamel fluorosis in a fluoridated population. Am J Epidemiol. 1994;140(5):461–471. doi: 10.1093/oxfordjournals.aje.a117268. [DOI] [PubMed] [Google Scholar]
- 16.Osuji OO, Leake JL, Chipman ML, Nikiforuk G, Locker D, Levine N. Risk factors for dental fluorosis in a fluoridated community. J Dent Res. 1988;67(12):1488–1492. doi: 10.1177/00220345880670120901. [DOI] [PubMed] [Google Scholar]
- 17.Pendrys DG, Katz RV. Risk of enamel fluorosis associated with fluoride supplementation, infant formula, and fluoride dentrifrice use. Am J Epidemiol. 1989;130(6):1199–1208. doi: 10.1093/oxfordjournals.aje.a115448. [DOI] [PubMed] [Google Scholar]
- 18.Ismail AI, Messer JG. The risk of fluorosis in students exposed to a higher than optimal concentration of fluoride in well water. J Public Health Dent. 1996;56(1):22–27. doi: 10.1111/j.1752-7325.1996.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 19.Holm AK, Andersson R. Enamel mineralization disturbances in 12-year-old children with known early exposure to fluorides. Community Dent Oral Epidemiol. 1982;10(6):335–339. doi: 10.1111/j.1600-0528.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumar JV, Green EL, Wallace W, Carnahan T. Trends in dental fluorosis and dental caries prevalences in Newburgh and Kingston NY. Am J Public Health. 1989;79(5):565–569. doi: 10.2105/ajph.79.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton JL, Messer LB. Dental caries and fluorosis in breast-fed and bottle-fed children. Caries Res. 1981;15(2):124–137. doi: 10.1159/000260511. [DOI] [PubMed] [Google Scholar]
- 22.Rock WP, Sabieha AM. The relationship between reported toothpaste usage in infancy and fluorosis of permanent incisors. Br Dent J. 1997;183(5):165–170. doi: 10.1038/sj.bdj.4809456. [DOI] [PubMed] [Google Scholar]
- 23.Levy SM. Review of fluoride exposures and ingestion. Community Dent Oral Epidemiol. 1994;22(3):173–180. doi: 10.1111/j.1600-0528.1994.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 24.Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: nutritional consequences. J Am Diet Assoc. 1999;99(4):436–441. doi: 10.1016/S0002-8223(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council, Committee on Fluoride in Drinking Water. Fluoride in Drinking Water: a Scientific Review of EPA’s Standards. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 26.American Dental Association. Infants, formula and fluoride. JADA. 2007;138(1):132. doi: 10.14219/jada.archive.2007.0031. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Background: Infant formula and the risk for enamel fluorosis. www.cdc.gov/fluoridation/safety/infant_formula.htm. Accessed Aug. 31, 2010.
- 28.Hujoel PP, Zina LG, Moimaz SA, Cunha-Cruz J. Infant formula and enamel fluorosis: a systematic review. JADA. 2009;140(7):841–854. doi: 10.14219/jada.archive.2009.0278. [DOI] [PubMed] [Google Scholar]
- 29.Marshall TA, Levy SM, Warren JJ, Broffitt B, Eichenberger-Gilmore JM, Stumbo PJ. Associations between intakes of fluoride from beverages during infancy and dental fluorosis of primary teeth. J Am Coll Nutr. 2004;23(2):108–116. doi: 10.1080/07315724.2004.10719350. [DOI] [PubMed] [Google Scholar]
- 30.Warren JJ, Levy SM, Kanellis MJ. Dental caries in the primary dentition: assessing prevalence of cavitated and noncavitated lesions. J Public Health Dent. 2002;62(2):109–114. doi: 10.1111/j.1752-7325.2002.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 31.Levy SM, Warren JJ, Broffitt B, Hillis SL, Kanellis MJ. Fluoride, beverages and dental caries in the primary dentition. Caries Res. 2003;37(3):157–165. doi: 10.1159/000070438. [DOI] [PubMed] [Google Scholar]
- 32.Marshall TA, Levy SM, Broffitt B, et al. Dental caries and beverage consumption in young children. Pediatrics. 2003;112(3 pt 1):e184–e191. doi: 10.1542/peds.112.3.e184. [DOI] [PubMed] [Google Scholar]
- 33.Levy SM, Kohout FJ, Kiritsy MC, Heilman JR, Wefel JS. Infants’ fluoride ingestion from water, supplements and dentifrice. JADA. 1995;126(12):1625–1632. doi: 10.14219/jada.archive.1995.0110. [DOI] [PubMed] [Google Scholar]
- 34.Levy SM, Kiritsy MC, Slager SL, Warren JJ, Kohout FJ. Patterns of fluoride dentifrice use among infants. Pediatr Dent. 1997;19(1):50–55. [PubMed] [Google Scholar]
- 35.Levy SM, Warren JJ, Davis CS, Kirchner HL, Kanellis MJ, Wefel JS. Patterns of fluoride intake from birth to 36 months. J Public Health Dent. 2001;61(2):70–77. doi: 10.1111/j.1752-7325.2001.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 36.Levy SM, Kohout FJ, Guha-Chowdhury N, Kiritsy MC, Heilman JR, Wefel JS. Infants’ fluoride intake from drinking water alone, and from water added to formula, beverages, and food. J Dent Res. 1995;74(7):1399–1407. doi: 10.1177/00220345950740071201. [DOI] [PubMed] [Google Scholar]
- 37.Van Winkle S, Levy SM, Kiritsy MC, Heilman JR, Wefel JS, Marshall T. Water and formula fluoride concentrations: significance for infants fed formula. Pediatr Dent. 1995;17(4):305–310. [PubMed] [Google Scholar]
- 38.Kiritsy MC, Levy SM, Warren JJ, Guha-Chowdhury N, Heilman JR, Marshall T. Assessing fluoride concentrations of juices and juice-flavored drinks. JADA. 1996;127(7):895–902. doi: 10.14219/jada.archive.1996.0347. [DOI] [PubMed] [Google Scholar]
- 39.Heilman JR, Kiritsy MC, Levy SM, Wefel JS. Fluoride concentrations of infant foods. JADA. 1997;128(7):857–863. doi: 10.14219/jada.archive.1997.0335. [DOI] [PubMed] [Google Scholar]
- 40.Heilman JR, Kiritsy MC, Levy SM, Wefel JS. Assessing fluoride levels of carbonated soft drinks. JADA. 1999;130(11):1593–1599. doi: 10.14219/jada.archive.1999.0098. [DOI] [PubMed] [Google Scholar]
- 41.Levy SM, Kiritsy MC, Slager SL, Warren JJ. Patterns of dietary fluoride supplement use during infancy. J Public Health Dent. 1998;158(3):228–233. doi: 10.1111/j.1752-7325.1998.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 42.Hamasha AA, Levy SM, Broffitt B, Warren JJ. Patterns of dietary fluoride supplement use in children from birth to 96 months of age. J Public Health Dent. 2005;65(1):7–13. doi: 10.1111/j.1752-7325.2005.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 43.Franzman MR, Levy SM, Warren JJ, Broffitt B. Tooth-brushing and dentifrice use among children ages 6 to 60 months. Pediatr Dent. 2004;26(1):87–92. [PubMed] [Google Scholar]
- 44.Pendrys DG. The fluorosis risk index: a method for investigating risk factors. J Public Health Dent. 1990;50(5):291–298. doi: 10.1111/j.1752-7325.1990.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 45.Russell AL. The differential diagnosis of fluoride and nonfluoride enamel opacities. J Public Health Dent. 1961;21(4):143–146. [Google Scholar]
- 46.Milsom K, Mitropoulos CM. Enamel defects in 8-year-old children in fluoridated and non-fluoridated parts of Cheshire. Caries Res. 1990;24(4):286–289. doi: 10.1159/000261284. [DOI] [PubMed] [Google Scholar]
- 47.Pang DT, Phillips CL, Bawden JW. Fluoride intake from beverge consumption in a sample of North Carolina Children. J Dent Res. 1992;71(7):1382–1388. doi: 10.1177/00220345920710070601. [DOI] [PubMed] [Google Scholar]
- 48.Griffin SO, Gooch BF, Lockwood SA, Tomar SL. Quantifying the diffused benefit from water fluoridation in the United States. Community Dent Oral Epidemiol. 2001;29(2):120–129. doi: 10.1034/j.1600-0528.2001.290206.x. [DOI] [PubMed] [Google Scholar]
- 49.Chankanka O, Levy SM, Warren JJ, Chalmers JM. A literature review of aesthetic perceptions of dental fluorosis and relationships with psychosocial aspects/oral health-related quality of life. Community Dent Oral Epidemiol. 2010;38(2):97–109. doi: 10.1111/j.1600-0528.2009.00507.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


