Abstract
BACKGROUND
In 2013, New York began requiring hospitals to follow protocols for the early identification and treatment of sepsis. However, there is controversy about whether more rapid treatment of sepsis improves outcomes in patients.
METHODS
We studied data from patients with sepsis and septic shock that were reported to the New York State Department of Health from April 1, 2014, to June 30, 2016. Patients had a sepsis protocol initiated within 6 hours after arrival in the emergency department and had all items in a 3-hour bundle of care for patients with sepsis (i.e., blood cultures, broad-spectrum antibiotic agents, and lactate measurement) completed within 12 hours. Multilevel models were used to assess the associations between the time until completion of the 3-hour bundle and risk-adjusted mortality. We also examined the times to the administration of antibiotics and to the completion of an initial bolus of intravenous fluid.
RESULTS
Among 49,331 patients at 149 hospitals, 40,696 (82.5%) had the 3-hour bundle completed within 3 hours. The median time to completion of the 3-hour bundle was 1.30 hours (interquartile range, 0.65 to 2.35), the median time to the administration of antibiotics was 0.95 hours (interquartile range, 0.35 to 1.95), and the median time to completion of the fluid bolus was 2.56 hours (interquartile range, 1.33 to 4.20). Among patients who had the 3-hour bundle completed within 12 hours, a longer time to the completion of the bundle was associated with higher risk-adjusted inhospital mortality (odds ratio, 1.04 per hour; 95% confidence interval [CI], 1.02 to 1.05; P<0.001), as was a longer time to the administration of antibiotics (odds ratio, 1.04 per hour; 95% CI, 1.03 to 1.06; P<0.001) but not a longer time to the completion of a bolus of intravenous fluids (odds ratio, 1.01 per hour; 95% CI, 0.99 to 1.02; P=0.21).
CONCLUSIONS
More rapid completion of a 3-hour bundle of sepsis care and rapid administration of antibiotics, but not rapid completion of an initial bolus of intravenous fluids, were associated with lower risk-adjusted in-hospital mortality.
More than 1.5 million cases of sepsis occur in the United States annually, and many patients with sepsis present to the emergency department.1 International clinical practice guidelines and the Centers for sepsis present to the emergency department.1 International clinical practice guidelines and the Centers for Medicare and Medicaid Services (CMS) recommend the prompt identification of sepsis and treatment with broad-spectrum antibiotic agents and intravenous fluids.2,3 These recommendations are supported by preclinical and observational studies suggesting that early treatment with antibiotics and intravenous fluids could reduce the number of avoidable deaths.4,5
Yet, considerable controversy exists about how rapidly sepsis must be treated.6 Some clinicians question the potential benefit of rapid treatment, citing the absence of data from randomized trials, the potential for adverse effects, and the challenging implementation of these efforts in environments where staff are often overworked. Using data from New York,7 where hospitals are required to implement protocols and report on the treatment of sepsis, we examined the association between the timing of treatment and risk-adjusted mortality.
METHODS
STUDY DESIGN AND POPULATION
In early 2013, the New York State Department of Health (NYSDOH) began requiring hospitals to submit and follow evidence-informed protocols for the early identification and treatment of severe sepsis or septic shock (New York Codes, Rules, and Regulations parts 405.2 and 405.4). Although protocols could be tailored by each hospital, all the protocols were required to include a 3-hour bundle consisting of receipt of the following care within 3 hours: obtaining of a blood culture before the administration of antibiotics, measurement of the serum lactate level, and the administration of broad-spectrum antibiotics. Protocols were also required to include a 6-hour bundle, consisting of the administration of a bolus of 30 ml of intravenous fluids per kilogram of body weight in patients with hypotension or a serum lactate level of 4.0 mmol or more per liter, the initiation of vasopressors for refractory hypotension, and the remeasurement of the serum lactate level within 6 hours after the initiation of the protocol. Details about the treatment bundles are provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
We performed a retrospective study involving 185 hospitals in the NYSDOH database, including data from April 1, 2014, to June 30, 2016. All the hospitals were required to report patient-level data for patients with sepsis and septic shock to the Department of Health using electronic case-report forms that included data on demographic characteristics, coexisting conditions, characteristics of sepsis and septic shock, illness severity, and outcomes. Date and time stamps for protocol initiation and the elements of 3-hour and 6-hour bundled care were required for patients in whom a sepsis protocol was initiated. The state performed audits on a 10% random sample of hospitals using manual chart review and provided feedback to hospitals regarding data quality and completeness. Audit results are provided in Table S2 in the Supplementary Appendix. Patient-level data were linked to hospital characteristics with the use of the NYSDOH administrative database. This study was approved with a waiver of informed consent by the NYSDOH institutional review boards.
SELECTION OF PATIENTS
Eligible encounters included those with patients who were older than 17 years of age and who had severe sepsis or septic shock, as defined with the use of criteria suggested in the 2001 International Sepsis Definitions Conference (Sepsis-2).8 In order to study only patients with community-acquired sepsis, we focused on patients who had a sepsis protocol initiated in the emergency department within 6 hours after arrival at the hospital. To remove outliers, we excluded patients in whom the 3-hour bundle was completed more than 12 hours after the initiation of the protocol. We also excluded patients in whom bundled care could be clinically contraindicated, patients with advance directives that limited treatment, patients who declined interventions, and patients who were enrolled in a clinical trial. We excluded 36 hospitals that had fewer than 50 cases of sepsis in order to remove spurious findings in reliability-adjusted models.9
Hospitals varied in their sepsis-identification strategies (see the Methods section in the Supplementary Appendix). These strategies included positive screening for sepsis on the basis of clinical assessment only (suspected or confirmed infection and two or more criteria for the systemic inflammatory response syndrome, with supporting laboratory test results optional); positive screening based on both clinical criteria and abnormal laboratory values; and a “code sepsis or rapid response” strategy that led to a positive screening based on clinical criteria. The regulations permitted hospitals to have flexibility with regard to case identification in order to facilitate broad adoption. Cases were not identified with the use of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) because these definitions were released after the implementation of the regulations was under way,10 and it was not possible to use post hoc adjudication. More than 98% of the patients with data entered in the database were confirmed to have had severe sepsis or septic shock on manual audit (Table S2 in the Supplementary Appendix). Cases that were found to have been entered erroneously could be removed by hospitals.
VARIABLES
The primary outcome was in-hospital mortality. The primary exposure was the time to completion of the 3-hour bundle, which was defined as the time in hours from the initiation of the protocol until all the elements of the 3-hour bundle were performed (i.e., blood cultures obtained, broad-spectrum antibiotics administered, and serum lactate level measured). If any element of the 3-hour bundle was performed before the start of the protocol, the patient was considered to have adhered to the protocol with regard to that element within the first hour. The time to the administration of broad-spectrum antibiotics was defined in a similar fashion. The time to the completion of the initial bolus of intravenous fluid was measured as the time from the initiation of the protocol until the completed administration of 30 ml of crystalloid fluid per kilogram, but only among patients who had a serum lactate level of 4.0 mmol or more per liter or who had hypotension (systolic blood pressure, <90 mm Hg).
Covariates included variables that were specified a priori as potential confounders between time to treatment and outcome on the basis of clinical experience and previous studies.10,11 These variables included demographic factors such as age, race or ethnic group, payer, burden of coexisting conditions, site of infection (e.g., respiratory, urinary, or skin), admission source (e.g., clinic, skilled nursing facility, or home), and measures of illness severity such as the presence of shock, serum lactate level, platelet count, or mechanical ventilation at admission. We developed a risk-adjustment model for in-hospital mortality using the above covariates with multivariable logistic regression that included a 90% random sample of the cohort. Internal validation of the model on the 10% remaining sample revealed adequate calibration (Hosmer–Lemeshow goodness-of-fit test with group size of 150, P = 0.97) (Fig. S2 in the Supplementary Appendix) and discrimination (area under the receiver-operating-characteristic curve [C statistic], 0.77).
SENSITIVITY ANALYSES
We assessed the robustness of our analyses by repeating the primary analysis using the time to treatment as measured from the earliest recorded time of the presentation in the emergency department.6 We also assessed models in prespecified subgroups of patients. We repeated models with the subgroup of patients who had a protocol initiated up to 24 hours after arrival in the emergency department and with the subgroup of patients who had up to 24 hours between protocol initiation and completion of the 3-hour bundle.12 We repeated models with patients who were discharged to hospice care classified as dead at discharge and models that excluded any patients who had an element of the 3-hour bundle, administration of antibiotics, or completion of bolus of intravenous fluids before protocol initiation.
In supporting analyses, we measured the association of other elements of the 3-hour bundle with mortality, including the time to obtaining of a blood culture and the time to serum lactate measurement. We performed quantitative bias analysis to assess the magnitude of a hypothetical, unmeasured confounder that would be necessary to account for the association between the time to completion of the 3-hour bundle and risk-adjusted in-hospital mortality (see the Supplementary Appendix).13
STATISTICAL ANALYSIS
We performed bivariate analyses of the characteristics of the patients who had the 3-hour bundle in the emergency department completed within 3 hours and those who did not have the 3-hour bundle completed within that time window. Continuous data are expressed as means with standard deviations or as medians with interquartile ranges, depending on normality. Categorical variables are shown as proportions. The range and variability in the times to treatments are shown with the use of histograms and cumulative proportions.
Multivariable modeling of the association between the time to treatment and in-hospital mortality was performed with the use of logistic regression, with adjustment for covariates. Binary variables were modeled as indicator covariates, and continuous variables were included as linear covariates, after assessment for nonlinear relationships with the use of fractional polynomials (P>0.05 for all models).14 We used multilevel regression with a random effect of hospital to account for hospital-level clustering. Each exposure (i.e., time to completion of the 3-hour bundle, time to the administration of broad-spectrum antibiotics, and time to completion of initial bolus of intravenous fluids) was evaluated separately. The risk of in-hospital death across the range of time to treatment was generated for the “typical” patient with the use of predictive margins that were adjusted for an average of the independent variables, as appropriate. We show adjusted risk estimates that are derived from the nonlinear models in order to show changes in risk over time.14
We used empirical Bayesian methods to determine the hospital-level rate of completion of the 3-hour bundle within 3 hours, administration of antibiotics within 3 hours, and completion of the initial bolus of intravenous fluids within 6 hours.9 We show the ranked order of adjusted rates across hospitals in caterpillar plots. All the analyses were performed with the use of Stata software, version 14.2 (StataCorp).
RESULTS
POPULATION OF PATIENTS AND TIME TO TREATMENT
Of 111,816 patients at 185 hospitals, we excluded 21,046 patients (18.8%) who were ineligible, 32,665 (29.2%) who had protocols initiated outside the emergency department, 3648 (3.3%) who had protocols initiated after 6 hours, and 5126 (4.6%) who did not have the 3-hour bundle completed within 12 hours (Fig. S1 and Table S3 in the Supplementary Appendix). Of the remaining 49,331 eligible patients in the emergency department at 149 hospitals, most (40,696 patients [82.5%]) had the 3-hour bundle completed within 3 hours.
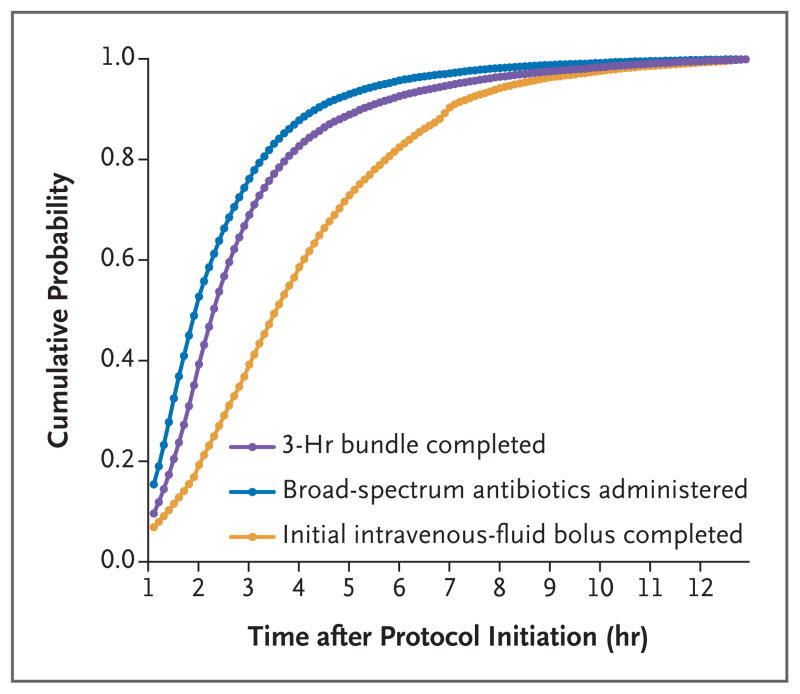
The median time to the completion of the 3-hour bundle was 1.30 hours (interquartile range, 0.65 to 2.35), the median time to the administration of broad-spectrum antibiotics was 0.95 hours (interquartile range, 0.35 to 1.95), and the median time to the completion of the initial bolus of intravenous fluids was 2.56 hours (interquartile range, 1.33 to 4.20) (Fig. 1). The characteristics of the patients who had the 3-hour bundle completed within 3 hours were similar to those who had the bundle completed during hours 3 through 12 (Table 1, and Table S4 in the Supplementary Appendix).
Figure 1. Cumulative Probability of Completion of the 3-Hour Bundle, Administration of Broad-Spectrum Antibiotics, and Completion of the Initial Intravenous-Fluid Bolus after the Time That the Sepsis Protocol Was Initiated.
The 3-hour bundle for the care of patients with sepsis or septic shock had to include receipt of the following care within 3 hours: obtaining of a blood culture before the administration of antibiotics, measurement of the serum lactate level, and the administration of broad-spectrum antibiotics; however, protocols could be tailored by each hospital. We also assessed the time to the administration of broad-spectrum antibiotics and the time to the completion of an initial bolus of intravenous fluids.
Table 1.
Characteristics of the Patients.
| Characteristic | All Patients (N = 49,331) | 3-Hr Bundle Completed in 3 Hr | P Value* | |
|---|---|---|---|---|
| Yes (N = 40,696) | No (N = 8635) | |||
| Percentage of patients | 100.0 | 82.5 | 17.5 | — |
|
| ||||
| Age at admission — yr | <0.001 | |||
|
| ||||
| Median | 73 | 73 | 71 | |
|
| ||||
| Interquartile range | 60–83 | 61–84 | 59–82 | |
|
| ||||
| Female sex — no. (%) | 23,634 (47.9) | 19,157 (47.1) | 4477 (51.8) | <0.001 |
|
| ||||
| Race — no. (%)† | <0.001 | |||
|
| ||||
| White | 33,075 (67.0) | 27,605 (67.8) | 5470 (63.3) | |
|
| ||||
| Black | 8,269 (16.8) | 6,487 (15.9) | 1782 (20.6) | |
|
| ||||
| Asian | 2,167 (4.4) | 1,774 (4.4) | 393 (4.6) | |
|
| ||||
| Other | 5,820 (11.8) | 4,830 (11.9) | 990 (11.5) | |
|
| ||||
| Hispanic ethnic group — no. (%)† | 4,851 (9.8) | 4,022 (9.9) | 829 (9.6) | 0.39 |
|
| ||||
| Coexisting condition — no. (%) | ||||
|
| ||||
| Chronic respiratory failure | 5,738 (11.6) | 4,656 (11.4) | 1082 (12.5) | 0.004 |
|
| ||||
| Congestive heart failure | 10,092 (20.5) | 8,311 (20.4) | 1781 (20.6) | 0.67 |
|
| ||||
| End-stage renal disease | 5,207 (10.6) | 4,109 (10.1) | 1098 (12.7) | <0.001 |
|
| ||||
| Admission source — no. (%) | <0.001 | |||
|
| ||||
| Home | 33,464 (67.8) | 27,306 (67.1) | 6158 (71.3) | |
|
| ||||
| Skilled nursing facility | 13,233 (26.8) | 11,247 (27.6) | 1986 (23.0) | |
|
| ||||
| Other‡ | 2,634 (5.3) | 2,143 (5.3) | 491 (5.7) | |
|
| ||||
| Site of infection — no. (%) | <0.001 | |||
|
| ||||
| Urinary | 13,439 (27.2) | 10,963 (26.9) | 2476 (28.7) | |
|
| ||||
| Respiratory | 19,839 (40.2) | 16,806 (41.3) | 3033 (35.1) | |
|
| ||||
| Gastrointestinal | 4,649 (9.4) | 3,580 (8.8) | 1069 (12.4) | |
|
| ||||
| Other§ | 11,404 (23.1) | 9,347 (23.0) | 2057 (23.8) | |
|
| ||||
| Positive blood cultures — no. (%) | 14,574 (29.5) | 12,322 (30.3) | 2252 (26.1) | <0.001 |
|
| ||||
| Serum lactate — mmol/liter | <0.001 | |||
|
| ||||
| Median | 2.7 | 2.8 | 2.5 | |
|
| ||||
| Interquartile range | 1.7–4.4 | 1.8–4.4 | 1.6–4.1 | |
|
| ||||
| Septic shock — no. (%) | 22,336 (45.3) | 18,393 (45.2) | 3943 (45.7) | 0.43 |
|
| ||||
| Teaching facility — no. (%) | 40,257 (81.6) | 7,739 (19.0) | 7300 (84.5) | <0.001 |
|
| ||||
| In-hospital death — no. (%) | 11,251 (22.8) | 9,213 (22.6) | 2038 (23.6) | 0.05 |
P values are based on Pearson’s chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Race and ethnic group were determined from medical records.
Other locations include clinic or unknown.
Other site of infection includes skin, central nervous system, and unknown.
PRIMARY ANALYSES
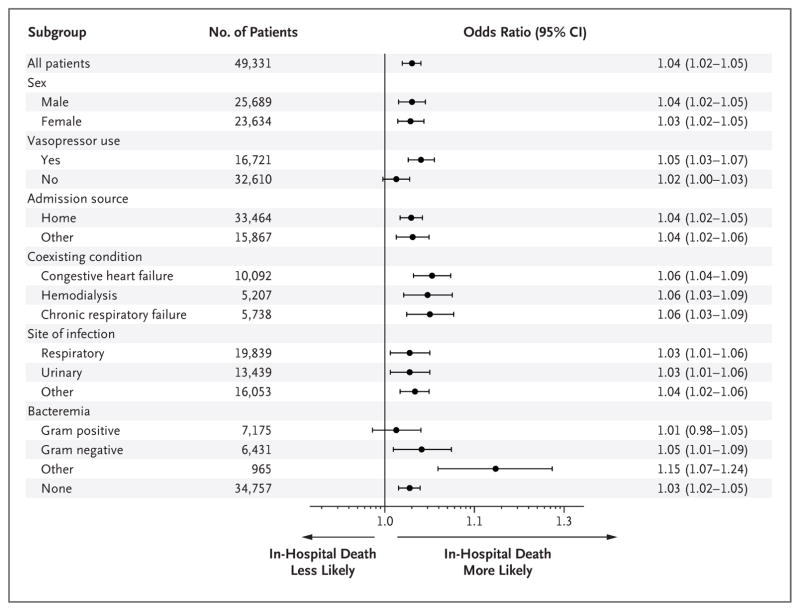
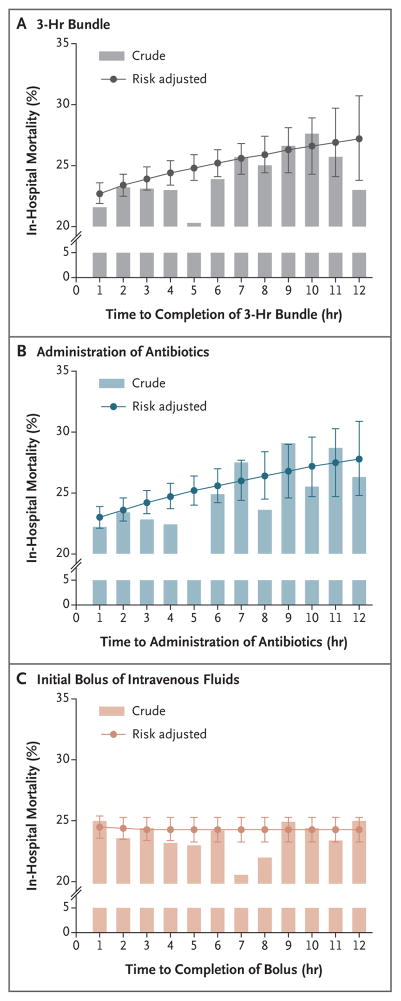
In a multivariable model, each hour of time to the completion of the 3-hour bundle was associated with higher mortality (odds ratio of death until completion of 3-hour bundle, 1.04 per hour; 95% confidence interval [CI], 1.02 to 1.05; P<0.001) (Fig. 2, and Table S5 in the Supplementary Appendix). Patients who had the bundle completed during hours 3 through 12 had 14% higher odds of in-hospital death than patients in whom all three items in the 3-hour bundle were completed in 3 hours (odds ratio, 1.14; 95% CI, 1.07 to 1.21; P<0.001). The association between the time to the administration of antibiotics and in-hospital mortality was similar (odds ratio of death until antibiotics were administered, 1.04 per hour; 95% CI, 1.03 to 1.06; P<0.001) (Fig. S3 in the Supplementary Appendix). Patients who received antibiotics in hours 3 through 12 had 14% higher odds of in-hospital death than those who received antibiotics within 3 hours (odds ratio, 1.14; 95% CI, 1.06 to 1.22; P = 0.001). These associations appeared to be stronger among patients receiving vasopressors than among those who were not receiving vasopressors (Fig. 2, and Fig. S3 in the Supplementary Appendix). Figure 3 shows the crude and predicted risks of in-hospital death across a range of times to treatment in typical patients who presented to the emergency department. On average, the completion of the 3-hour bundle at 6 hours was associated with mortality that was approximately 3 percentage points higher than the mortality associated with completion of the bundle within the first hour.
Figure 2. Risk-Adjusted Odds Ratios of In-Hospital Death in the Primary Model and Prespecified Subgroups.
Shown are odds ratios, with 95% confidence intervals, for in-hospital death for each hour that it took to complete the 3-hour bundle. Other site of infection includes gastrointestinal, skin, central nervous system, and unknown.
Figure 3. Crude In-Hospital Mortality and Predicted Risks of In-Hospital Death.
Shown are the crude in-hospital mortality and predicted risks of in-hospital death, with adjustment for covariates across a range of time after protocol initiation, for the completion of the 3-hour bundle of sepsis care (Panel A), the administration of broad-spectrum antibiotics (Panel B), and the completion of the initial bolus of intravenous fluids (Panel C) in a typical patient. I bars represent 95% confidence intervals.
Among the 26,978 patients who were eligible for and had the bolus of intravenous fluids completed within 12 hours, the time to completion of the fluid bolus was not associated with inhospital mortality (odds ratio of death until fluid bolus was completed, 1.01 per hour; 95% CI, 0.99 to 1.02; P = 0.21) (Fig. S4 in the Supplementary Appendix). Patients who had the initial fluid bolus completed during hours 6 through 12 had an odds of in-hospital death that was similar to that among patients who had the initial fluid bolus completed within 6 hours (odds ratio of death for >6 hours to complete intravenous-fluid bolus, 1.02; 95% CI, 0.92 to 1.14; P = 0.65). We found no interaction between time to the administration of antibiotics and time to completion of the initial bolus of intravenous fluids (P = 0.88).
ADDITIONAL ANALYSES
A sensitivity analysis that used the earliest time of arrival in the emergency department to measure the time to treatment showed an association that was similar to that in the primary analyses. The results were unchanged when hospice discharges were reclassified as in-hospital deaths or when we excluded patients who had treatments completed before protocol initiation. When the time window for protocol initiation or completion of the 3-hour bundle was relaxed to 24 hours, the association between completion of the bolus of intravenous fluids and mortality became significant, albeit of very small magnitude (odds ratio 1.001; 95% CI, 1.000 to 1.002; P = 0.03). Details are provided in Table S6 in the Supplementary Appendix.
In supporting analyses, we found that the time to obtaining a blood culture was associated with mortality (odds ratio, 1.04 per hour; 95% confidence interval, 1.02 to 1.06; P<0.001). Similar findings were observed for each hour until serum lactate measurement (Figs. S5 and S6 in the Supplementary Appendix). The quantitative bias analysis indicated that our results would be robust unless an unmeasured confounder was at least twice as prevalent among patients who had the 3-hour bundle completed later as among those who had it completed 1 hour earlier and unless the unmeasured confounder increased the odds of in-hospital death by more than 1.35 times (Fig. S7 in the Supplementary Appendix).
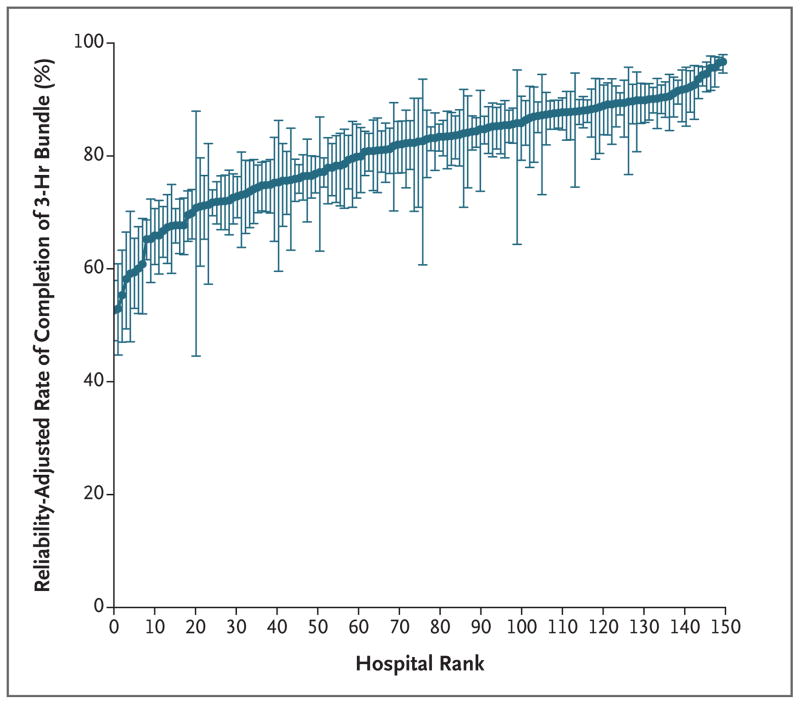
The risk-adjusted and reliability-adjusted rates of completing the 3-hour bundle ranged from 53 to 97% (median, 83%; interquartile range, 75 to 88) (Fig. 4, and Fig. S8 in the Supplementary Appendix). After we ranked hospitals from the lowest to greatest likelihood of completing the 3-hour bundle, the hospitals in the highest decile, despite similar illness severity among their patients, were 1.5 times as likely to complete the 3-hour bundle as hospitals in the lowest decile (94.3% vs. 64.1%). Hospitals that had a higher rate of bundle completion within 3 hours were somewhat smaller and less likely to be teaching hospitals than those that took longer than 3 hours to complete the bundle (Table S7 in the Supplementary Appendix).
Figure 4. Reliability-Adjusted Rate for Each Hospital for Completion of the 3-Hour Bundle in 3 Hours, According to Hospital Rank.
The 149 hospitals that were included in the study were ranked from lowest to highest, with higher numbers indicating a greater likelihood of completing the 3-hour bundle within 3 hours. I bars represent 95% confidence intervals.
DISCUSSION
Our findings support an association between time to treatment and outcome among patients with sepsis or septic shock treated in the emergency department during a statewide initiative mandating protocolized care. We found that a longer time to completion of a 3-hour bundle of care for patients with sepsis and the administration of broad-spectrum antibiotics were each associated with higher risk-adjusted in-hospital mortality. In our primary analysis, we did not find an association between the time to completion of the initial bolus of intravenous fluids and inhospital mortality. The time to treatment varied widely across hospitals.
Our findings are consistent with multiple smaller, observational studies.5,15,16 A recent meta-analysis of 11 observational studies, however, showed no significant mortality benefit of the administration of antibiotics within 3 hours, as compared with after 3 hours, after triage in the emergency department (odds ratio, 1.16; 95% CI, 0.92 to 1.46) or within 1 hour after the recognition of shock (odds ratio, 1.46; 95% CI, 0.89 to 2.40).6 The odds ratios we report are similar, but the confidence intervals are narrower given the much larger sample size that was included in our study.
This study complements a patient-level meta-analysis of goal-directed therapy in severe sepsis and septic shock, the Protocolized Resuscitation in Sepsis Meta-Analysis (PRISM) trial.17 More than three of four patients in the PRISM trial received elements of the 3-hour bundle before randomization, after which the various trials composing the PRISM trial tested whether protocolized resuscitation strategies improved outcomes. Our study asked a different question: does timing matter for these earliest and most basic elements of care? These population-level data also place in context the relatively high compliance with these steps in the control groups of the various trials composing the PRISM trial before randomization. Only half the hospitals in the statewide database performed near this level.
There are several biologic explanations for the association between the time to completion of a 3-hour treatment bundle and outcome. First, more rapid administration of antibiotics reduces pathogen burden, modifies the host response, and could reduce the incidence of subsequent organ dysfunction. Second, clinicians who decide more quickly to measure the serum lactate level may identify heretofore unrecognized shock and are more prepared to deliver lactate-guided resuscitation than clinicians who are slower to measure the serum lactate level — a strategy that may improve outcome in randomized trials.18 Third, physicians have broad variation in how they identify sepsis, even when they are presented with similar cases.19 Fast delivery of sepsis treatment, even within the structure of mandated protocols, requires a prompt clinical suspicion of both infection and worsening organ dysfunction.
Although we found no association between the time to completion of the initial bolus of intravenous fluids and outcome in our primary analysis, these data should not be interpreted as evidence in favor of abandoning early fluid resuscitation. The analysis of the time to completion of the initial fluid bolus is most prone to confounding by indication (e.g., sicker patients will receive fluids sooner and are also more likely to die).20 A greater volume of fluids given at rapid pace may also contribute to adverse effects such as pulmonary edema, volume overload, and longer duration of organ support in selected patients.21 Causal inference will require investigation in randomized clinical trials, and our analysis contributes to the clinical equipoise needed for such trials.
We found a variation of 1 to 2 times across hospitals with regard to the rates of completing the 3-hour bundle, the administration of antibiotics, and the completion of a bolus of intravenous fluids in the emergency department. Adherence, in general, ranged from 60 to 90%, and was greater than in comparable quality-improvement programs for stroke treatment in New York.22 Such performance may stem from increasing public awareness and advocacy about sepsis and national quality-improvement initiatives led by CMS.23 Adherence was greatest in the emergency departments at smaller nonteaching hospitals, a finding that differs from a previous cohort study.24 These hospitals may have fewer clinicians to train, a lower census in the emergency department, and a different case mix as compared with larger referral centers, which perhaps facilitates the more rapid implementation of sepsis protocols.
Our study has several limitations. First, this was not a randomized trial, so the results may be biased by confounding. Of greatest concern may be the lack of data about the appropriateness of broad-spectrum antibiotics. The appropriateness of the initial choice of an antibiotic agent has been associated with risk-adjusted mortality25 but may be measurable only in the minority of patients with positive cultures and may differ according to local pathogen and antimicrobial resistance profiles. The hospitals included in this study were limited to a single state that may have epidemiologic features of sepsis that are distinct from those in other geographic regions.26 The start time for measuring delays may not be accurate in all cases. To address this, we evaluated models that used the earliest time of arrival in the emergency department and found no change in associations.
Our statewide evaluation showed that the times to the completion of a 3-hour bundle and the administration of broad-spectrum antibiotics were associated with greater in-hospital mortality among patients with severe sepsis and septic shock in the emergency department. We found no association between the time to completion of the initial bolus of intravenous fluids and outcome. If the relationship is causal, prompt recognition and faster treatment of sepsis and septic shock in the context of emergency care may reduce the incidence of avoidable deaths.
Supplementary Material
Acknowledgments
Funded by the National Institutes of Health and others.
Supported by grants (R35GM119519, to Dr. Seymour; and K08 GM115859, to Dr. Prescott) from the National Institutes of Health, by a grant (11-109 13-079, to Dr. Iwashyna) from the Veterans Affairs Health Services Research and Development Investigator-Initiated Research program, and by IPRO (to Mr. Phillips).
We thank the members of the New York State Sepsis Advisory Workgroup for assistance with the development and implementation of the New York State Sepsis initiative.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the view of the U.S. government or the Department of Veterans Affairs.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care — reasons for caution. N Engl J Med. 2014;370:1673–6. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Haery C, Paladugu B, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193:251–8. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43:1907–15. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson Reuters; 2017. New York codes, rules and regulations: 405.4 medical staff. ( https://govt.westlaw.com/nycrr/Document/I4fe39657cd1711dda432a117e6e0f345?viewType=FullText&%3BoriginationContext=documenttoc&%3BtransitionType=CategoryPageItem&%3BcontextData=(sc.Default)(last.)=Default=(sc.Default)) [Google Scholar]
- 8.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45:1614–29. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banta JE, Joshi KP, Beeson L, Nguyen HB. Patient and hospital characteristics associated with inpatient severe sepsis mortality in California, 2005–2010. Crit Care Med. 2012;40:2960–6. doi: 10.1097/CCM.0b013e31825bc92f. [DOI] [PubMed] [Google Scholar]
- 12.Reineck LA, Pike F, Le TQ, Cicero BD, Iwashyna TJ, Kahn JM. Hospital factors associated with discharge bias in ICU performance measurement. Crit Care Med. 2014;42:1055–64. doi: 10.1097/CCM.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data. New York: Springer-Verlag; 2009. [Google Scholar]
- 14.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology — with an emphasis on fractional polynomials. Methods Inf Med. 2005;44:561–71. [PubMed] [Google Scholar]
- 15.Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39:2066–71. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017 Mar 27; doi: 10.1164/rccm.201609-1848OC. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The PRISM Investigators. Early, goal-directed therapy for septic shock — a patient-level meta-analysis. N Engl J Med. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 18.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multi-center, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 19.Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care. 2016;20:89. doi: 10.1186/s13054-016-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 22.Gropen TI, Gagliano PJ, Blake CA, et al. Quality improvement in acute stroke: the New York State Stroke Center Designation Project. Neurology. 2006;67:88–93. doi: 10.1212/01.wnl.0000223622.13641.6d. [DOI] [PubMed] [Google Scholar]
- 23.Cooke CR, Iwashyna TJ. Sepsis mandates: improving inpatient care while advancing quality improvement. JAMA. 2014;312:1397–8. doi: 10.1001/jama.2014.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189:548–55. doi: 10.1164/rccm.201311-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez-Guillamet C, Scolari M, Zilberberg MD, Shorr AF, Micek ST, Kollef M. Using the number needed to treat to assess appropriate antimicrobial therapy as a determinant of outcome in severe sepsis and septic shock. Crit Care Med. 2014;42:2342–9. doi: 10.1097/CCM.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 26.Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G. National variation in United States sepsis mortality: a descriptive study. Int J Health Geogr. 2010;9:9. doi: 10.1186/1476-072X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.