Abstract
Purpose
We used the Female Sexual Function Index (FSFI) to investigate the prevalence of sexual dysfunction (SD) and factors associated with diminished sexual functioning in early stage endometrial cancer (EC) patients treated with simple hysterectomy and adjuvant brachytherapy.
Methods and Materials
A cohort of 104 patients followed in a radiation oncology clinic completed questionnaires to quantify current levels of sexual functioning. The time interval between hysterectomy and questionnaire completion ranged from <6 months to >5 years. Multivariate regression was performed using the FSFI as a continuous variable (score range, 1.2–35.4). SD was defined as an FSFI score of <26, based on the published validation study.
Results
SD was reported by 81% of respondents. The mean (±standard deviation) domain scores in order of highest-to-lowest functioning were: satisfaction, 2.9 (±2.0); orgasm, 2.5 (±2.4); desire, 2.4 (±1.3); arousal, 2.2 (±2.0); dryness, 2.1 (±2.1); and pain, 1.9 (±2.3). Compared to the index population in which the FSFI cut-score was validated (healthy women ages 18–74), all scores were low. Compared to published scores of a postmenopausal population, scores were not statistically different. Multivariate analysis isolated factors associated with lower FSFI scores, including having laparotomy as opposed to minimally invasive surgery (effect size, −7.1 points; 95% CI, −11.2 to −3.1; P<.001), lack of vaginal lubricant use (effect size, −4.4 points; 95% CI, −8.7 to −0.2, P = .040), and short time interval (<6 months) from hysterectomy to questionnaire completion (effect size, −4.6 points; 95% CI, −9.3–0.2; P = .059).
Conclusions
The rate of SD, as defined by an FSFI score <26, was prevalent. The postmenopausal status of EC patients alone is a known risk factor for SD. Additional factors associated with poor sexual functioning following treatment for EC included receipt of laparotomy and lack of vaginal lubricant use.
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States (1). It is curable when diagnosed at an early stage and treated with simple hysterectomy with or without adjuvant radiation therapy (RT). Due to its favorable risk profile compared to that of pelvic RT (2), intra-vaginal RT (IVRT) is a common form of adjuvant therapy offered to patients with early stage disease and intermediate risk features (3).
EC patients survive for many years (1), and therefore, as demonstrated in the Postoperative Radiation Therapy for Endometrial Carcinoma-2 (PORTEC-2) trial, it is critical to examine patients’ quality of life resulting from treatment (2). Sexual health is an important aspect of quality of life in cancer patients (4), and vaginal toxicity including stenosis following RT has been reported (5, 6). The need for better evaluation of patient-reported sexual effects in this patient group has been acknowledged by several authors (4, 7).
Recent tools have been developed and validated which may address this problem. The Female Sexual Function Index (FSFI) is a measure designed by Rosen and colleagues for self-reporting female sexual functioning (SF) (8). The FSFI is simply worded and assesses 6 domains of SF in women: (1) desire, (2) arousal, (3) dryness, (4) orgasm, (5) satisfaction, and (6) pain. It has been used previously with cervical cancer patients to compare outcomes resulting from treatment (9). Recently, there have been 2 investigations reporting on FSFI scores in early stage EC patients (10, 11), however, these were small studies in which only relatively few patients (13 and 29 patients, respectively) had received radiation.
In the present cross-sectional study, we sought to measure the prevalence of sexual dysfunction (SD) by using the FSFI in a large group of early stage EC patients, all of whom received simple hysterectomy and adjuvant IVRT. We aimed to characterize SF through the FSFI domain scores and isolate potential factors influencing SF in this unique population.
Methods and Materials
Patient cohort and study design
The names and medical record numbers of 473 patients with early stage EC (stage I–II) who had received IVRT following simple hysterectomy at Memorial Sloan-Kettering Cancer Center between January 1, 2003 and September 30, 2009, were identified through institutional and departmental databases. Patients were excluded due to receipt of chemotherapy/hormone therapy (n=123), pelvic RT (n=2), previous cancer diagnosis (n=59), death (n=22), or recurrent disease (n=21). Additional patients were excluded who did not speak English (n=10) or who were lost to follow-up (defined as no appointment in 2008 or 2009 [n=27]). The remaining 209 patients were eligible to participate in this cross-sectional study.
All eligible patients attending outpatient clinic visits during a prespecified time period, October 25, 2009–February 26, 2010, were approached in clinic and invited to complete the study questionnaire. All additional eligible patients without a scheduled appointment during the study period were identified via data extraction from the departmental scheduling system and were sent the identical study questionnaire by mail. The questionnaire itself had no patient identifiers. Patients participating in clinic placed the completed questionnaires in an unmarked box at the nursing station. The mailed questionnaires were returned in premarked postage-paid envelopes without any patient identifiers. A list with patient identifiers was used solely for determining patient eligibility for the study, was not linked in any manner to the de-identified questionnaires, and was destroyed at the conclusion of the study period. The anonymous study design was approved by the institutional review board under a waiver of authorization and consent.
Cancer treatment
Surgery for all patients was either total abdominal hysterectomy via laparotomy or, alternatively, minimally invasive surgery with either laparoscopic or robot-assisted technology. In the period 2001–2005, eligible patients at our institution were offered participation in the LAP-2 protocol of the Gynecologic Oncology Group (GOG) (12). For patients not enrolled on LAP-2, the surgical technique used depended upon the comfort/experience of the surgeon and the patient’s preference. Regardless of surgical technique, standard practice during this time period was to perform complete surgical lymph node staging when feasible.
All patients received IVRT with a single radiation oncologist (K.M.A.) via an after-loading high-dose-rate Ir-192 source, with a technique previously described (3). Cylinder diameter varied from 2.0–3.0 cm, and prescription was to 0.5 cm from the vaginal surface. IVRT dose was 2100 cGy in 3 fractions if a 3.0 cm cylinder was used and was decreased to 1800 cGy in three fractions for a cylinder diameter of <3.0 cm to minimize mucosal toxicity. The typical prescription length was 4 cm for stage IA–B grades 1 and 2; 5 cm for stage IC grade 1 and 2; 6 cm for stage II; and 7 cm for grade 3 disease. Dose optimization was performed in all cases to taper the dose distribution and improve the depth dose in the distal vagina.
Patients were followed with physical examination and vaginal cytology every 3–6 months for the first 2 years and every 6–12 months beyond 2 years. Women were routinely instructed to use vaginal dilators 3 times per week, starting 4 weeks after IVRT and continuing indefinitely.
Questionnaire content
To quantify participants’ current level of SF, the FSFI was chosen because it has been widely validated, is simply worded, and takes only a few minutes to complete (8). A sample question from the FSFI is, “Over the past four weeks, how satisfied have you been with your sexual relationship with your partner?” Response options are “Very Satisfied,” “Moderately satisfied,” “About equally satisfied and dissatisfied,” “Moderately dissatisfied,” or “Very dissatisfied.” In total, there are 19 multiple-choice questions assessing 6 domains of SF in women: (1) desire, (2) arousal, (3) dryness, (4) orgasm, (5) satisfaction, and (6) pain. Individual scores from each domain are combined to yield a composite score, with the highest possible score of 36. In a study demonstrating the validity and reliability of the FSFI, an analysis of sensitivity and specificity yielded a cut score of 26 for the identification of women with SD (13).
In addition to the FSFI, the anonymous questionnaire items included age group (<50, 50–59, 60–69, 70–79, 80+ years), ethnicity, marital status, educational level, time interval from hysterectomy, surgery type, performance status, and use of interventions for vaginal health including dilators, synthetic lubricants (ie, vitamin E, K-Y Jelly [McNEIL-PPC, Inc., Canada]), and moisturizers (ie, Replens [Lil’ Drug Store Products, Inc., Cedar Rapids, Iowa, USA]). To ensure anonymity of the participants, age group rather than exact age was collected.
Data analysis
FSFI scores were considered evaluable if more than 50% of questions were answered for each domain. For all analyses, FSFI scores were analyzed continuously (score range, 1.2–35.4). All variables except age were treated as categorical; age group was investigated as an ordinal predictor (<50, 50–59, 60–69, 70–79, 80+) because analyses using age group as a categorical variable showed a roughly monotonic relationship with FSFI. Age group was also investigated as a dichotomous variable with various cutoffs. All 3 ways of incorporating age group resulted in similar model estimates. Univariate analyses to examine associations between patient and treatment characteristics and FSFI scores were performed using linear regression. Multivariate regression was applied to identify factors independently associated with SF. The multivariate model was built based on variables significant in univariate analysis and on results in topical published literature. Fisher’s exact test (2-tailed) was used to compare characteristics between dilator use groups (ever vs never used, currently using vs not currently using, and recommended vs sporadic use). A P value of less than .05 was considered significant. All statistical analyses were performed with SAS version 9.2 and R 2.11.1 software.
Results
Patient characteristics
Of 209 questionnaires distributed, 159 (76%) were returned. Of these 159 respondents, 26 had all FSFI items left blank. Reasons for omitting the FSFI included a lack of interest in the sensitive subject matter (n=11), lack of time (n=3), and a lack of sexual activity (n=3). Nine patients provided no reason. An additional 29 patients completed parts of the FSFI, however, due to missing items, their scores were considered unevaluable. The remaining 104 patients (50% of the initial 209) had evaluable FSFI scores and are the subject of the present analyses (Fig. 1, flow diagram).
Fig. 1.
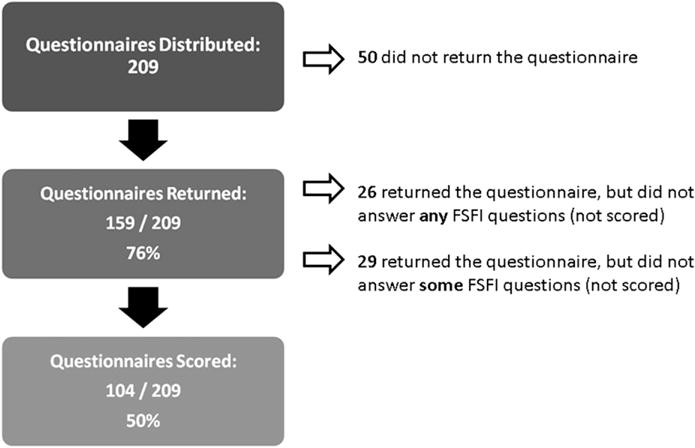
Flow diagram of EC patient cohort.
Among respondents, 74% were older than 60, 61% were married, 80% were white, and 57% had ≥1 comorbidity (diabetes, hypertension, depression). Surgical technique was minimally invasive in 51%. Patient responses were captured at a wide range of time from hysterectomy, varying from <6 months to >5 years. Patient characteristics are detailed in Table 1.
Table 1.
Patient characteristics, FSFI scores, and univariate results
| Covariate | Level | No. of patients (%) | Mean FSFI (±SD)* | Univariate P value† |
|---|---|---|---|---|
| Age group (y) | <50 | 6 (5.8) | 19.8 (12.2) | .096 |
| 50–59 | 21 (20.2) | 14.3 (10.3) | ||
| 60–69 | 44 (42.3) | 15.0 (11.2) | ||
| 70–79 | 24 (23.1) | 10.9 (9.6) | ||
| 80+ | 9 (8.7) | 12.2 (10.1) | ||
| Racial group | White | 83 (79.8) | 14.3 (10.6) | .599 |
| Black/African-American | 7 (6.7) | 10.5 (9.3) | ||
| Hispanic | 6 (5.8) | 9.2 (11.2) | ||
| Asian | 4 (3.8) | 16.6 (14.6) | ||
| Other | 4 (3.8) | 18.0 (11.8) | ||
| Marital status | Married | 63 (60.6) | 14.3 (10.4) | .683 |
| Divorced | 8 (7.7) | 12.5 (11.3) | ||
| Single | 18 (17.3) | 11.3 (10.4) | ||
| Widowed | 9 (8.7) | 15.3 (12.8) | ||
| Other | 6 (5.8) | 18.0 (11.5) | ||
| Education level | No degree | 3 (2.9) | 3.7 (2.2) | .359 |
| High school degree | 28 (27.2) | 13.4 (10.7) | ||
| College degree | 32 (31.1) | 15.2 (11.4) | ||
| Postgraduate degree | 40 (38.8) | 14.2 (10.4) | ||
| Diabetes | No | 86 (82.7) | 14.6 (10.7) | .146 |
| Yes | 18 (17.3) | 10.6 (10.3) | ||
| High bp | No | 63 (60.6) | 15.2 (11.3) | .137 |
| Yes | 41 (39.4) | 12.0 (9.5) | ||
| Anxiety | No | 87 (83.7) | 14.4 (10.8) | .319 |
| Yes | 17 (16.3) | 11.6 (9.7) | ||
| Karnofsky performance score | 80 or less | 10 (9.6) | 9.4 (6.2) | .159 |
| 90 or 100 | 94 (90.4) | 14.4 (10.9) | ||
| Time from hysterectomy (binary) | <6 mo | 25 (24.3) | 12.2 (9.9) | .325 |
| ≥6 mo | 78 (75.7) | 14.7 (10.9) | ||
| Time from hysterectomy | <6 mo | 25 (24.3) | 12.2 (9.9) | .816 |
| 6 mo–1 y | 10 (9.7) | 17.1 (12.0) | ||
| 1–2 y | 15 (14.6) | 16.0 (10.4) | ||
| 2–3 y | 9 (8.7) | 16.5 (12.6) | ||
| 3–4 y | 14 (13.6) | 14.4 (10.1) | ||
| 4–5 y | 10 (9.7) | 13.4 (11.6) | ||
| >5 y | 20 (19.4) | 12.4 (10.9) | ||
| Hysterectomy type | Laparotomy | 45 (45.9) | 10.3 (9.7) | <.001 |
| Minimally invasive surgery | 53 (54.1) | 17.8 (10.5) | ||
| Ever use vaginal dilator | No | 25 (24.3) | 12.3 (11.1) | .346 |
| Yes | 78 (75.7) | 14.6 (10.5) | ||
| Lubricant use since hysterectomy | No | 68 (65.4) | 11.7 (10.7) | .002 |
| Yes | 36 (34.6) | 18.2 (9.2) | ||
| Vaginal moisturizer use since hysterectomy | No | 72 (69.2) | 14.1 (10.8) | .794 |
| Yes | 32 (30.8) | 13.5 (10.6) | ||
| Topical estrogen use since hysterectomy | No | 96 (92.3) | 14.0 (10.7) | .989 |
| Yes | 8 (7.7) | 13.9 (10.4) |
Abbreviation: bp = blood pressure; FSFI = Female Sexual Function Index; SD = standard deviation.
Mean values reflect the FSFI according to each patient characteristic.
P value represents an association between each covariate with FSFI score as a continuous variable.
Sexual functioning scores
The distribution of FSFI scores for all patients is shown in Figure 2. The overall prevalence of SD, as defined by an FSFI score <26, was 80.77%. The mean (± standard deviation) domain scores in order of highest-to-lowest functioning were satisfaction, 2.9 (±2.0); orgasm, 2.5 (±2.4); desire, 2.4 (±1.3); arousal, 2.2 (±2.0); dryness, 2.1 (±2.1); and pain, 1.9 (2.3).
Fig. 2.
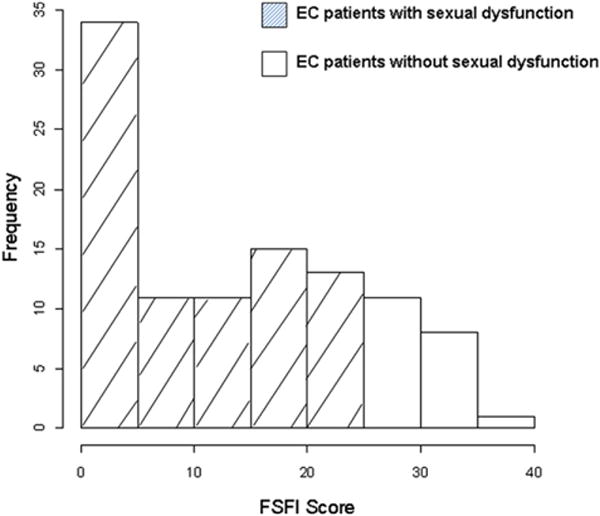
Distribution of FSFI scores in EC patients. A score <26 is considered sexual dysfunction.
The FSFI domain scores for the cohort are given in Figure 3 and, for comparison, are plotted against the domain scores of the index population of women (ages 18–74) in which the FSFI cut-score was established (13). As shown in Figure 3, all domain scores were substantially lower than those of the index population. Also plotted are the FSFI domain scores of a postmenopausal population without EC taken from the literature (14). Compared to this latter group, all domain scores were similar, although dryness, satisfaction, and pain appeared slightly worse in EC patients.
Fig. 3.
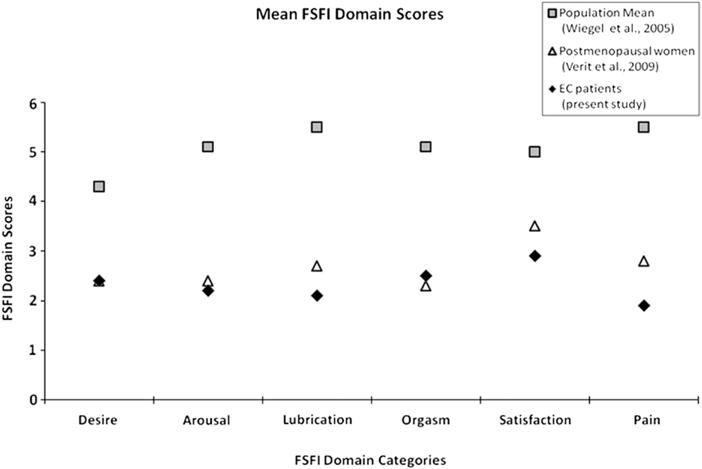
Comparison of mean FSFI domain scores among EC patients, postmenopausal women, and a control population of healthy women (ages 18–74). A higher score indicates a higher level of functioning.
Table 1 lists mean FSFI scores according to patient characteristics and univariate regression results relating patient characteristics with FSFI score. On univariate regression, FSFI score was significantly associated with hysterectomy type (P=.0008) and lubricant use (P=.002), while there was a trend toward an association with patient age group (P=.096).
Multivariate analysis is presented in Table 2. The model included age, hysterectomy type, lubricant use, diabetes, and time interval since hysterectomy. The first 3 variables were included as they had a P value of <.1 on univariate analysis, while the latter 2 variables were included as they had previously been found in the literature to significantly impact SF and quality of life after hysterectomy, respectively (15, 16). The effects of both hysterectomy type and lubricant use on SF were significant, with laparotomy associated with an average 7.1-point decrease in FSFI compared to minimally invasive surgery (95% confidence interval [CI], −11.2 to −3.1); P=.0008), and no lubricant use was associated with an average 4.4-point decrease in FSFI compared to use (95% CI, −8.7 to −0.2); P=.040). Patients who completed the questionnaire within 6 months of hysterectomy had marginally decreased FSFI scores compared to those beyond 6 months (effect size, 4.6-point decrease; 95% CI, −9.3–0.2; P=.059).
Table 2.
Multivariate analysis of factors influencing SF*
| Covariate | Estimated effect on FSFI (95% CI) | P value |
|---|---|---|
| Intercept | 25.345 (19.853–30.836) | <.0001 |
| Age† | −1.596 (−3.699–0.506) | .135 |
| Diabetes | −1.802 (−7.165–3.560) | .506 |
| No lubricant use | −4.444 (−8.682– −0.207) | .040 |
| 6 Less than mo since hysterectomy | −4.568 (−9.314–0.178) | .059 |
| Laparotomy (vs minimally invasive surgery) | −7.122 (−11.181– −3.064) | .0008 |
Ninety-seven of 104 women with evaluable FSFI scores were included in the multivariate model because 7 women were missing covariate information (6 were missing hysterectomy type, 1 woman was missing time from hysterectomy).
Age was recorded as an ordinal variable (<50, 50–59, 60–69, 70–79, 80+). The regression coefficient for age represents the effect of moving into the next age group.
Baseline FSFI scores prior to treatment were not available due to the cross-sectional study design. To approximate baseline sexual activity, patients were asked to recall whether or not they had been sexually active prior to their cancer diagnosis. Sixty-nine patients (66%) reported being sexually active at baseline, and an estimated 71% of those who reported being sexually active prior to their diagnosis remained sexually active at the time of survey completion. Adding patients’ baseline sexual activity to the multivariate model did not substantially change the results.
Rates of vaginal dilator use
Vaginal dilator use (ever vs never used) did not appear to impact SF on univariate analysis (Table 1). We observed that any use of dilators (including use ≥ once per week, ≥ once per month, and less than once per month) as well as recommended dilator use (defined as once per week) both dropped off substantially at 2 years after the patient’s hysterectomy (Fig. 4). Potential predictors of compliance with dilator use were examined, including, age, Karnofsky performance status (KPS), marital status, education, ethnicity, comorbidities (diabetes, hypertension, anxiety), type of hysterectomy, estrogen, moisturizer and lubricant use, and previous sexual activity. Dilator use was associated with higher KPS score (P=.012), lubricant use (P=.029), and moisturizer use (P<.001).
Fig. 4.
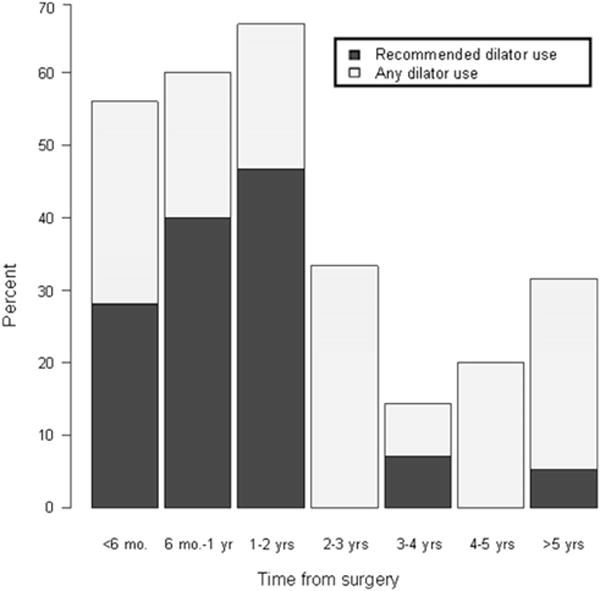
Dilator use with time from RT. Average rate of any dilator use before 2 years is 60%, after is 25%, P<.001. Average rate of recommended dilator use (>1 time/week) before 2 years is 36%, after is 4%, P<.001.
Discussion
Using the published cut-score for SD (FSFI score, <26), we found that the rate of SD in EC patients following hysterectomy and adjuvant IVRT was 81%. This agrees with a recent report which found SD in 89% of EC patients, of whom only a minority (18%) had received radiation (10). Similarly, researchers from the University of Pennsylvania found that “sexual changes” including vaginal dryness, shrinkage, or painful intercourse, were the most common perceived late effects reported by uterine cancer survivors (7). These findings highlight a need for further study of sexual health outcomes after EC treatments.
The high prevalence of SD in EC survivors may be striking; however, when considering their older age and multiple medical comorbidities, the low scores may not be unexpected. Previous studies of postmenopausal women (14) and those with diabetes (15) and other female cancer populations (9) have reported a similarly high prevalence of SD, using the FSFI. Thus, while it is important to be aware of the high risk of SD in EC, one must also consider the multiple potential contributing factors. Onujiogu et al (10) confirmed an association between certain demographic and disease-related factors and FSFI scores in EC, including relationship status, mental health, diabetes, and histologic grade. In our study, we identified treatment-related factors associated with FSFI scores, including, vaginal lubricant use, type of hysterectomy, and time interval from hysterectomy. The association of these factors with SF deserves future study.
Lubricant use was significantly associated with SF on the multivariate analysis, and this might reflect the problem of vaginal dryness faced by these women. While all FSFI domain scores were low, dryness and pain scores were particularly low (Fig. 3). Friedman et al (17) analyzed the Sexual Function-Vaginal Changes Questionnaire in a similar population of EC patients who had undergone IVRT. They reported that 58% of those surveyed reported vaginal dryness, and 23% reported pain. Those findings together suggest there may be an important role for psycho-educational interventions in this population, focused on use of vaginal dilators, lubricants, and moisturizers.
On our multivariate analysis, the factor associated with the largest observed negative effect on SF was having an open hysterectomy as opposed to minimally invasive surgery, which was associated with a 7-point drop in FSFI score (P<.001). There have been several recent prospective randomized studies which have found advantages to laparoscopy compared to laparotomy for EC with regard to technical feasibility, perioperative morbidity, and other quality-of-life endpoints (16, 18); however, those studies did not specifically address SF. To our knowledge, ours is the first study to suggest an association between sexual morbidity and receipt of laparotomy vs minimally invasive surgery in EC patients who had received adjuvant radiation. We hypothesize that the poorer SF scores observed in the laparotomy group may be secondary to an increased likelihood of adhesions and scarring, worse cosmesis, and poorer body image. Additionally, it is possible that the amount of upper vagina resected differed between the surgical techniques and that laparotomies were associated with shorter residual vaginal lengths and possibly a greater percentage of irradiated vagina. Certainly, factors unrelated to treatment, including psychological and social concerns, may account for decreased SF in EC patients (17); therefore, caution is needed in interpreting this retrospective data.
In looking at time from hysterectomy as a variable, we had hypothesized that late survivors would have the poorest functioning due to late toxicities of RT (5, 6). In fact, we found that it was the earliest survivors, patients surveyed within 6 months of their hysterectomy, who had the lowest FSFI scores. It has been shown that there may be physical and psychological effects of treatment which affect quality of life within the first 6 months after surgery (2, 16). Future studies investigating the impact of therapies on sexual morbidity in EC likely should exclude patients who are within the first 6 months since their treatment.
There were several study limitations. The main limitation was a lack of a control group. While FSFI results were compared to those in the literature, an internal comparison group with which to draw conclusions about the impact of local therapies on SF would have been useful. We are currently conducting a study of EC patients treated with hysterectomy with or without IVRT to specifically assess the impact of vaginal irradiation on SF. Second, an inherent limitation of the anonymous study design was the inability to account for dose or length of vagina irradiated, which may influence SF (6). Nonetheless, the anonymous study design may have allowed more patients to respond to questions which might otherwise have been deemed too personal. A third limitation was the lack of baseline FSFI scores. While the cross-sectional study design allowed identification of potential factors influencing SF, prospective longitudinal validation is necessary. Finally, there are several drawbacks of the FSFI instrument in this population. It does not permit the patient to specify whether a reported lack of sexual activity is due to treatment/disease or is a lack of sexual activity in general. Additionally, the standard cut value of 26 is based on a younger population of healthy women ages 18–74 (13). Although this is the only published cut value for defining SD using the FSFI, its applicability to the EC population may be limited, given its older age and medical comorbidities. Future survivorship research would benefit from establishing FSFI norms in different cancer populations.
Conclusions
In summary, SD, defined as an FSFI score of <26, was prevalent among EC patients treated with hysterectomy and adjuvant IVRT. Dryness and pain domains were most affected. There were likely multiple contributing factors to poor SF in this group including patients’ advanced age, postmenopausal status, and medical comorbidities. Treatment-related variables, such as lubricant use, type of surgery, and time from surgery, appear to be associated with SF as well. As treatment options for early stage EC continue to evolve with new combinations of surgery, radiation, and systemic therapies under study, attention to the impact of therapy on adverse symptom reporting, including SF, is worthwhile.
Summary.
A total of 104 patients treated with adjuvant intra-vaginal brachytherapy for early stage endometrial cancer participated in a cross-sectional study to assess level of sexual functioning after treatment. Sexual functioning scores were quantified by the Female Sexual Function Index (FSFI). Sexual dysfunction was present in 81% of the cohort. Receipt of laparotomy (vs minimally invasive surgery) and lack of vaginal lubricant use were associated with poorer FSFI scores.
Footnotes
This study was presented in part at the 53rd Annual Meeting of the American Society for Radiation Oncology (ASTRO), Miami Beach, FL, Oct 2–6, 2011.
Conflict of interest: Mario Leitao, MD, is a consultant for Intuitive Surgical.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009;27:3547–3556. doi: 10.1200/JCO.2008.20.2424. [DOI] [PubMed] [Google Scholar]
- 3.Alektiar KM, Venkatraman E, Chi DS, et al. Intravaginal brachytherapy alone for intermediate-risk endometrial cancer. Int J Radiat Oncol Biol Phys. 2005;62:111–117. doi: 10.1016/j.ijrobp.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Katz A. The sounds of silence: sexuality information for cancer patients. J Clin Oncol. 2005;23:238–241. doi: 10.1200/JCO.2005.05.101. [DOI] [PubMed] [Google Scholar]
- 5.Bruner DW, Lanciano R, Keegan M, et al. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1993;27:825–830. doi: 10.1016/0360-3016(93)90455-5. [DOI] [PubMed] [Google Scholar]
- 6.Bahng AY, Dagan A, Bruner DW, et al. Determination of prognostic factors for vaginal mucosal toxicity associated with intravaginal high-dose rate brachytherapy in patients with endometrial cancer. Int J Radiat Oncol Biol Phys. 2012;82:667–673. doi: 10.1016/j.ijrobp.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 7.Grover S, Hill-Kayser C, Vachani C, et al. Patient reported late effects of gynecological cancer treatment. Gyn Onc. 2012;124:399–403. doi: 10.1016/j.ygyno.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 9.Frumovitz M, Sun CC, Schover LR, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 10.Onujiogu N, Johnson T, Seo S, et al. Survivors of endometrial cancer: who is at risk for sexual dysfunction? Gyn Onc. 2011;123:356–359. doi: 10.1016/j.ygyno.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gyn Onc. 2011;121:169–173. doi: 10.1016/j.ygyno.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 14.Verit FF, Verit A, Billurcu N. Low sexual function and its associated risk factors in pre- and postmenopausal women without clinically significant depression. Maturitas. 2009;64:38–42. doi: 10.1016/j.maturitas.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Olarinoye J, Olarinoye A. Determinants of sexual function among women with type 2 diabetes in a Nigerian population. J Sex Med. 2008;5:878–886. doi: 10.1111/j.1743-6109.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 16.Kornblith AB, Huang HQ, Walker JL, et al. Quality of life of patients with endometrial cancer undergoing laparoscopic International Federation of Gynecology and Obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:5337–5342. doi: 10.1200/JCO.2009.22.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys. 2010;80:751–757. doi: 10.1016/j.ijrobp.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 18.Janda M, Gebski V, Brand A, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol. 2010;11:772–780. doi: 10.1016/S1470-2045(10)70145-5. [DOI] [PubMed] [Google Scholar]


