Abstract
Key points
High work of breathing and exercise‐induced arterial hypoxaemia (EIAH) can decrease O2 delivery and exacerbate exercise‐induced quadriceps fatigue in healthy men.
Women have a higher work of breathing during exercise, dedicate a greater fraction of whole‐body towards their respiratory muscles and develop EIAH.
Despite a greater reduction in men's work of breathing, the attenuation of quadriceps fatigue was similar between the sexes.
The degree of EIAH was similar between sexes, and regardless of sex, those who developed the greatest hypoxaemia during exercise demonstrated the most attenuation of quadriceps fatigue.
Based on our previous finding that women have a greater relative oxygen cost of breathing, women appear to be especially susceptible to work of breathing‐related changes in quadriceps muscle fatigue.
Abstract
Reducing the work of breathing or eliminating exercise‐induced arterial hypoxaemia (EIAH) during exercise decreases the severity of quadriceps fatigue in men. Women have a greater work of breathing during exercise, dedicate a greater fraction of whole‐body towards their respiratory muscles, and demonstrate EIAH, suggesting women may be especially susceptible to quadriceps fatigue. Healthy subjects (8 male, 8 female) completed three constant load exercise tests over 4 days. During the first (control) test, subjects exercised at ∼85% of maximum while arterial blood gases and work of breathing were assessed. Subsequent constant load exercise tests were iso‐time and iso‐work rate, but with EIAH prevented by inspiring hyperoxic gas or work of breathing reduced via a proportional assist ventilator (PAV). Quadriceps fatigue was assessed by measuring force in response to femoral nerve stimulation. For both sexes, quadriceps force was equally reduced after the control trial (−27 ± 2% baseline) and was attenuated with hyperoxia and PAV (−18 ± 1 and −17 ± 2% baseline, P < 0.01, respectively), with no sex difference. EIAH was similar between the sexes, and regardless of sex, subjects with the lowest oxyhaemoglobin saturation during the control test had the greatest quadriceps fatigue attenuation with hyperoxia (r 2 = 0.79, P < 0.0001). For the PAV trial, despite reducing the work of breathing to a greater degree in men (men: 60 ± 5, women: 75 ± 6% control, P < 0.05), the attenuation of quadriceps fatigue was similar between the sexes (36 ± 4 vs. 37 ± 7%). Owing to a greater relative of the respiratory muscles in women, less of a change in work of breathing is needed to reduce quadriceps fatigue.
Keywords: hypoxaemia, oxygen delivery, work of breathing
Key points
High work of breathing and exercise‐induced arterial hypoxaemia (EIAH) can decrease O2 delivery and exacerbate exercise‐induced quadriceps fatigue in healthy men.
Women have a higher work of breathing during exercise, dedicate a greater fraction of whole‐body towards their respiratory muscles and develop EIAH.
Despite a greater reduction in men's work of breathing, the attenuation of quadriceps fatigue was similar between the sexes.
The degree of EIAH was similar between sexes, and regardless of sex, those who developed the greatest hypoxaemia during exercise demonstrated the most attenuation of quadriceps fatigue.
Based on our previous finding that women have a greater relative oxygen cost of breathing, women appear to be especially susceptible to work of breathing‐related changes in quadriceps muscle fatigue.
Abbreviation
arterial oxygen content
fraction of oxyhaemoglobin
- EFL
expiratory flow limitation
- EMG
electromyogram
arterial carbon dioxide tension
arterial oxygen tension
- PAV
proportional assist ventilator
- RMS
root mean square
arterial oxygen saturation
expired minute ventilation
maximum oxygen uptake
respiratory muscle oxygen uptake
whole‐body oxygen uptake
Introduction
In healthy young men, the respiratory system can influence the development of locomotor muscle fatigue during intense exercise in two ways. First, the exercise‐induced arterial hypoxaemia (EIAH) observed in some endurance trained men (Dempsey et al. 1984) contributes to quadriceps muscle fatigue, and alleviating EIAH by breathing a mild hyperoxic inspirate attenuates the severity of fatigue (Romer et al. 2006a). Second, when the work of breathing is reduced, quadriceps muscle fatigue is attenuated (Romer et al. 2006b). The reduction in locomotor muscle fatigue resulting from experimental manipulations (i.e. hyperoxia or reduced work of breathing) is thought to result from increased oxygen delivery to the working muscles. When EIAH is reversed with a mild hyperoxic inspirate (∼0.26), arterial oxyhaemoglobin saturation () is increased to ≥ 98% while arterial oxygen tension () remains near resting values. Consequently, arterial oxygen content () and oxygen delivery are increased. When the work of breathing is reduced, blood flow is redirected towards the active locomotor muscles (Harms et al. 1997) via the respiratory metaboreflex, a sympathetically mediated reflex originating in the respiratory muscles (Dempsey et al. 2006). The effect of respiratory muscle work on blood flow redistribution is only elicited during intense exercise (> 85% of maximum oxygen uptake; ), where the work of breathing is high and there is sufficient competition between vascular beds for the finite cardiac output (Harms et al. 1997; Wetter et al. 1999; Calbet et al. 2004). Increasing oxygen delivery is thought to attenuate fatigue in contracting skeletal muscle by enhancing Ca2+ release and uptake at the sarcoplasmic reticulum (Duhamel et al. 2004) and by lessening the accumulation of metabolic byproducts such as H+ (Adams & Welch, 1980) and inorganic phosphate (Hogan et al. 1999). A concern with the previous studies is the predominant or exclusive use of male subjects, which is pertinent given the recent awareness of important sex‐based differences in exercise physiology (Joyner, 2017).
Compared to men, height matched women have smaller lungs and airways and fewer alveoli (Mead, 1980; Thurlbeck, 1982). Even when matched for absolute lung size, women still have smaller conducting airways (Sheel et al. 2009). Thus, some aspects of the pulmonary response to acute whole‐body exercise differ between the sexes. For example, when minute ventilation () exceeds 60 l min−1, women have a greater total work of breathing (Wanke et al. 1991; Guenette et al. 2007), primarily due to greater resistive rather than viscoelastic work (Guenette et al. 2009; Dominelli et al. 2015a). As a consequence of the higher work of breathing, women have a higher oxygen cost of breathing () for comparable absolute submaximal (i.e. > 55 l min−1 (Dominelli et al. 2015b). It follows that the respiratory muscles in women demand a greater portion of whole‐body () at maximal exercise (Dominelli et al. 2015b), and based on the Fick equation, it is reasonable to suggest that women's respiratory muscles would also command a greater fraction of total cardiac output. Similar to men, women may develop significant EIAH during intense exercise (Harms et al. 1998; Dominelli et al. 2013). Unlike men, however, both moderately and highly trained women can develop EIAH due, in part, to mechanical ventilatory constraints (McClaran et al. 1998; Dominelli et al. 2013), and even untrained trained women have been shown to develop EIAH (Harms et al. 1998; Dominelli et al. 2012). However, we caution that the true incidence of EIAH in both sexes using arterial blood gases is not known.
Given the aforementioned effects of EIAH and high work of breathing on locomotor muscle fatigue coupled with sex differences in the pulmonary physiological response to exercise, we sought to address two questions. First, to what extent does eliminating EIAH attenuate quadriceps muscle fatigue in healthy men and women? Second, will reducing work of breathing during intense exercise attenuate quadriceps muscle fatigue similarly in both sexes? We hypothesized that, regardless of sex, subjects who develop the lowest during exercise will have the greatest attenuation of quadriceps fatigue when EIAH is prevented. We further hypothesized that when work of breathing is reduced, women will show a greater attenuation of quadriceps fatigue.
Methods
Subjects
After providing written informed consent, 16 healthy subjects (8 men) participated in the study. All procedures were approved by the Clinical Research Ethics Board at the University of British Columbia, and adhered to the Declaration of Helsinki. Subjects had a range of exercise participation (recreational to national calibre athletics). All subjects were free of cardiorespiratory ailments, and spirometric values were commensurate with age‐ and sex‐predicted values (American Thoracic Society, 1995). The female subjects were tested randomly throughout their menstrual cycle (MacNutt et al. 2012) and four were using oral contraceptives.
Experimental design
Subjects were tested on four days (Days 1–4), each separated by 48 h to 7 d. Day 1 consisted of a maximal incremental exercise test for the determination of subsequent work rates, whereas Days 2–4 comprised a constant load exercise test. On Days 2–4, the femoral nerve was magnetically stimulated to determine contractile function of the quadriceps femoris before exercise and at 3, 10, 30 and 60 min after exercise. Data collection was similar for all three constant load exercise tests (Days 2–4), except that arterial blood gas analysis was performed on Day 2 only. The constant load exercise test on Day 2 served as a control, and was used to determine exercise duration for subsequent days. The constant load exercise test on Days 3 and 4 involved the experimental manipulations and was performed for an identical duration and work rate as Day 2. On Days 3 and 4, either EIAH was reversed using a hyperoxic inspirate or work of breathing was reduced using a proportional assist ventilator (PAV). For Days 3 and 4, the order of manipulations (reversal of EIAH or reduction in work of breathing) was randomized and counterbalanced. Familiarization sessions were performed prior to the PAV trial to ensure the subjects were accustomed to the sensation of assisted breathing during exercise and were coached to relax and allow the PAV to assist inspiration.
Maximal exercise (Day 1)
To obtain maximal work rate and the full range of values for work of breathing, a step‐wise incremental exercise test on a cycle ergometer (VeloTron Pro, RacerMate, Seattle, WA, USA) was performed to the limit of tolerance after placement of an oesophageal balloon‐tipped catheter. Men began at 120 W and women at 80 W, with a 20 W increase every 2 min for both groups. A step‐wise exercise protocol was selected in order to assess respiratory mechanics at discrete work rates and the duration of the test was longer than a typical ramp protocol (20–30 W min−1). Accordingly, due to the slow‐component of O2 kinetics, the values were above values commensurate with similar work rates but a shorter duration test. Cardiorespiratory function and respiratory mechanics were assessed using customized hardware and software (Dominelli et al. 2015a).
Constant load exercise tests (Days 2–4)
Subjects exercised on a similar cycle ergometer, maintained seat height/handle bar position and used the same pedalling cadence across all tests. Subjects remained seated throughout all of the tests. On Day 2, subjects performed a self‐selected warm‐up and on Days 3 and 4 this warm‐up was replicated. Exercise intensity was >85% of the maximal work rate achieved during the test performed on Day 1. On Day 2, the subjects exercised for as long as possible while maintaining >60 rpm (range 8.7–21.5 min). On Days 3 and 4 the constant load exercise test was performed for an identical duration and external work rate as on Day 2. For each constant load exercise test, cardiorespiratory variables were collected as per Day 1 with the addition of quadriceps electromyogram (EMG; see below).
Hyperoxia
The hyperoxic constant load exercise test was performed while inspiring humidified hyperoxic gas ( 0.23–0.30). The level of hyperoxia was individually selected to reverse any hypoxaemia during exercise while maintaining at physiological levels. Since exercise‐induced changes in arterial blood gases are reproducible and consistent across differing exercise protocols (Romer et al. 2006a; Dominelli et al. 2012), we used the measured values from Day 2 to create a unique level of hyperoxia for each subject to return to 98%. Hyperoxic gas mixtures were made using a previously described system (Dominelli et al. 2014).
Proportional assist ventilator (PAV)
A PAV was used to partially unload the respiratory muscles during a constant load exercise test. The PAV apparatus has been previously detailed (Dominelli et al. 2016) and elicits comparable effects to other devices (Younes et al. 1987; Gallagher & Younes, 1989; Lua et al. 2001; Babcock et al. 2002; Romer et al. 2006b; Amann et al. 2007). Briefly, using a proportional solenoid valve and compressed air, positive airway pressure was generated during inspiration in proportion to the subject's inspiratory effort, thereby unloading the respiratory muscles and reducing the work of breathing in a controlled manner. Maximal positive inspiratory pressure was set to 40 cmH2O and was typically between 20 and 30 cmH2O. The degree of unloading was set to the maximal level tolerated by each subject. Before the constant load exercise test on the PAV, subjects completed several familiarization trials on separate days.
Electromyography
Surface EMG of the rectus femoris, vastus lateralis, and right and left costal diaphragm were recorded using surface electrodes (F‐E15D160, Grass Technologies, Warwick, RI, USA). The electrodes were connected with non‐amplified leads (F‐SL48 and F‐P5IC3/REV1, Grass Technologies) to an amplifier (P511 Series, Grass Technologies). Electrodes for the rectus femoris and vastus lateralis were placed on the muscle bellies in parallel to the fibre orientation. The diaphragm electrodes were placed on the anterior axillary line in the sixth to eighth intercostal spaces. Ground electrodes were placed on the bony process of the anterior superior iliac spine and patella. Peak‐to‐peak amplitude and integrated area of the M‐waves were measured for each twitch. EMG signals were amplified (200×) and band‐pass filtered (30 and 2000 Hz). The analog signals were A/D converted (PowerLab/16SP model ML 795, ADInstruments, Colorado Springs, CO, USA) and recorded simultaneously (10 kHz) using data acquisition software (LabChart v8.1, ADInstruments).
Quadriceps fatigue
An inelastic strap was tightly wrapped around the malleoli of the subject's right leg. The strap was connected to a load cell (SML 1000, Interface, Scottsdale, AZ, USA) via a metal cable and carabineer. The load cell was attached to a large wooden chair and was adjusted in three planes through customized hardware (80/20 Inc., East Columbia City, IN, USA). Adjustments were made to ensure that traction along the load cell was parallel to the ground. Using a goniometer, we ensured that the subject's knee was in 90 deg flexion and the hip–knee–ankle alignment was similar across trials. During the assessment of quadriceps fatigue, the subject was seated upright on the chair and secured via a seat‐belt across the hips and shoulders to ensure only the quadriceps were activated. Quadriceps fatigue assessment was always performed before diaphragm fatigue. The femoral nerve was stimulated using a commercially available magnetic stimulator (MagStim 200 Mono Pulse, MagStim, Whitland, UK) and custom‐made large ‘figure‐of‐eight’ coil. To ensure the stimulator coil was in the same orientation and angle throughout testing, we used both indelible ink and a three‐dimensional accelerometer (ADXL335, Adafruit, New York, NY, USA) that was securely fastened to the shaft of the stimulator coil. The accelerometer output voltage signals were in proportion to the three‐dimensional orientation of the device attached to the stimulator coil. The signals were available in real‐time to the investigators through the use of custom‐built software (LabView 8.0, National Instruments, Austin, TX, USA). Once satisfied with the initial coil placement, the orientation was marked in the software so that the location could be easily referenced for pre‐ and post‐exercise twitch assessment.
At baseline, the location of stimulation that elicited the greatest quadriceps twitch was located and marked. An incremental protocol to verify whether stimulation was supramaximal was then performed. Briefly, we collected three twitches, each separated by 30 s, at 60, 70, 80, 85, 90, 95 and 100% of the stimulator's output. Thereafter, subjects performed a series of static maximal voluntary contractions (MVCs) of their quadriceps. Immediately (∼1 s) after each contraction a single twitch at 100% of stimulator output was delivered. Subjects performed a minimum of six and maximum of nine MVCs per twitches and the first two were discarded because maximal potentiation was not achieved. The series of potentiated twitches was performed before, immediately after (3 min) and 10, 30 and 60 min after each constant load exercise test. The entire fatigue assessment, including rigorous identification of the largest twitch and the ramp protocol, was performed after each constant load exercise test (Days 2–4). Quadriceps muscle fatigue was defined as a significant post‐exercise reduction in twitch force amplitude compared to pre‐exercise baseline values.
Diaphragm fatigue
We were concerned about the PAV's ability to eliminate diaphragm fatigue, and therefore we assessed diaphragm fatigue in a subset of subjects (n = 4, 2 women) using cervical magnetic stimulation (Similowski et al. 1989) as we have previously described (Guenette et al. 2010). As lung volume can influence twitch amplitude (Smith & Bellemare, 1987), all twitches were performed at end‐expiratory lung volume as verified using end‐expiratory oesophageal pressure prior to each stimulation. The protocols for determining supramaximal stimulation and contractile fatigue were identical to those described above for the quadriceps.
Arterial blood sampling
On Day 2, arterial blood samples were obtained from the radial artery as described previously (Dominelli et al. 2013; Foster et al. 2014) and analysed immediately with a commercial blood gas analyser (ABL80 CO‐OX, Radiometer, Copenhagen, Demark). The catheter was kept patent through the use of pressurized saline. Offline, blood gases were corrected for core temperature (Severinghaus, 1966) as measured by a rapid response thermistor (Ret‐1, Physitemp Instruments, Clinton, NJ, USA) placed in the oesophagus at a similar depth as the oesophageal balloon catheter.
Data analysis
To ensure nerve stimulation was consistent, excitability of the muscles was inferred from the peak‐to‐peak amplitude and area of the M‐waves for each potentiated twitch before and after exercise. The amplitude of each twitch was determined by subtracting the baseline force/pressure from the peak. Raw EMG signals from the vastus lateralis corresponding to each muscle contraction during the constant load exercise trials were processed to determine electrical activation of the muscle. Signals were band‐pass filtered between 30 and 2000 Hz. Thereafter, the root mean square (RMS) was determined for each contraction using 200 ms moving averaging (Blouin et al. 2007). The peak RMS of each contraction was extracted then averaged over 30 s windows. Values are expressed as a percentage of the first minute of each constant load exercise trial (Amann et al. 2007).
The mechanical work of breathing was determined as previously described (Dominelli & Sheel, 2012). For all presented data, the work of breathing was calculated using oesophageal pressure–volume loop integration and inspiratory force was inferred using oesophageal pressure–time product. The method of assessing the work of breathing was selected because Campbell diagrams and integrating trans‐pulmonary pressure–volume loops are not valid during assisted ventilation (i.e. PAV (Dominelli et al. 2016)). All other cardiorespiratory variables were collected continuously using an online system (Dominelli et al. 2015b). Pressure, flow and force signals were A/D converted and recorded simultaneously (200 Hz) using data acquisition software (LabChart v8.1, PowerLab; ADInstruments).
Repeat trials
Additional constant load exercise tests (and associated fatigue measures) were performed to verify the reproducibility of our findings. Specifically, we repeated the control condition (Day 2) after the initial three experimental days in two subjects (one man, one woman). We also repeated the PAV trials with the same and varying degrees of unloading in one man and one woman. None of the repeat trials were used for any subsequent analysis other than what is presented in the ‘Repeat trials’ section (see below).
Statistics
Descriptive and maximal exercise test variables between the sexes were compared using Student's unpaired t test. A 2 × 3 (sex [male and female] by condition [control, hyperoxia, PAV]) repeated measures ANOVA was used to test for differences in cardiorespiratory variables for the constant load exercise tests. For quadriceps fatigue, a 3 × 5 (condition [control, hyperoxia, PAV] by time (pre‐ and 3, 10, 30, 60 min post‐exercise) repeated measures ANOVA was used to test for differences in quadriceps twitch force. When significant F ratios were detected, Tukey's post hoc test was used to determine the location of group mean differences. Pearson's product moment correlation was used to determine the relationship between (i) and predicted and (ii) fraction of oxyhaemoglobin () and a difference in quadriceps fatigue immediately post‐exercise between the control and hyperoxia trial. Significance was set at P < 0.05 and data are presented as means ± SEM.
Results
Subjects
Subject characteristics, pulmonary function and maximal exercise data (Day 1) are presented in Table 1. Both groups were of similar age, and pulmonary function was within normal limits (Tan et al. 2011). For the incremental exercise test, men achieved a higher peak work rate and higher absolute and relative . The were no differences in aerobic power when values were expressed as a percentage of predicted (Jones et al. 1985). Despite a higher in men, operational lung volumes, expiratory flow limitation (EFL) and oesophageal pressures were similar between sexes; however, the absolute work of breathing (in J min−1) was significantly higher in men (Table 1).
Table 1.
Subject characteristics, pulmonary function and maximal exercise data
| Men | Women | |
|---|---|---|
| Age (years) | 29 ± 3 | 28 ± 2 |
| Mass (kg) | 74 ± 2 | 60 ± 2* |
| Height (cm) | 179 ± 2 | 166 ± 3* |
| FVC (l) | 5.4 ± 0.2 | 4.0 ± 0.2* |
| FVC (% predicted) | 98 ± 4 | 101 ± 5 |
| FEV1 (l) | 4.4 ± 0.2 | 3.2 ± 0.1* |
| FEV1 (% predicted) | 98 ± 3 | 96 ± 3 |
| FEV1/FVC (%) | 82 ± 3 | 81 ± 3 |
| Work rate (W) | 338 ± 17 | 235 ± 7* |
| Test time (min) | 22 ± 2 | 16 ± 1* |
| HR (beats min−1) | 186 ± 3 | 185 ± 4 |
| (l min−1) | 4.5 ± 0.2 | 3.1 ± 0.1* |
| (ml kg−1 min−1) | 60.5 ± 1.6 | 52.7 ± 2.7* |
| (% predicted) | 135 ± 5 | 145 ± 8 |
| (l min−1) | 4.7 ± 0.1 | 3.3 ± 0.1* |
| RER | 1.09 ± 0.02 | 1.05 ± 0.02 |
| V T (l) | 3.2 ± 0.2 | 2.1 ± 0.1* |
| f b (beats min−1) | 57 ± 3 | 59 ± 4 |
| V T/FVC (%) | 59 ± 1 | 53 ± 3 |
| V T/T I (l sec−1) | 6.2 ± 0.2 | 4.1 ± 0.2* |
| V T/T E (l sec−1) | 5.9 ± 0.2 | 3.9 ± 0.2* |
| T I/T tot (%) | 49 ± 1 | 49 ± 1 |
| (l min−1) | 180 ± 6 | 120 ± 5* |
| / | 40 ± 1 | 39 ± 1 |
| / | 38 ± 1 | 37 ± 1 |
| ERV (% FVC) | 34 ± 2 | 38 ± 2 |
| ∆P oe (cmH2O) | 56 ± 4 | 47 ± 4 |
| WOB (J min−1) | 625 ± 42 | 366 ± 42* |
| WOB/ (J l−1) | 3.5 ± 0.2 | 3.0 ± 0.2 |
| PTPI (cmH2O l−1 s−1) | 595 ± 35 | 552 ± 50 |
| (l min−1) | 212 ± 7 | 160 ± 9* |
| / (%) | 85 ± 1 | 76 ± 5 |
| EFL (n) | 6/8 | 5/8 |
| EFL (%) | 26 ± 1 | 21 ± 5 |
Values are means ± SEM. EFL, expiratory flow limitation defined as percentage overlap of tidal breaths on the maximal expiratory flow volume curve; ERV, expiratory reserve volume; f b, breathing frequency; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, heart rate; ∆P oe, oesophageal pressure swing; PTPI, inspiratory pressure–time product; RER, respiratory exchange ratio; T E, expiratory time; T I, inspiratory time; , carbon dioxide output; , ventilatory capacity; , oxygen uptake; V T, tidal volume; WOB, work of breathing. *Significantly different from men, P < 0.05.
Femoral nerve stimulation
Both sexes demonstrated a plateau in quadriceps twitch force and M‐wave amplitude with increasing stimulation intensity (Fig. 1). Furthermore, there were no differences in absolute twitch force or M‐wave amplitude for both muscles combined between any of the experimental days (control, hyperoxia, PAV) at any stimulation intensity, regardless of sex. Men had a greater baseline potentiated quadriceps twitch force and MVC force, but neither sex showed a difference between constant load exercise trials for either variable (Table 2). The coefficients of variation within an experimental trial were not different between sexes or trials (Table 2). Between‐day coefficients of variation for baseline potentiated twitch force were not different between the sexes (3.3 ± 0.8 vs. 4.9 ± 0.6% for men and women, respectively; range: men 1.3–8.5%, women 2.7–7.0%). For the quadriceps muscles and sexes combined, there was no difference in M‐wave amplitude (97 ± 3, 98 ± 2 and 100 ± 4% baseline) or area (99 ± 3, 105 ± 4 and 101 ± 2% baseline) when compared to pre‐exercise values for all three experimental trials (control, hyperoxia and PAV, respectively) (P > 0.05). There were no differences between the sexes at any time point for M‐wave amplitude or area.
Figure 1. Group mean quadriceps twitch force (A and B) and M‐wave amplitude (C and D) in response to magnetic stimulation of the femoral nerve at different stimulator outputs for men (A and C) and women (B and D).
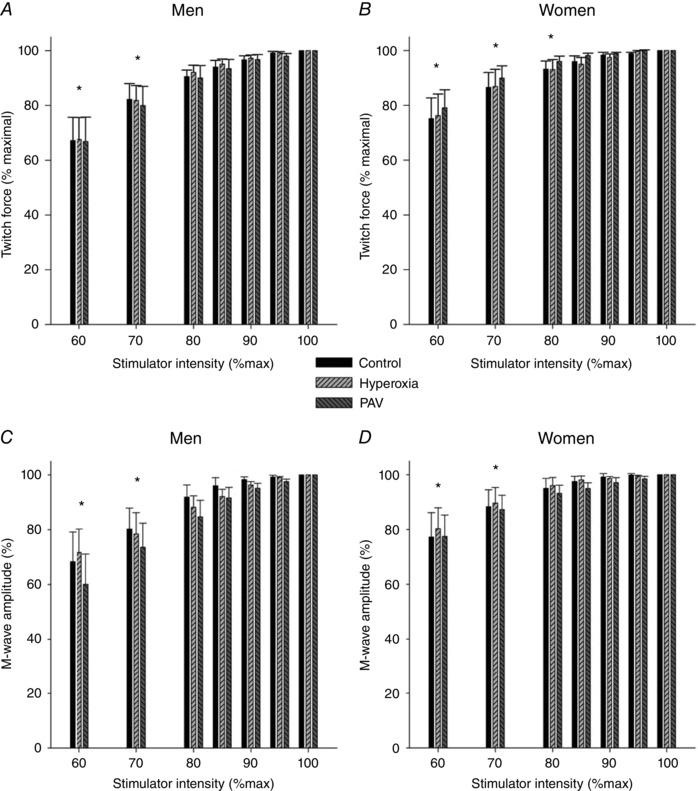
There was no difference between control, hyperoxia or PAV for men or women at any stimulator intensity for twitch force or M‐wave amplitude. PAV, proportional assist ventilator. *Significantly different for all conditions from 100%. P < 0.05.
Table 2.
Cardiorespiratory variables averaged over the final 50% of the three constant load exercise trials and quadriceps function variables at baseline
| Control | Hyperoxia | PAV | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | W | M | W | M | W | Sex | Trial | Interaction | |
| Cardiorespiratory | |||||||||
| Work rate (W) | 287 ± 16 | 203 ± 6* | — | — | — | — | |||
| Work rate (W kg−1) | 3.78 ± 0.2 | 3.44 ± 0.2 | 3.79 ± 0.2 | 3.44 ± 0.2 | 3.79 ± 0.2 | 3.44 ± 0.2 | NS | NS | NS |
| Cadence (rpm) | 97 ± 3 | 88 ± 2* | 98 ± 3 | 89 ± 2* | 97 ± 3 | 90 ± 2* | 0.046 | NS | NS |
| RMS EMG (% 1st min) | 141 ± 6 | 134 ± 6 | 129 ± 4 | 123 ± 7† | 124 ± 6 | 127 ± 6† | NS | 0.003 | NS |
| HR (beats min−1) | 179 ± 3 | 181 ± 3 | 175 ± 4 | 180 ± 4 | 173 ± 4 | 181 ± 3§ | NS | 0.018 | 0.026 |
| (l min−1) | 4.3 ± 0.2 | 2.9 ± 0.1* | 4.1 ± 0.1 | 2.9 ± 0.1*, ‡ | 3.9 ± 0.1 | 2.8 ± 0.1*, † | < 0.001 | < 0.001 | 0.028 |
| (% control) | — | — | 98 ± 1 | 98 ± 2‡ | 92 ± 1 | 94 ± 2 | NS | < 0.001 | NS |
| (ml kg−1 min−1) | 58 ± 2 | 49 ± 3* | 56 ± 1 | 49 ± 3*, ‡ | 53 ± 1 | 46 ± 2*, † | 0.016 | < 0.001 | NS |
| (% max) | 96 ± 1 | 94 ± 1 | 94 ± 2 | 92 ± 2‡ | 88 ± 1 | 88 ± 2† | NS | < 0.001 | NS |
| (l min−1) | 4.3 ± 0.2 | 2.9 ± 0.1* | 4.1 ± 0.1 | 2.9 ± 0.1*, ‡ | 3.9 ± 0.2 | 2.8 ± 0.1*, † | < 0.001 | < 0.001 | 0.03 |
| RER | 1.00 ± 0.02 | 1.00 ± 0.01 | 1.02±.01 | 1.02 ± 0.01 | 1.00 ± 0.01 | 1.02 ± 0.03 | NS | NS | NS |
| V T (l) | 3.1 ± 0.1 | 2.0 ± 0.1* | 3.2 ± 0.2 | 2.1 ± 0.1*, ‡ | 3.3 ± 0.1 | 2.4 ± 0.1*, † | < 0.001 | < 0.001 | NS |
| f b (breaths min−1) | 51 ± 2 | 54 ± 3 | 43 ± 2 | 48 ± 2† | 47 ± 2 | 48 ± 2† | NS | < 0.001 | NS |
| (l min−1) | 158 ± 7 | 108 ± 4* | 135 ± 7 | 100 ± 4*, †, ‡ | 153 ± 5 | 114 ± 4* | < 0.001 | < 0.001 | NS |
| (% max) | 85 ± 2 | 91 ± 3* | 75 ± 4 | 84 ± 3*, †, ‡ | 83 ± 3 | 96 ± 5* | NS | < 0.001 | NS |
| / | 37 ± 1 | 37 ± 1 | 33 ± 2 | 35 ± 1†, ‡ | 39 ± 1 | 42 ± 2† | NS | < 0.001 | NS |
| / | 37 ± 1 | 37 ± 1 | 33 ± 2 | 34 ± 1†, ‡ | 39 ± 1 | 41 ± 1† | NS | < 0.001 | NS |
| ∆P oe (cmH2O) | 48 ± 2 | 41 ± 3 | 39 ± 2 | 37 ± 2†, ‡ | 32 ± 3 | 32 ± 3† | NS | < 0.001 | NS |
| PTP (cmH2O l−1 s1) | 568 ± 28 | 499 ± 36 | 459 ± 13 | 456 ± 27†, ‡ | 303 ± 29 | 360 ± 38† | NS | < 0.001 | 0.004 |
| ∆PTP (% control) | — | — | 84 ± 4 | 92 ± 5‡ | 54 ± 4 | 70 ± 4 | < 0.001 | < 0.001 | < 0.001 |
| (%) | Room air | 26.1 ± 0.5 | 25.5 ± 0.2 | Room air | |||||
| Quadriceps function | |||||||||
| Baseline twitch (N) | 192 ± 8 | 130 ± 7* | 189 ± 9 | 133 ± 6* | 190 ± 8 | 129 ± 4* | < 0.001 | NS | NS |
| Range | 158–234 | 113–174 | 160–230 | 108–157 | 155–229 | 116–149 | |||
| Within occasion CV (%) | 1.0 ± 0.2 | 1.4 ± 0.3 | 2.1 ± 0.9 | 1.7 ± 0.3 | 1.6 ± 0.3 | 2.0 ± 0.4 | NS | NS | NS |
| Baseline MVC (N) | 640 ± 21 | 430 ± 27* | 615 ± 17 | 446 ± 32* | 618 ± 21 | 456 ± 34* | < 0.001 | NS | NS |
Abbreviations: M, men; W, women; CV, coefficient of variation; f b, breathing frequency; , fraction of inspired oxygen; HR, heart rate; MVC, maximal voluntary contraction; ∆P oe, oesophageal pressure swing; PTP, pressure–time product; RER, respiratory exchange ratio; RMS EMG, root mean square of EMG for vastus lateralis; , carbon dioxide output; , expired minute ventilation; , oxygen uptake; V T, tidal volume; WOB, work of breathing. *Significant main effect of sex; †significantly different from control (both sexes pooled); ‡significantly different from PAV (both sexes pooled); §significant interaction between sexes; P < 0.05.
Arterial blood gases
Mean and individual values for arterial blood gases and oesophageal temperature during the control constant load exercise test are presented in Figs 2 and 3, respectively. During exercise, oesophageal temperature and arterial pH decreased in a time‐dependent manner (Fig. 2 A and C), but did not differ between the sexes. Although the group mean remained near pre‐exercise resting values (Fig. 2 B), there was considerable inter‐subject variability (Fig. 3 A) and no differences between the sexes. Some subjects maintained within 5 mmHg of resting values for the duration of the constant load exercise test. Other subjects exhibited a considerable decrease in , with a male and female subject each having a reduction of 25–30 mmHg relative to pre‐exercise values; these two subjects were the most aerobically fit ( 65 and 64 ml kg−1 min−1 for the man and woman, respectively). Overall, there was a significant relationship between end‐exercise and percentage predicted (r = 0.50, P < 0.05). For both sexes, the major contributor (> 80%) to the change in oxyhaemoglobin saturation was due to temperature, pH and related shifts in the oxyhaemoglobin dissociation curve, rather than to changes in . However, both sexes had some subjects (n = 2 each) whose reduction in was responsible for ∼50% of the change in . Table 3 displays the changes in blood gas variables from rest to exercise termination. Men had a higher resting haemoglobin concentration and haematocrit, which resulted in greater . Despite a lower at exercise termination, haemoconcentration occurred in both groups, resulting in a greater at termination compared to baseline (Table 3). If no desaturation had occurred, the group average increase in would have been 0.7 ml dl−1 greater. Both sexes demonstrated the anticipated changes in other variables, with no differences in the changes from baseline between sexes (Table 3).
Figure 2. Group mean arterial blood gas and oesophageal temperature throughout the control (Day 2) constant load exercise trial.
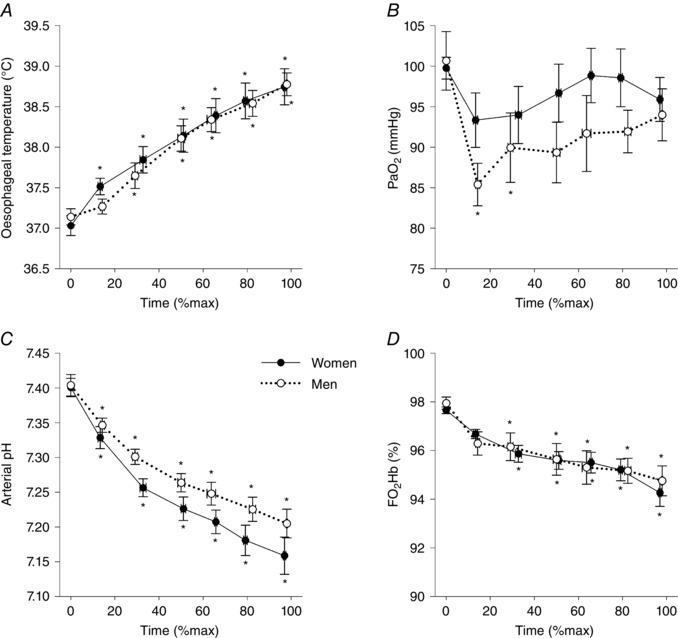
, arterial oxygen tension; , oxyhaemoglobin saturation. *Significantly different from baseline, P < 0.05.
Figure 3. Individual subject changes in arterial oxygen tension, oxyhaemoglobin saturation and arterial oxygen content throughout the control constant load exercise trial.
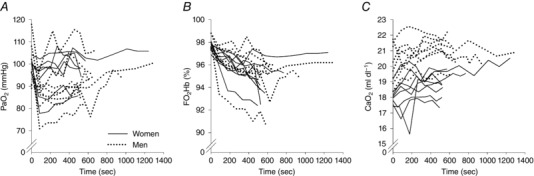
Table 3.
Arterial blood variables at baseline and near exercise termination for control constant load exercise trial
| Baseline | Termination | Change from baseline | ||||
|---|---|---|---|---|---|---|
| M | W | M | W | M | W | |
| [K+] (mmol l−1) | 4.0 ± 0.2 | 3.8 ± 0.1 | 5.5 ± 0.1† | 5.4 ± 0.1† | 1.4 ± 0.3 | 1.6 ± 0.1 |
| [Ca2+] (mmol l−1) | 1.19 ± 0.01 | 1.17±.01 | 1.25 ± 0.02† | 1.26 ± 0.01† | 0.05 ± 0.03 | 0.09 ± 0.02 |
| [Na+] (mmol l−1) | 142 ± 0.1 | 142 ± 5 | 145 ± 0.9† | 146 ± 0.9† | 3.1 ± 0.9 | 3.8 ± 0.8 |
| [Cl−] (mmol l−1) | 108 ± 1 | 110 ± 1 | 112 ± 0.9† | 112 ± 0.9† | 3.6 ± 1.0 | 2.3 ± 0.5 |
| [Hb] (g dl−1) | 14.5 ± 0.3 | 12.9 ± 0.2* | 15.6 ± 0.3† | 14.1 ± 0.2†, * | 1.2 ± 0.3 | 1.1 ± 0.2 |
| Hct (%) | 44 ± 1 | 40 ± 1* | 48 ± 1† | 43 ± 1†, * | 3.5 ± 0.8 | 3.4 ± 0.5 |
| FHHb (%) | 1.3 ± 0.2 | 1.5 ± 0.2 | 4.4 ± 0.6† | 4.2 ± 0.6† | 3.1 ± 0.7 | 2.7 ± 0.7 |
| [HCO3 −] (mmol l−1) | 22.4 ± 0.3 | 20.3 ± 0.5* | 12.1 ± 0.9† | 10.6 ± 0.9†, * | −10.2 ± 1.0 | −9.6 ± 1.4 |
| Base excess (mmol l−1) | −1.4 ± 0.4 | −3.2 ± 0.3* | −14.6 ± 1.2† | −16.9 ± 1.3† | −13.2 ± 1.2 | −13.7 ± 1.2 |
| (mmHg) | 99 ± 4 | 100 ± 1 | 94 ± 3 | 96 ± 2 | −5 ± 5 | −3 ± 3 |
| (range) | (89–117) | (93–104) | (81–111) | (86–106) | (−23–21) | (−17–8) |
| (mmHg) | 37 ± 1 | 34 ± 2 | 31 ± 1† | 31 ± 1† | − ± 2 | −3 ± 1 |
| (range) | (29–41) | (26–38) | (24–34) | (28–33) | (−17–0) | (−6–2) |
| (mmHg) | 110 ± 3 | 110 ± 2 | 117 ± 3† | 117 ± 1† | 7 ± 3 | 7 ± 1 |
| (range) | (100–124) | (106–120) | (113–123) | (113–120) | (−5–22) | (0–10) |
| A‐a (mmHg) | 11 ± 1 | 9 ± 2 | 23 ± 3† | 21 ± 3† | 12 ± 3 | 11 ± 3 |
| (range) | (5–15) | (7–17) | (12–33) | (8–34) | (1–23) | (−1–25) |
| (ml dl−1) | 20.1 ± 0.5 | 18.1 ± 0.2* | 21.0 ± 0.3† | 18.9 ± 0.4†, * | 0.9 ± 0.4 | 0.7 ± 0.2 |
| 1 (ml dl−1) | 20.1 ± 0.5 | 18.1 ± 0.2* | 21.7 ± 0.4† | 19.6 ± 0.4†, * | 1.6 ± 0.4 | 1.4 ± 0.2 |
Values are means ± SEM. M, men; W, women; A‐a, alveolar‐to‐arterial oxygen gradient; , arterial oxygen content; 1 , ideal if no desaturation was present; FHHb, fraction of deoxyhaemoglobin; Hb, haemoglobin; Hct, haematocrit; , arterial oxygen tension; , alveolar oxygen tension. *Significantly different from men; †significantly different from baseline; P < 0.05.
Cardiorespiratory responses during constant load exercise
Cardiorespiratory variables for the latter half of each constant load exercise tests are presented in Table 2. The latter 50% of each constant load exercise test was chosen because it represented a relative steady‐state in and a sustained elevated (> 50% of maximal ) and work of breathing. Compared to control, was lower during hyperoxia and not different vs. PAV for both sexes. However, the PAV trial resulted in a lower average for both sexes, despite the work rates being identical to the control. Despite men achieving a greater , the oesophageal pressure swing and pressure–time product were not different between men and women, but were significantly lower during the PAV trial for both sexes.
Quadriceps muscle exercise response
Men adopted a significantly higher pedal cadence than women, but there was no difference across trials for either sex (Table 2). Compared to the control trial, RMS EMG amplitude was reduced for both the hyperoxia and PAV trial, with no difference between the sexes (Table 2). The percentage change in quadriceps twitch force across conditions for both sexes is presented in Fig. 4. Relative to the control trial, the PAV and hyperoxia trials attenuated quadriceps fatigue to a similar degree in men and women. The differences in quadriceps fatigue were still evident at 60 min after exercise. Hyperoxia resulted in a 31 ± 5% attenuation of quadriceps fatigue immediately after exercise, and this response did not differ on the basis of sex. However, the degree of attenuation was variable between subjects (range ∼0–60%). The degree of attenuation with hyperoxia was significantly related to the nadir during the control trial (Fig. 5). That is, subjects with the lowest in the control trial had the greatest increase in their post‐exercise twitch amplitude,
Figure 4. Changes in quadriceps twitch force across time for each condition and sex.
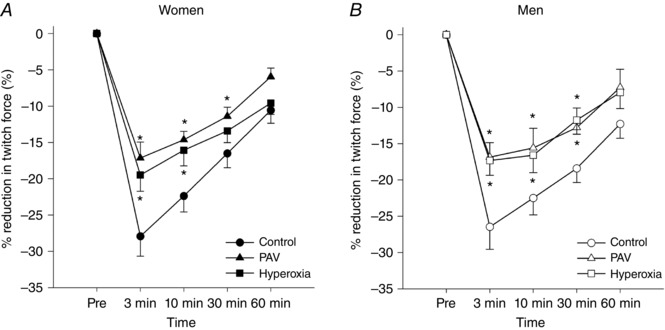
PAV, proportional assist ventilator. *Significantly different vs. control, P < 0.05.
Figure 5. Correlations between nadir oxyhaemoglobin saturation () and quadriceps function between the hyperoxia and control constant load exercise trials.
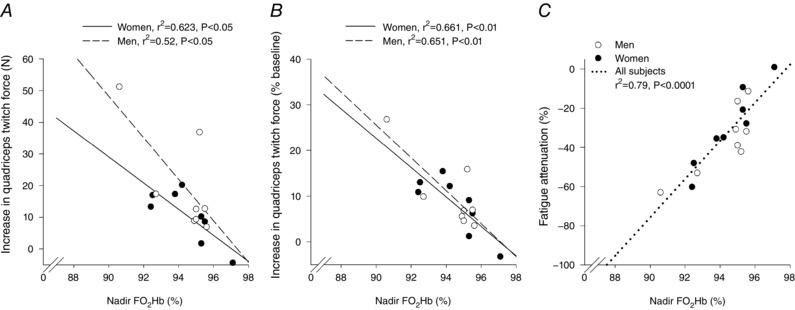
For all panels, quadriceps function was assessed using the average potentiated twitch amplitude 3 min post‐exercise. A and B show the absolute and relative increase in quadriceps twitch force, respectively. C shows the percentage attenuation in quadriceps fatigue after the hyperoxia trial.
with the slope greater in men (Fig. 5 A). However, when the increase in twitch amplitude was expressed as a relative increase, the sexes were identical (Fig. 5 B). Overall, those who developed the lowest exhibited the greatest attenuation of fatigue in the hyperoxia trial. The PAV trial resulted in a significantly reduced work of breathing (Fig. 6 A and B), but there was considerable variability in this decrease. There was no significant difference in for either sex during the PAV trial compared to control (P > 0.05; Fig. 7), but men had a significantly greater reduction in absolute work of breathing compared to women (75 ± 19 vs. 60 ± 5% of control, P < 0.05; Fig. 7). When the work of breathing was expressed as work unit per unit , however, all subjects decreased during the PAV trial in a similar manner (Fig. 6 C). Overall, relative to control, exercise on the PAV resulted in a significant attenuation of quadriceps fatigue (37 ± 4%) and a lower with no difference between sexes (Fig. 7). Both sexes demonstrated a significant relationship between percentage change in work of breathing from control to PAV trial and percentage attenuation in quadriceps fatigue (P < 0.05). The difference between slopes of the regressions approached statistical significance (0.82 ± 0.3 vs. 0.33 ± 0.1 for women and men, respectively, P = 0.08).
Figure 6. Work of breathing for the control trial and the proportional assist ventilator (PAV) trial.
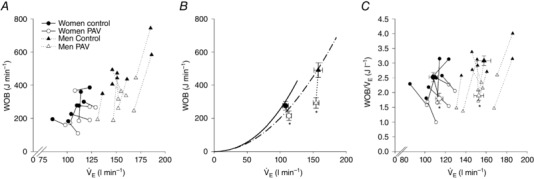
A shows individual data points for each subject, whereas B shows the group mean. Regression lines in B are redrawn from maximal exercise, with the solid and broken lines representing women and men, respectively. C shows both individual subject (small symbols) and group means (large symbol). , ventilation; PAV, proportional assist ventilator; WOB, work of breathing. *Significantly different from control trial, P < 0.05.
Figure 7. Quadriceps fatigue, work of breathing, ventilation and whole‐body oxygen uptake during the PAV trial as a percentage of control.
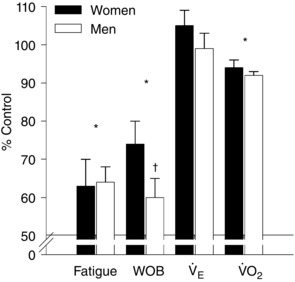
, ventilation; , oxygen uptake; WOB, work of breathing. *Significantly different from control for both sexes; †significantly different from women; P < 0.05.
Diaphragm fatigue
In the subset of subjects (n = 4) in which diaphragm fatigue was assessed, potentiated transdiaphragmatic twitch pressure amplitude was significantly reduced (82 ± 5% of baseline) immediately after the control constant load exercise. The PAV trial resulted in attenuation of diaphragm fatigue (95 ± 3% of baseline, P < 0.05 compared to control) for all of the subjects. Baseline potentiated diaphragm twitches were not different between control and PAV (33.6 ± 1.0 vs. 32.6 ± 1.6 cmH2O, respectively; P = 0.65).
Repeat trials
To verify the reproducibility of the change in quadriceps fatigue for the control trial, a male and female subject completed an additional control trial within 7 days of the final experimental trial. The absolute and relative differences between the control trials are presented in Table 4. To determine the reproducibility of the effect of PAV on quadriceps fatigue, a different male subject completed a second PAV trial 7 days after the initial trial. For the second PAV trial, with a similar reduction in work of breathing, the attenuation of quadriceps fatigue was within 1% of the first trial. To verify the dose response of the PAV on quadriceps fatigue, another subject completed an additional PAV trial where the reduction in work of breathing was intentionally minimal (work of breathing > 90% of control). The lesser unloading resulted in a post‐exercise reduction in quadriceps twitch amplitude similar to that for the control trial (30 vs. 31% reduction). Conversely, when the subject performed the PAV trial with maximal unloading (work of breathing > 60% of control), the pre‐ to post‐exercise quadriceps twitch was attenuated (24% of the control condition).
Table 4.
Absolute and relative differences in quadriceps twitch and respiratory variables for the two repeated control constant load exercise trials
| Subject A | Subject B | |
|---|---|---|
| (male) | (female) | |
| Absolute baseline twitch (N) | 3.8 | 7.6 |
| % change | 1.6 | 5.5 |
| Absolute 3 min post twitch (N) | 1.9 | 7.1 |
| % change | 1.1 | 4.9 |
| Absolute (l min−1) | 0.05 | 0.09 |
| % change | 1.0 | 2.6 |
| Absolute (ml kg−1 min−1) | 0.7 | 0.1 |
| % change | 1.3 | 0.2 |
| Absolute (l min−1) | 1 | 6 |
| % change | 0.6 | 4.7 |
ventilation; , oxygen uptake.
Discussion
Major findings
The findings from our study are threefold. First, regardless of sex, those who had the greatest decline in with exercise also showed the greatest attenuation of quadriceps fatigue when EIAH was experimentally prevented. We interpret this finding to mean that, independent of sex, the hypoxaemia that can accompany intense exercise contributes to the development of quadriceps fatigue. Second, during high‐intensity cycle exercise we observed that quadriceps fatigue occurred to a similar degree between men and women. This observation differs from other studies, which have demonstrated that women are typically more fatigue resistant during small muscle mass exercise. We suggest that the absence of a difference in quadriceps fatigue may imply that any native sex differences are masked by the many other physiological adjustments that accompany intense exercise that are known to contribute to the development of muscular fatigue. Third, despite reducing the work of breathing to a greater degree in men, both sexes showed similar attenuation of quadriceps fatigue. Thus, the similar quadriceps fatigue attenuation with different degrees of unloading between the sexes indicates that the high work of breathing associated with exercise contributes to locomotor muscle fatigue to a greater extent in women relative to men. Specifically, less of a decrease in work of breathing is needed in women to obtain the same attenuation of fatigue. Overall, we have demonstrated that EIAH contributes to quadriceps fatigue equally in men and women, whereas high work of breathing has a relatively greater effect in women.
Exercise‐induced arterial hypoxaemia
All subjects demonstrated progressive hyperthermia and acidosis, with considerable between‐subject variability with respect to blood gas homeostasis in both sexes. We noted that some subjects had a progressive decline in during exercise, whereas others had an immediate drop that either returned to baseline or stayed depressed throughout exercise. As such, comparing changes in blood gases from baseline to exercise termination may not best represent the temporal appearance or magnitude of gas exchange impairment, as suggested by previous reports (Romer et al. 2006a; Dominelli et al. 2013). Given that blood gas values can vary within an individual during intense exercise, we elected to use the nadir observed during the constant load exercise trial to characterize the hypoxaemia of exercise. We found that those subjects who had the lowest during the control constant load exercise trial demonstrated the greatest attenuation of quadriceps fatigue when breathing hyperoxic gas and this was equally the case for men and women (Fig. 5 C). Specifically, the absolute increase in twitch amplitude after the hyperoxia trial was significantly related to , with men having a greater slope (Fig. 5 A). However, when the change in twitch amplitude was expressed as a percentage of the pre‐exercise twitch (to eliminate the sex effect of muscle strength), there was no difference between the sexes (Fig. 5 B). Thus, while there is clearly individual variability in the extent of oxyhaemoglobin desaturation during constant load exercise, the reversal appears to improve all subjects quadriceps function similarly, regardless of sex.
When breathing “a hyperoxic gas mixture,” oxyhaemoglobin saturation remained >98% for the duration of the constant load exercise tests and the increased would have raised . As EIAH in a single subject has been shown to be reproducible between exercise trials on different days (Dominelli et al. 2012), those who had the lowest during the control constant load exercise trial would have the greatest increase in with hyperoxia. It is important to note that at exercise termination for the control constant load exercise trial did not drop below baseline values (Fig. 3 C). Rather, due to an increase in haemoglobin concentration, rose progressively during exercise (+0.7 ml dl−1) despite a fall in . The relative haemoconcentration we observed is well known to occur during exercise (Astrand et al. 1964; Sjogaard & Saltin, 1982) and could be due to splenic erythrocyte release (Stewart et al. 2003), a decrease in plasma volume (Kargotich et al. 1998), or both. As such, during the hyperoxic trial the increase in was doubled relative to control (+1.5 ml dl−1) owing to increases in both haemoglobin and (Table 3). While the absolute increase in is relatively modest, if we assume a similar O2 extraction, the greater would increase mixed venous , which is an important determinant of pulmonary gas exchange (Wagner, 1982).
The current study was not designed to address sex differences in EIAH, but this point does merit brief comment. In the current study, we found no difference in the arterial blood gas variables between the sexes (Fig. 2, Table 3). Yet, we (Dominelli et al. 2013) and others (Harms et al. 1998) have found that women appear to develop EIAH more readily and to a greater degree than men, though this is not a universal finding (Hopkins et al. 2000; Olfert et al. 2004). There are several potential explanations for the apparent discrepancy. First, the exercise modality in the current study was cycling, which is known to elicit a smaller decrease in compared to running (Hopkins et al. 2000). Second, the aforementioned studies investigating EIAH typically used an incremental stage to elicit . Conversely, our study utilized a constant load exercise protocol, which was designed to induce quadriceps muscle fatigue in part due to the sustained high quadriceps work, acidosis and rise in core temperature, rather than a test to progressively stress pulmonary gas exchange. Finally, even within a relatively homogeneous population in terms of sex, age and aerobic fitness, the appearance and severity of EIAH is variable (Dempsey & Wagner, 1999). Regardless of any sex‐specific effect on the prevalence or severity of EIAH, we clearly show that the reversal of any hypoxaemia has a similar effect on quadriceps fatigue between the sexes.
Sex difference in muscle fatigue
We found no differences in the development of quadriceps muscle fatigue between the sexes after the control constant load exercise trial. Yet, others have found that women are typically more fatigue resistant and this finding persists even when subjects are matched for absolute strength (Hunter et al. 2004). The important difference in many of these studies relates to the relative muscle mass, whether upper or lower limb muscles were studied, and if the exercise was static or dynamic (Hunter, 2009, 2014, 2016). Typically, the exercise paradigm where a sex difference is present involves small muscle mass and isometric contractions in the upper limb (Senefeld et al. 2013). In contrast, when large muscles (including quadriceps) perform dynamic contractions, there appears to be little difference between the sexes (Hunter, 2016; Sundberg et al. 2017), which is consistent with our observation.
While we show no difference in fatigability between the sexes, we cannot fully exclude that there may still be a sex difference in muscle fatigue. Rather, we suggest that during dynamic whole‐body exercise there are many other factors that can influence the development of fatigue and may outweigh any sex‐specific influence. For example, differences in muscle training status result in changes in total force achievable that can compress microvasculature (Hunter & Enoka, 2001), altered muscle capillarization (Saltin et al. 1968) and changes in muscle fibre type (Yan et al. 2011), all of which can influence fatigue and potentially outweigh any sex differences. Furthermore, during intense dynamic whole‐body exercise there are many cardiorespiratory responses that are variable between subjects, including blood flow competition (Harms et al. 1997; Calbet et al. 2004), blood gas homeostasis (Dempsey & Wagner, 1999) and work of breathing (Dominelli et al. 2015a). Although not specifically tested, we suggest that the above confounding variables have a greater influence on fatigue development than any innate sex differences.
Respiratory muscle work
Lowering the mechanical work of breathing via PAV resulted in a consistent attenuation of quadriceps fatigue (17–74% decrease compared to control at 3 min post‐exercise; Fig. 6). The effect of lowered work of breathing on fatigue has been previously demonstrated in men (Romer et al. 2006b; Amann et al. 2007), but has not been shown in women. We found that despite lowering men's work of breathing to a significantly greater degree, the attenuation of quadriceps fatigue was similar between the sexes (Fig. 7). Likewise, the slope of the relationship between work of breathing and percentage attenuation in quadriceps fatigue was 2.5× greater in women (P = 0.07), providing further evidence that a lesser change of work of breathing is needed for similar fatigue attenuation in women. The proposed mechanism behind quadriceps fatigue attenuation resulting from lowering the work of breathing involves sympathetically mediated redistribution of blood flow (Harms et al. 1997) and presumably increased O2 delivery to the working limb. However, when blood flow increases the locomotor muscles, whole body will decrease because less work is performed by the respiratory muscles during a constant load exercise trial (Table 2) (Harms et al. 1997). The decrease in is because the locomotor muscles are already at near‐maximal levels of extraction and locomotor muscle increases minimally (Harms et al. 1997). Conversely, during PAV exercise, decreases considerably. Specifically, in men, the high during intense exercise results in a corresponding to ∼10–12% of (Aaron et al. 1992b). According to the Fick equation, the fraction of total cardiac output directed to an active tissue bed is proportional to the of that tissue. Thus, the respiratory musculature would command ∼10–12% of cardiac output (Dempsey, 2012). Since is linearly related to work of breathing (Aaron et al. 1992a; Dominelli et al. 2015b), any reduction in the work of breathing results in a proportional change in . The blood flow that was previously directed towards the respiratory musculature can now be redirected to the active muscle tissue (Harms et al. 1997). The increased blood flow would serve to lessen fatigue due to an increase in convective O2 delivery (Amann & Calbet, 2008) and reduced metabolite accumulation (Barclay, 1986; Hogan et al. 1998). Compared to men, the for women during intense exercise ( > 75% of max) represents a greater fraction of whole‐body (∼15%) (Dominelli et al. 2015b). Therefore, despite less of a reduction in the work of breathing in women compared to men (75 vs 60% of control), the fraction of associated with the respiratory musculature was similar between the sexes for the PAV trial. Specifically, based on our / estimates at maximum exercise (Dominelli et al. 2015b), a 25 and 40% reduction in the work of breathing for women and men would result in 3.5 and 3.8% reduction in no longer associated with the respiratory muscles. Since the change in as a percentage of was similar between the sexes, it follows that a comparable fraction of cardiac output would have been redirected towards the locomotor muscles. A comparable increase in blood flow to the locomotor muscles would explain why the extent of quadriceps fatigue attenuation was not different between the sexes.
Consistent with the reduction in work of breathing with the PAV is the decrease in whole‐body . We and others (Romer et al. 2006b) found that whole‐body decreased from 96% of during the control trial to 88% for the PAV constant load exercise trial. The reduction in whole‐body is the result of lower work of breathing and therefore , rather than a change in leg , which increases minimally (Harms et al. 1997). Critically, the change in during the PAV trial was similar between the sexes (Table 2). The similar change in despite a different work of breathing attenuation provides further evidence that a similar amount of cardiac output could be redirected to the quadriceps resulting in similar fatigue attenuation.
During the hyperoxia constant load exercise trial, was consistently reduced, which also resulted in a lower pressure–time product (Table 2). The depressed response with hyperoxia has been observed by others and is likely to be the result of decreased lactate accumulation (Romer et al. 2006a), as the increase in with our modest increase in would not have been excessive (< 150 mmHg). In contrast, changes in during the PAV trial were variable and not statistically different from the control constant load exercise trial for either sex. The relatively small change in work of breathing between control and PAV constant load exercise trial is due to the curvilinear relationship between and work of breathing (Otis, 1954). With a high (> ∼80% of max) there is often an inflection point above which any change in results in disproportionate increase in work of breathing. However, when work of breathing is expressed as work per litre of , all subjects demonstrated a consistent decrease during the PAV trial (Fig. 6 C). Specifically, the work of breathing unit cost of the increased was less than what it would have been without the PAV. Therefore, the modest decline in work of breathing during the PAV constant load exercise trial in the subjects who increased their relative to control was still much lower than the work of breathing would have been without the PAV.
All the subjects who increased their during the PAV trial would have had EFL during their control constant load exercise trial (+11 ± 2 vs. −10 ± 3 l min−1 for the EFL and non‐EFL group). To determine if subjects experienced EFL during the control trial, we used operational lung volumes from the maximal incremental test as a reference. Unlike other methods to unload the respiratory muscles (helium), the PAV does not permit expansion of the maximal expiratory flow–volume curve. Without the PAV, the sustained elevated in the EFL subjects would increase considerably, which would be unsustainable at the fixed external work rate. Rather, the PAV allows for a that would otherwise be energetically unachievable. The greater is beneficial because of the raised alveolar ventilation, which, with other factors being similar, would raise and lower . The raised would increase , and the lower would increase pH, both of which would help to alleviate locomotor fatigue. Mechanical ventilatory constraint limiting effective hyperpnoea has been demonstrated previously in men (Johnson et al. 1992) and women (McClaran et al. 1998). We have previously shown that when flow‐limited subjects are given heliox, their , and subsequently increase, whereas those who are not flow‐limited show no change in (Dominelli et al. 2013). We observed a similar finding with regards to EFL in the current study, except the increase in would have been achieved via reduction in work of breathing rather than expansion of the maximal expiratory flow–volume curve.
Diaphragm fatigue
In a subset (n = 4) of subjects, the diaphragmatic twitch force was significantly reduced from baseline after the control trial, but was not different from baseline after the PAV trials. Our finding of reduced diaphragm fatigue is similar to others who also used a PAV to reduce the work of breathing during intense exercise (Babcock et al. 2002). As such it emphasizes the importance of work specific to the respiratory musculature, rather than locomotor muscles, in the development of diaphragm fatigue (Babcock et al. 1995). High respiratory muscle work resulting in metabolite accumulation stimulates type III and IV afferents, which causes increased sympathetic activity, blood flow redistribution and ultimately exacerbated locomotor fatigue (Dempsey et al. 2006). The prevention of diaphragm fatigue after the PAV trial further supports our hypothesis regarding blood flow redistribution through the metaboreflex.
Technical considerations
Our findings are dependent on our ability to detect small changes in quadriceps fatigue. Accordingly, we undertook several steps to ensure our results are representative of physiological changes rather than experimental variation. First, prior to each constant load exercise test, we were rigorous in our identification of the anatomical area which evoked the greatest twitch and performed a ramp protocol to confirm the stimulus was supramaximal. Second, to ensure that the stimulator coil was placed in a similar position we outlined the coil placement in indelible ink, used an accelerometer to verify the coil orientation and measured the coil distance from known anatomical landmarks that were also marked in ink. Our within‐occasion (Table 2) and between‐day coefficients of variation (< 5%) were similar to those reported by others (Romer et al. 2006a).
While a PAV permits some degree of customization with respect to the degree of unloading, we were unable to reduce the work of breathing to a similar extent in all subjects. On average, we were unable to unload women to a similar extent to men, but there was overlap. The variability in the degree of unloading arises from differences in subject tolerance, respiratory mechanics, and breathing patterns. Notably for women, both the relatively smaller tidal volume and the greater breathing frequency decreased the total time available to unload. Specifically, the PAV has an inherent fixed delay (20–50 ms) in the ability to generate positive mouth pressure. Thus, when the reduced inspiratory time (greater breathing frequency) in women is coupled with a fixed delay, the fraction of a breath that is unloaded in women is less.
Conclusions
Three conclusions can be drawn from this study. First, although the extent and susceptibility of EIAH vary between (and within) the sexes, their implications for quadriceps fatigue are not different. Specifically, regardless of sex, those who develop the most severe EIAH demonstrate the most fatigue attenuation when EIAH is reversed. Second, during high‐intensity whole‐body dynamic cycle exercise with no experimental manipulations, the development of quadriceps muscle fatigue is similar between the sexes. During heavy exercise with a ventilator, the work of breathing is reduced to a greater extent in men and yet attenuation of quadriceps fatigue is similar between the sexes. We attribute the similar fatigue attenuation from unloading the work of breathing in women to a lesser extent to their greater relative . Overall, both EIAH and high work of breathing in both sexes can influence the development of quadriceps fatigue. However, owing to their greater (Dominelli et al. 2015b), alterations in work of breathing appear to influence quadriceps fatigue to a greater extent in women.
Additional information
Competing interests
None declared.
Author contributions
Conception of study: P.B.D., J.S.B., G.E.F., L.M.R., M.S.K. and A.W.S. Design of experiments: P.B.D., Y.M.S., W.R.H., G.S.D., J.S.B., G.E.F., L.M.R., M.S.K. and A.W.S. Data collection, analysis and interpretation, drafting of article: P.B.D., Y.M.S., D.E.G., C.M.P., J.S.B., M.S., G.S.D., W.R.H., G.E.F., L.M.R., M.S.K. and A.W.S. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the Natural Science and Engineering Research Council of Canada (NSERC). P.B.D., Y.M.S. and C.M.P. were supported by a graduate scholarship from NSERC. P.B.D. was also supported by the Canadian Thoracic Society.
Acknowledgements
We are grateful for the perseverance and enthusiastic participation by our research subjects. We thank Dr Jerome A. Dempsey (University of Wisconsin‐Madison) for critical review of the manuscript.
References
- Aaron EA, Johnson BD, Seow CK & Dempsey JA (1992a). Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol 72, 1810–1817. [DOI] [PubMed] [Google Scholar]
- Aaron EA, Seow KC, Johnson BD & Dempsey JA (1992b). Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol 72, 1818–1825. [DOI] [PubMed] [Google Scholar]
- Adams RP & Welch HG (1980). Oxygen uptake, acid‐base status, and performance with varied inspired oxygen fractions. J Appl Physiol 49, 863–868. [DOI] [PubMed] [Google Scholar]
- Amann M & Calbet JAL (2008). Convective oxygen transport and fatigue. J Appl Physiol 104, 861–870. [DOI] [PubMed] [Google Scholar]
- Amann M, Pegelow DF, Jacques AJ & Dempsey JA (2007). Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293, R2036–R2045. [DOI] [PubMed] [Google Scholar]
- Astrand P, Cuddy T, Saltin B & Stenberg J (1964). Cardiac output during submaximal and maximal work. J Appl Physiol 19, 268–274. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society (1995). Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152, 1107–1136. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, Harms CA & Dempsey JA (2002). Effects of respiratory muscle unloading on exercise‐induced diaphragm fatigue. J Appl Physiol 93, 201–206. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, McClaran SR, Suman OE & Dempsey JA (1995). Contribution of diaphragmatic power output to exercise‐induced diaphragm fatigue. J Appl Physiol 78, 1710–1719. [DOI] [PubMed] [Google Scholar]
- Barclay JK (1986). A delivery‐independent blood flow effect on skeletal muscle fatigue. J Appl Physiol 61, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Blouin J‐S, Siegmund GP & Inglis JT (2007). Interaction between acoustic startle and habituated neck postural responses in seated subjects. J Appl Physiol 102, 1574–1586. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Jensen‐Urstad M, van Hall G, Holmberg H‐C, Rosdahl H & Saltin B (2004). Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol 558, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA (2012). New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol 590, 4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Hanson PG & Henderson KS (1984). Exercise‐induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J & Smith C (2006). Consequences of exercise‐induced respiratory muscle work. Resp Physiol Neurobiol 151, 242–250. [DOI] [PubMed] [Google Scholar]
- Dempsey JA & Wagner PD (1999). Exercise‐induced arterial hypoxemia. J Appl Physiol 87, 1997–2006. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC & Sheel AW (2013). Exercise‐induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591, 3017–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Foster GE, Dominelli GS, Querido JS, Henderson WR, Koehle MS & Sheel AW (2012). Repeated exercise‐induced arterial hypoxemia in a healthy untrained woman. Resp Physiol Neurobiol 183, 201–205. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Henderson WR & Sheel AW (2016). A proportional assist ventilator to unload respiratory muscles experimentally during exercise in humans. Exp Physiol 101, 754–767. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Molgat‐Seon Y, Bingham D, Swartz PM, Road JD, Foster GE & Sheel AW (2015a). Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol 119, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Render JN, Molgat‐Seon Y, Foster GE, Romer LM & Sheel AW (2015b). Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593, 1965–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Render JN, Molgat‐Seon Y, Foster GE & Sheel A (2014). Precise mimicking of exercise hyperpnea to investigate the oxygen cost of breathing. Resp Physiol Neurobiol 201, 14–23. [DOI] [PubMed] [Google Scholar]
- Dominelli PB & Sheel AW (2012). Experimental approaches to the study of the mechanics of breathing during exercise. Resp Physiol Neurobiol 180, 147–161. [DOI] [PubMed] [Google Scholar]
- Duhamel TA, Green HJ, Sandiford SD, Perco JG & Ouyang J (2004). Effects of progressive exercise and hypoxia on human muscle sarcoplasmic reticulum function. J Appl Physiol 97, 188–196. [DOI] [PubMed] [Google Scholar]
- Foster GE, Koehle MS, Dominelli PB, Mwangi FM, Onywera VO, Boit MK, Tremblay JC, Boit C & Sheel AW (2014). Pulmonary mechanics and gas exchange during exercise in Kenyan distance runners. Med Sci Sports Exerc 46, 702–710. [DOI] [PubMed] [Google Scholar]
- Gallagher CG & Younes M (1989). Effect of pressure assist on ventilation and respiratory mechanics in heavy exercise. J Appl Physiol 66, 1824–1837. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Querido JS, Eves ND, Chua R & Sheel AW (2009). Sex differences in the resistive and elastic work of breathing during exercise in endurance‐trained athletes. Am J Physiol Regul Integr Comp Physiol 297, R166–R175. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC & Sheel AW (2010). Sex differences in exercise‐induced diaphragmatic fatigue in endurance‐trained athletes. J Appl Physiol 109, 35–46. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Witt JD, McKenzie DC, Road JD & Sheel AW (2007). Respiratory mechanics during exercise in endurance‐trained men and women. J Physiol 581, 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB & Dempsey JA (1998). Exercise‐induced arterial hypoxaemia in healthy young women. J Physiol 507, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Gladden LB, Grassi B, Stary CM & Samaja M (1998). Bioenergetics of contracting skeletal muscle after partial reduction of blood flow. J Appl Physiol 84, 1882–1888. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS & Haseler LJ (1999). Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P‐MRS study. J Appl Physiol 86, 1367–1373. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S & Wagner PD (2000). Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol 89, 721–730. [DOI] [PubMed] [Google Scholar]
- Hunter SK (2009). Sex differences and mechanisms of task‐specific muscle fatigue. Exerc Sport Sci Rev 37, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK (2014). Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiologica 210, 768–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK (2016). Sex differences in fatigability of dynamic contractions. Exp Physiol 101, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin I‐S & Enoka RM (2004). Men are more fatigable than strength‐matched women when performing intermittent submaximal contractions. J Appl Physiol 96, 2125–2132. [DOI] [PubMed] [Google Scholar]
- Hunter SK & Enoka RM (2001). Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91, 2686–2694. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Saupe KW & Dempsey JA (1992). Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 73, 874–886. [DOI] [PubMed] [Google Scholar]
- Jones NL, Makrides L, Hitchcock C, Chypchar T & McCartney N (1985). Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131, 700–708. [DOI] [PubMed] [Google Scholar]
- Joyner MJ (2017). Physiological limits to endurance exercise performance: influence of sex. J Physiol 595, 2949–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargotich S, Goodman CL, Keast D & Morton AR (1998). The influence of exercise‐induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med 26, 101–117. [DOI] [PubMed] [Google Scholar]
- Lua AC, Shi KC & Chua LP (2001). Proportional assist ventilation system based on proportional solenoid valve control. Med Eng Physics 23, 381–389. [DOI] [PubMed] [Google Scholar]
- MacNutt MJ, De Souza MJ, Tomczak SE, Homer JL & Sheel AW (2012). Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol 112, 737–747. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF & Dempsey JA (1998). Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 84, 1872–1881. [DOI] [PubMed] [Google Scholar]
- Mead J. (1980). Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121, 339–342. [DOI] [PubMed] [Google Scholar]
- Olfert IM, Balouch J, Kleinsasser A, Knapp A, Wagner H, Wagner PD & Hopkins SR (2004). Does gender affect human pulmonary gas exchange during exercise? J Physiol 557, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB (1954). The work of breathing. Physiol Rev 34, 449–458. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF & Dempsey JA (2006a). Effect of exercise‐induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol 290, R365–R375. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF & Dempsey JA (2006b). Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Wildenthal K, Chapman CB, Frenkel E, Norton W, Siperstein M, Suki W, Vastagh G & Prengler A (1968). A longitudinal study of adaptive changes in oxygen transport and body composition. Circulation 38, VII‐1–VII‐78. [Google Scholar]
- Senefeld J, Yoon T, Bement MH & Hunter SK (2013). Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve 48, 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinghaus JW (1966). Blood gas calculator. J Appl Physiol 21, 1108–1116. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S & Coxson HO (2009). Evidence for dysanapsis using computed tomographic imaging of the airways in older ex‐smokers. J Appl Physiol 107, 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Similowski T, Fleury B, Launois S, Cathala HP, Bouche P & Derenne JP (1989). Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol 67, 1311–1318. [DOI] [PubMed] [Google Scholar]
- Sjogaard G & Saltin B (1982). Extra‐ and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol Regul Integr Comp Physiol 12, R271–R280. [DOI] [PubMed] [Google Scholar]
- Smith J & Bellemare F (1987). Effect of lung volume on in vivo contraction characteristics of human diaphragm. J Appl Physiol 62, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Stewart IB, Warburton DER, Hodges ANH, Lyster DM & McKenzie DC (2003). Cardiovascular and splenic responses to exercise in humans. J Appl Physiol 94, 1619–1626. [DOI] [PubMed] [Google Scholar]
- Sundberg CW, Hunter SK & Bundle MW (2017). Rates of performance loss and neuromuscular activity in men and women during cycling: evidence for a common metabolic basis of muscle fatigue. J Appl Physiol 122, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Bourbeau J, Hernandez P, Chapman K, Cowie R, FitzGerald M, Aaron S, Marciniuk D, Maltais F, O'Donnell D, Goldstein R, Sin D, Chan‐Yeung M, Manfreda J, Anthonisen N, Tate R, Sears M, Siersted H, Becklake M, Ernst P, Bowie D, Sweet L & Til LV (2011). Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can Resp J 18, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM (1982). Postnatal human lung growth. Thorax 37, 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD (1982). Influence of mixed venous PO2 on diffusion of O2 across the pulmonary blood: gas barrier. Clin Physiol 2, 105–115. [DOI] [PubMed] [Google Scholar]
- Wanke T, Formanek D, Schenz G, Popp W, Gatol H & Zwick H (1991). Mechanical load on the ventilatory muscles during an incremental cycle ergometer test. Eur Respir J 4, 385–392. [PubMed] [Google Scholar]
- Wetter TJ, Harms CA, Nelson WB, Pegelow DF & Dempsey JA (1999). Influence of respiratory muscle work on VO2 and leg blood flow during submaximal exercise. J Appl Physiol 87, 643–651. [DOI] [PubMed] [Google Scholar]
- Yan Z, Okutsu M, Akhtar YN & Lira VA (2011). Regulation of exercise‐induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110, 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Bilan D, Jung D & Kroker H (1987). An apparatus for altering the mechanical load of the respiratory system. J Appl Physiol 62, 2491–2499. [DOI] [PubMed] [Google Scholar]


