Abstract
Background
Tests to determine serum antibody levels—the 2-tier sonicate immunoglobulin M (IgM) and immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) and Western blot method or the IgG of the variable major protein-like sequence-expressed (VlsE) sixth invariant region (C6) peptide ELISA method—are the major tests available for support of the diagnosis of Lyme disease. However, these tests have not been assessed prospectively.
Methods
We used these tests prospectively to determine serologic responses in 134 patients with various manifestations of Lyme disease, 89 patients with other illnesses (with or without a history of Lyme disease), and 136 healthy subjects from areas of endemicity and areas in which the infection was not endemic.
Results
With 2-tier tests and the C6 peptide ELISA, only approximately one-third of 76 patients with erythema migrans had results that were positive for IgM or IgG seroreactivity with Borrelia burgdorferi in acute-phase samples. During convalescence, 3–4 weeks later, almost two-thirds of patients had seroreactivity with the spirochete B. burgdorferi. The frequencies of seroreactivity were significantly greater among patients with spirochetal dissemination than they were among those who lacked evidence of disseminated disease. Of the 44 patients with Lyme disease who had neurologic, heart, or joint involvement, all had positive C6 peptide ELISA results, 42 had IgG responses with 2-tier tests, and 2 patients with facial palsy had only IgM responses. However, among the control groups, the IgG Western blot was slightly more specific than the C6 peptide ELISA. The differences between the 2 test systems (2-tier testing and C6 peptide ELISA) with respect to sensitivity and specificity were not statistically significant.
Conclusions
Except in patients with erythema migrans, both test systems were sensitive for support of the diagnosis of Lyme disease. However, with current methods, 2-tier testing was associated with slightly better specificity.
Lyme disease, which, in the United States, is caused by the tick-borne spirochete Borrelia burgdorferi, usually begins with an expanding skin lesion, erythema migrans (EM) [1]. Within days to weeks, the spirochete often disseminates to other sites, particularly the nervous system, heart, or joints. Soon after dissemination, patients may have acute neurologic or cardiac abnormalities. Months later, untreated patients often have monoarticular or oligoarticular arthritis. In rare instances, a late encephalopathy or neuropathy may develop.
Tests to determine serum antibody levels are the major tests available for support of the diagnosis of Lyme disease. The Centers for Disease Control and Prevention (CDC) recommends a 2-tier approach of sonicate IgM and IgG ELISA, followed by Western blotting [2]. For results to be considered positive, IgM blots, which are only to be used during the first 4 weeks of infection, are required to have at least 2 of 3 specific bands, and IgG blots, which are applicable at any time in the illness, are required to have at least 5 of 10 specific bands [2]. In the search for better Lyme disease tests that use recombinant antigens or synthetic peptides, an IgG ELISA that employs a 26-mer peptide from the sixth invariant region (C6) of the variable major protein-like sequence-expressed (VlsE) lipoprotein of the spirochete B. burgdorferi has shown particular promise [3,4]. However, the performance of these serologic tests has not been assessed prospectively for patients with Lyme disease.
In this study, we used the standard 2-tier tests and an IgG VlsE C6 peptide ELISA to determine prospectively the serologic responses in patients with various manifestations of Lyme disease and in control subjects.
PATIENTS AND METHODS
Study subjects
From 1999 through 2001, 2 primary care physicians at field sites in East Lyme, Connecticut, or Wakefield, Rhode Island, recruited 97 patients with EM for this study. During the same period, 147 patients with later manifestations of Lyme disease or other illnesses who were seen in the Lyme Disease Clinic at New England Medical Center (Boston, Massachusetts) were invited to participate. The study was approved by the Human Investigations Committees at New England Medical Center for the period 1999–2001 and at Massachusetts General Hospital (Boston, Massachusetts) for the period 2002–2007, and informed consent was obtained.
Inclusion criteria for patients with EM
Patients were required to meet the criteria of the CDC for Lyme disease [5]. In patients with EM, a 1.5-mm punch skin biopsy was performed for culture of B. burgdorferi [6], and an EDTA-anti-coagulated blood sample was obtained for PCR detection of B. burgdorferi, Anaplasma phagocytophilum, and Babesia microti DNA [7]. In addition, an acute-phase blood sample was obtained, and 3–4 weeks later, at the conclusion of antibiotic therapy, a convalescent-phase serum sample was obtained for serologic testing for these 3 organisms. Of the 97 patients with EM who were initially evaluated, 79 (81%) had a culture positive for B. burgdorferi, and 3 patients had a PCR result positive for a coinfecting agent or IgM or IgG seroconversion to a coinfecting agent [7]. Thus, for this analysis, only the 76 culture-positive patients who did not have evidence of coinfection were included. In these patients, disseminated disease was defined by a PCR result positive for B. burgdorferi DNA in blood or by multiple EM skin lesions.
Inclusion criteria for patients with later manifestations of the infection
Neuroborreliosis was defined clinically as meningitis, cranial neuropathy, peripheral neuropathy, or radiculoneuropathy. Except for patients with cranial neuropathy, these patients were required to have CSF pleocytosis or electromyographic evidence of an axonal polyneuropathy [8]. Cardiac involvement was defined by the presence of acute atrioventricular nodal block. Lyme arthritis was defined as inflammatory arthritis in ≥1 large joint. In all patients with neurologic, cardiac, or joint involvement, a serologic result positive for B. burgdorferi by ELISA and Western blot was required for case inclusion [5]. All patients who met the criteria for early or late manifestations of Lyme disease were treated with courses of oral or intravenous antibiotic therapy, as recommended by the Infectious Diseases Society of America [9].
Post–Lyme disease symptoms were defined as subjective pain and neurocognitive or fatigue symptoms occurring within 6 months after receipt of recommended antibiotic therapy for an objective manifestation of Lyme disease [9]. Patients with other illnesses, with or without a past history of Lyme disease, did not meet the clinical criteria for this infection. For all patients, including those seen at field or hospital clinics, serologic testing was performed on fresh samples that were obtained during the course of medical evaluation. The results of 2-tier testing were reported to clinicians and patients, but the results of the VlsE C6 ELISA, a research test, were not reported.
Control groups
The control group from an area in which Lyme disease was endemic consisted of healthy individuals without a history of the infection whom physicians at the Connecticut or Rhode Island field sites saw for well visits. The control group from an area in which the infection was not endemic consisted of healthy individuals who were blood donors in Fort Collins, Colorado; Dr. Barbara Johnson at the CDC provided these frozen, archival samples.
Serologic methods
Serologic results were determined by 2- tier sonicate ELISA and Western blot and by VlsE C6 peptide ELISA, as described elsewhere [6, 10–12]. Both ELISAs were noncommercial, in-house tests. For the sonicate ELISA, the antigen preparation was derived from B. burgdorferi strain G39/ 40 [10]. The cutoff value for a positive response was defined as 3 SDs for IgG and 5 SDs for IgM above the mean absorbance of 8 negative control samples included on each plate, which were previously shown to be representative of values obtained from 50 healthy control subjects [12]. As recommended by the CDC, Western blotting was performed only on samples that had positive or indeterminate responses by ELISA, using a commercial test system that employed B. burgdorferi sonicate (strain B31) (MarDx). Positive results were interpreted according to the CDC criteria [2]. The IgG VlsE C6 ELISA employed the originally described 26-mer sequence from the sixth invariant region of Borrelia garinii [3]. An absorbance of 0.45, which was 3 SDs above the mean absorbance of 8 negative control samples included on each plate, was defined as a positive response.
Statistics
The numbers of patients with positive results according to 2-tier testing or the VlsE C6 peptide ELISA were compared by χ2 analysis or, if appropriate, by Fisher’s exact test. All P values were 2-tailed.
RESULTS
Patients with EM
With the sonicate ELISA and Western blot, 22 of the 76 culture-positive patients with EM (29%) had positive IgM or IgG antibody responses to B. burgdorferi in acute-phase samples, and 49 patients (64%) had such reactivity in convalescent-phase serum samples that were obtained 3–4 weeks later, at the conclusion of antibiotic therapy. Of the 76 patients, 40 (53%) had disseminated disease, defined by the presence of multiple EM skin lesions or a PCR result positive for B. burgdorferi DNA in blood, whereas the other 36 patients lacked evidence of disseminated infection. In both groups, samples were obtained a median of 4 days (range, 1–30 days) after disease onset.
Among the 36 patients who lacked evidence of dissemination, only 6 (17%) had positive IgM or IgG antibody responses to B. burgdorferi in acute-phase samples by sonicate ELISA and Western blot (table 1). Four of the 6 patients had only IgM responses, and 2 patients who had a history of previous EM had only IgG responses. In contrast, 17 of the 40 patients (43%) who had evidence of spirochetal dissemination had positive IgM or IgG responses to B. burgdorferi in acute-phase samples. During convalescence, 3–4 weeks later, 19 (53%) of the 36 patients who lacked evidence of dissemination had IgM or IgG responses to the spirochete B. burgdorferi, whereas 30 (75%) of the 40 patients who had disseminated disease had such responses. These differences reached statistical significance for the frequency of IgM reactivity as determined by 2-tier testing, which was greater among patients with disseminated disease, both in acute-phase and convalescent-phase samples.
Table 1.
Prospective study comparing an ELISA that employs a 26-mer peptide from the sixth invariant region (C6) of the variable major protein-like sequence-expressed (VlsE) lipoprotein of the spirochete Borrelia burgdorferi with B. burgdorferi sonicate ELISA and Western blot for the serologic diagnosis of patients with Lyme disease and in control subjects.
| Proportion (%) of patients with positive result, by test(s) |
||||
|---|---|---|---|---|
| Sonicate 2-test approach
|
||||
| Variable | VlsE C6 peptide ELISA |
ELISA and Western blot IgM |
ELISA and Western blot IgG |
ELISA and Western blot IgM or IgG |
| Patients with Lyme disease | ||||
| Skin infection (stage 1) | ||||
| Erythema migrans without evidence of disseminated disease | ||||
| Acute | 7/36 (19) | 4/36 (11)a | 2/36 (6)b | 6/36 (17) |
|
| ||||
| Convalescent, after antibiotics | 17/36 (47) | 14/36 (39)a | 6/36 (17)b | 19/36 (53) |
|
| ||||
| Erythema migrans with evidence of disseminated diseasec | ||||
| Acute phase | 15/40 (38) | 15/40 (38)a | 6/40 (15)b | 17/40 (43) |
|
| ||||
| Convalescent phase (after receipt of antibiotics) | 25/40 (63) | 28/40 (70)a | 8/40 (20)b | 30/40 (75) |
|
| ||||
| Disseminated infection (stage 2) | ||||
| Acute neurologic or cardiac involvementd | 13/13 (100) | 11/13 (85) | 11/13 (85) | 13/13 (100) |
|
| ||||
| Persistent infection (stage 3) | ||||
| Arthritis or chronic neurologic involvemente | 31/31 (100) | 7/31 (23) | 31/31 (100) | 31/31 (100) |
|
| ||||
| Post-Lyme disease symptoms | 6/14 (43) | 7/14 (50) | 5/14 (36) | 10/14 (71) |
|
| ||||
| Patients with another illness | ||||
| And previous Lyme disease | 9/14 (64) | 1/14 (7) | 10/14 (71) | 11/14 (79) |
|
| ||||
| Not Lyme diseasef | 1/75 (1) | 0 | 0 | 0 |
|
| ||||
| Healthy subjects | ||||
| Area of Lyme disease endemicity | 4/86 (5) | 1/86 (1) | 1/86 (1) | 2/86 (2) |
|
| ||||
| Area in which Lyme disease is not endemic | 1/50 (2) | 0 | 0 | 0 |
With 2-tier testing, patients with erythema migrans who had evidence of disseminated disease had positive IgM responses significantly more often than did patients who lacked evidence of dissemination, both in acute-phase and convalescent-phase samples (in each instance, P = .02).
In patients with erythema migrans, the IgG VlsE C6 peptide ELISA became positive before IgG reactivity with ≥5 bands developed with 2-tier testing. In acute-phase samples, this difference approached statistical significance for patients who did not have evidence of dissemination (P = .15) and was statistically significant for patients who did have evidence of dissemination (P = .04). In comparison, the differences during the convalescent phase were still greater, both for patients who did not have evidence of dissemination (P = .003) and for those who did (P< .001).
Of the 40 patients, 25 had a PCR result positive for B. burgdorferi DNA in blood alone, 4 had multiple erythema migrans lesions alone, and 11 had both of these findings.
Of the 13 patients, 4 had meningitis and facial palsy (and in 1 case, radiculoneuritis), 4 had facial palsy alone, 1 had anterior optic neuritis, and 4 had high-degree atrioventricular nodal block. Of the 4 patients with heart block, 1 also had unilateral paralysis of the phrenic nerve, and another had radiculoneuritis. Seven of the 13 patients had erythema migrans, and 6 experienced flu-like symptoms several weeks prior to the onset of neurologic or cardiac abnormalities. They did not receive antibiotic therapy at that time, but they were treated successfully when they had neurologic or cardiac involvement.
Of the 31 patients, 30 had arthritis (in 1 case accompanied by radiculoneuropathy), and 1 patient had radiculoneuropathyalone. Six patients had erythema migrans, and 5 experienced flu-like symptoms months prior to the onset of arthritis or radiculoneuropathy; in the remaining 20 patients, arthritis was the initial manifestation of the illness. Twelve (40%) of the 30 patients with arthritis had a result positive for B. burgdorferi DNA in joint fluid. One patient with erythema migrans was treated with oral amoxicillin for 30 days, but the remaining patients did not receive antibiotics prior to the onset of joint or neurologic abnormalities.
Includes 37 patients with chronic fatigue syndrome or fibromyalgia, 19 with rheumatic diseases (such as rheumatoid arthritis or psoriatic arthritis), 11 with neurologic illnesses (including multiple sclerosis), 7 with other infections, and 1 with T cell lymphoma.
The only significant difference between the 2 test systems was that the VlsE C6 peptide ELISA, which is an IgG test, often had positive results before ≥5 IgG bands were detected on sonicate Western blots, and this difference was particularly significant during the convalescent period (table 1). However, when the results obtained with both the IgM and IgG sonicate tests were compared with the results obtained with the IgG VlsE C6 peptide ELISA, the percentage of patients with positive results did not differ significantly between the groups.
Patients with organ involvement
A median of 6 weeks after disease onset (range, 3–25 weeks), 13 patients had acute neurologic or cardiac abnormalities associated with Lyme disease. None of these patients had received antibiotic therapy prior to evaluation. All 13 patients had positive VlsE C6 peptide ELISA results (table 1). With 2-tier testing, 9 patients had positive IgM and IgG responses to B. burgdorferi; 2 patients (both with facial palsy alone) who were seen 4 and 6 weeks after disease onset, had only IgM reactivity with the spirochete B. burgdorferi, and 2 patients with carditis had only IgG reactivity. The patient with facial palsy who had only a positive IgM response to B. burgdorferi 6 weeks after disease onset had IgM and IgG reactivity with the 23-kD, 39-kD, and 41-kD spirochetal proteins and IgG reactivity with the VlsE C6 peptide. Because the IgM criteria are to be used only for the first 4 weeks after disease onset [2], this patient would not meet the CDC serologic criteria for the diagnosis of Lyme disease.
A median of 12 months after disease onset (range, 3–60 months), 30 patients had arthritis, usually affecting 1 or both knees; in 1 case, arthritis was accompanied by radiculoneuropathy, and 1 patient had radiculoneuropathy alone (table 1). All 31 patients had positive IgG responses to B. burgdorferi with 2-tier testing and with the VlsE C6 peptide ELISA.
Patients with post–Lyme disease symptoms or other illnesses
A median of 12 months (range, 2–144 months) after objective manifestations of Lyme disease, 14 patients were evaluated for subjective pain, neurocognitive, or fatigue symptoms that began within 6 months after EM (12 cases) or Lyme arthritis (2 cases). Of the 14 patients, 10 (71%) had a positive antibody response to B. burgdorferi with 2-tier testing, most commonly of the IgM isotype, and 6 (43%) had reactivity with the VlsE C6 peptide (table 1). Fourteen additional patients were evaluated who had experienced Lyme disease a median of 16 months previously (range, 3–120 months), but we thought that they now had other neurologic, dermatologic, or rheumatic diseases. Of these 14 patients, 11 (79%) had a positive antibody response to B. burgdorferi by 2-tier testing, most commonly of the IgG isotype, and 9 (64%) had reactivity with the VlsE C6 peptide. Seventy-five patients were referred for possible Lyme disease but did not meet clinical criteria for that infection. All 75 patients had negative results with 2-tier testing, but 1 patient had a positive result with the C6 ELISA (table 1). This patient did not meet clinical criteria for Lyme disease, and only the 41-kD and 58-kD bands were present on an IgG Western blot.
Healthy control subjects from areas of endemicity and areas of nonendemicity
Among the 86 healthy individuals from areas in which Lyme disease is endemic who did not have a history of the infection, 1 each had IgM or IgG reactivity with B. burgdorferi by 2-tier testing (table 1). The person with a positive IgG response also had reactivity with the VlsE C6 peptide, and 3 other individuals had responses to the VlsE peptide alone. Among the 50 healthy blood donors from an area in which Lyme disease was not endemic, none had positive results by 2-tier testing, but 1 had reactivity with the VlsE peptide alone.
Magnitude of antibody responses by ELISA
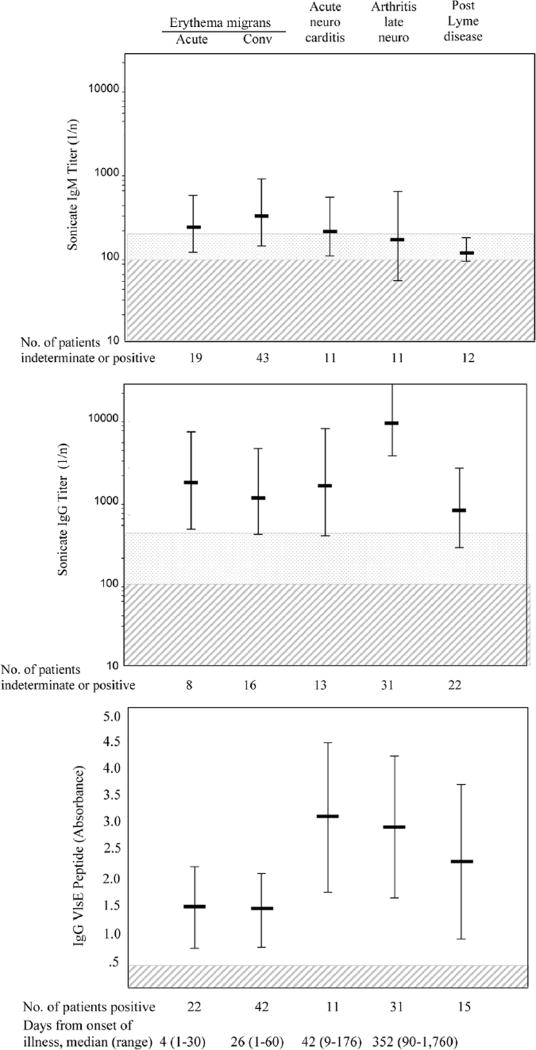
With the sonicate ELISA, IgM values were highest in patients with neurologic or heart abnormalities, a median duration of 6 weeks after disease onset, and the highest IgG responses were found later in the illness in patients with Lyme arthritis (figure 1). In comparison, IgG reactivity to the VlsE C6 peptide developed faster and was higher in patients with neurologic, heart, or joint abnormalities. With both tests, the antibody levels were lower in patients with post–Lyme disease symptoms or with past Lyme disease and another current illness.
Figure 1.
Antibody titers to Borrelia burgdorferi by sonicate IgM (A) or IgG (B) ELISA are shown for patients with erythema migrans (acute and convalescent [Conv] phase), acute-phase neurologic or cardiac involvement (Acute neuro carditis), arthritis or late neurologic abnormalities (Arthritis late neuro), and post-infection symptoms (Post Lyme disease). The antibody titers were plotted on a logarithmic scale. For IgG determinations, a titer of >1 :400 was defined as positive, a titer of 1:200 or 1:400 was defined as indeterminate, and a titer <1:100 was defined as negative. For IgM determinations, a titer>1:200 was defined as positive, a titer 1:100 was defined as indeterminate, and a titer <1:100 was defined as negative. The absorbance values with an ELISA that employs a 26-mer peptide from the sixth invariant region (C6) of the variable major protein-like sequence-expressed (VlsE) lipoprotein of the spirochete are shown for the same patient groups (C). The absorbance values are plotted on an arithmetic scale. In all 3 panels, the horizontal bars indicate mean values for patients with positive responses, vertical bars indicate 1 SD, stipled areas indicate indeterminate range, and cross-hatched areas indicate negative range.
Antibody to B. burgdorferi proteins on Western blot
In acute samples obtained from patients with EM, IgM and IgG responses, if present, were most commonly directed against the 23-kD outer-surface protein C and the 41-kD flagellar protein of B. burgdorferi and were less often directed against the 39-kD Borrelia membrane protein (table 2). Several weeks after disease onset, most patients with acute neurologic or heart involvement had IgG reactivity with these 3 B. burgdorferi proteins and several other spirochetal proteins. All patients with Lyme disease-associated arthritis or late neurologic involvement had IgG reactivity with the 18-kD decorin binding protein A and with most of the 10 proteins used in the diagnostic criteria.
Table 2.
Frequency of responses to the Borrelia burgdorferi proteins used in Centers for Disease Control and Prevention diagnostic criteria, as determined by Western blot, in patient or control samples that had positive or indeterminate responses by ELISA.
| Erythema migrans
|
Acute-phase neurologic or cardiac involvement |
Arthritis or late neurologic Involvement |
Previous Lyme disease, other current Illness |
Other illness, not Lyme disease |
Healthy subjects
|
|||
|---|---|---|---|---|---|---|---|---|
| Variable | Acute phase |
Convalescent phase |
Area of Endemlcity |
Area of nonendemlcity |
||||
| IgM response | ||||||||
| No. of patientsa | 19 | 42 | 11 | 11 | 12 | 7 | 7 | 0 |
|
| ||||||||
| Patients with positive band, % | ||||||||
| 23 kDa | 100 | 93 | 100 | 82 | 100 | 57 | 57 | 0 |
|
| ||||||||
| 39 kDa | 42 | 55 | 82 | 55 | 27 | 14 | 0 | 0 |
|
| ||||||||
| 41 kDa | 95 | 95 | 100 | 55 | 64 | 14 | 14 | 0 |
|
| ||||||||
| Median no. of bandsb | 2 | 2 | 3 | 2 | 2 | 1 | 1 | 0 |
|
| ||||||||
| IgG response | ||||||||
| No. of patientsa | 9 | 16 | 13 | 31 | 22 | 8 | 5 | 3 |
|
| ||||||||
| Patients with positive band, % | ||||||||
| 18 kDa | 75 | 79 | 69 | 100 | 68 | 12 | 20 | 0 |
|
| ||||||||
| 23 kDa | 88 | 100 | 100 | 65 | 41 | 12 | 20 | 0 |
|
| ||||||||
| 28 kDa | 50 | 29 | 23 | 90 | 27 | 0 | 0 | 0 |
|
| ||||||||
| 30 kDa | 25 | 29 | 54 | 90 | 41 | 0 | 0 | 0 |
|
| ||||||||
| 39 kDa | 63 | 100 | 100 | 90 | 73 | 12 | 20 | 0 |
|
| ||||||||
| 41 kDa | 100 | 100 | 100 | 94 | 91 | 88 | 60 | 0 |
|
| ||||||||
| 45 kDa | 88 | 79 | 62 | 68 | 41 | 12 | 20 | 0 |
|
| ||||||||
| 58 kDa | 63 | 86 | 62 | 97 | 68 | 38 | 0 | 0 |
|
| ||||||||
| 66 kDa | 63 | 71 | 54 | 74 | 59 | 0 | 0 | 0 |
|
| ||||||||
| 93 kDa | 25 | 7 | 38 | 84 | 59 | 0 | 0 | 0 |
|
| ||||||||
| Median no. of bandsb | 5 | 6 | 7 | 9 | 6 | 2 | 1 | 0 |
Western blot was performed only with patient or control samples that had positive or indeterminate responses by ELISA.
Median number of bands in samples in which bands were present.
Sensitivity and specificity of tests
Among the 76 patients with EM, the sensitivity of both IgM and IgG 2-tier tests and the IgG VlsE C6 peptide ELISA was low in acute-phase samples, but it was higher in convalescent-phase samples obtained 3–4 weeks later (table 3). Among the 44 patients with neurologic, heart, or joint abnormalities, all had positive IgM or IgG responses to B. burgdorferi with 2-tier testing, and all 44 had IgG reactivity with the VlsE C6 peptide. Thus, in patients with later manifestations of the infection, 2-tier testing had a sensitivity of 100% and a specificity of 99%. Similarly, the VlsE C6 peptide ELISA had a sensitivity of 100% but a specificity of 96%. These differences in sensitivity and specificity were not statistically significant.
Table 3.
Sensitivity and specificity of 2-tier testing, compared with that of an ELISA that employs a 26-mer peptide from the sixth invariant region (C6) of the variable major protein-like sequence-expressed (VlsE) lipoprotein of the spirochete Borrelia burgdorferi in a cohort of patients with various manifestations of Lyme disease.
| Two-tier testing with sonicate ELISA and Western blot
|
||||||||
|---|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
IgM or IgG
|
IgG VlsE C6 peptide ELISA |
|||||
| Variable | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| Erythema migrans | ||||||||
| Acute phase | 25 | 99 | 11 | 99 | 29 | 99 | 29 | 96 |
| Convalescent phase | 55 | 99 | 18 | 99 | 64 | 99 | 56 | 96 |
|
| ||||||||
| Acute neurologic or cardiac abnormalities | 85 | 99 | 85 | 99 | 100 | 99 | 100 | 96 |
|
| ||||||||
| Arthritis or chronic neurologic abnormalities | NA | … | 100 | 99 | 100 | 99 | 100 | 96 |
NOTE. Sensitivity was determined on the basis of serum samples from 76 patients with erythema migrans, 13 patients with acute neurologic or cardiac abnormalities, and 31 patients with arthritis or chronic neurologic abnormalities. Specificity was determined on the basis of serum samples from 86 healthy subjects from an area in which Lyme disease was endemic and serum samples from 50 subjects from an area in which Lyme disease was not endemic. NA, not applicable.
DISCUSSION
In this study, as in previous retrospective analyses [4, 12–14], the standard 2-tier tests (sonicate ELISA with Western blot) and the VlsE C6 peptide ELISA had high sensitivities and specificities in patients with Lyme disease, except in those patients with EM. It is problematic to determine the frequency of seroreactivity in patients with neurologic, cardiac, or joint manifestations of Lyme disease, because serologic confirmation is a part of the case definition [2]. However, B. burgdorferi DNA can be detected by PCR in the majority of patients with Lyme arthritis before antibiotic therapy [15, 16], and in our experience, all such patients have been seropositive for B. burgdorferi. Both culture and PCR have been low-yield procedures among patients with early or late neuroborreliosis, but positive cultures or PCR results have been reported for some of these patients [17, 18], and they have been seropositive for B. burgdorferi. Moreover, in animal models of Lyme disease, spirochetes have been seen in or cultured from nervous system, heart, or joint lesions, and these animals have been seropositive for B. burgdorferi [19, 20]. Although a small number of patients with symptoms of “seronegative late Lyme disease” were described in 1988 [21], this concept has been discredited as our understanding of Lyme disease has increased and as the standardization of serologic tests for Lyme disease has improved. Therefore, current thinking is that all patients with objective neurologic, cardiac, or joint abnormalities associated with Lyme disease have serologic responses to B. burgdorferi.
In contrast, subjective post–Lyme disease symptoms are not thought to result from active spirochetal infection [22, 23]. Antibody titers to B. burgdorferi decrease after antibiotic therapy [24, 25], and titers to the VlsE C6 peptide decrease faster than titers to sonicate antigens [11, 24,26]. However, with any of these tests, positive IgM or IgG responses may persist for years [24, 27]. Thus, antibody responses to B. burgdorferi were typically low in our patients with post-Lyme disease symptoms or in those with past Lyme disease and another current illness, but many patients were still seropositive for B. burgdorferi.
In this study, the sensitivity of 2-tier testing in patients with later manifestations of Lyme disease was 100%, and the specificity was 99%, although 1 patient with facial palsy still had only an IgM response to B. burgdorferi 6 weeks after disease onset (rather than at 4 weeks after disease onset, as required by CDC criteria [2]). Similarly, a patient with Lyme disease– associated meninigitis and radiculoneuritis was recently described who had only a positive IgM response 5 weeks after disease onset [28]. Thus, the period of infection in which IgM criteria may be used to support the diagnosis of Lyme disease should be extended by several weeks.
The specificity of 2-tier testing may even be >99%. Although 2 individuals in the control group from an area in which Lyme disease is endemic had positive IgM or IgG responses, asymptomatic B. burgdorferi infection, which occurs in ~10% of cases [29], may be the explanation for these responses. In contrast, such responses were not seen in the control group from a region in which Lyme disease was not endemic. Although patients with other illnesses were not included in the specificity calculations, none met the clinical criteria for Lyme disease or had positive serologic responses with 2-tier testing. Therefore, the inclusion of such patients would not have decreased this calculation.
As in previous retrospective analyses [3, 4, 30], we obtained results with an IgG VlsE C6 peptide ELISA (a single test) that were similar to those obtained with the 4 tests of the sonicate IgM and IgG ELISA and Western blot. In addition to ease of testing, the principal advantage of the C6 peptide ELISA is the early IgG response, which usually develops before ≥5 bands are detected on sonicate IgG Western blots. This difference is important, because 2-tier testing of patients with early Lyme disease relies heavily on IgM responses, and in clinical practice [31], the IgM criteria have not performed as well as reported here.
The major advantages of sonicate Western blot are its slightly greater specificity, compared with the C6 ELISA, and the fact that the degree of expansion of the antibody response (as assessed by Western blot) gives information about the duration of infection. A higher cutoff value may be used to increase the specificity of the C6 ELISA [3, 4] at the expense of sensitivity. Four current subjects in healthy control groups, including 1 individual from an area in which Lyme disease is not endemic, had robust responses to the VlsE C6 peptide alone, without reactivity with other spirochetal proteins. We do not think that these patients had asymptomatic B. burgdorferi infection, because 28 previous patients with asymptomatic infection had reactivity not only with the VlsE C6 peptide but also with other B. burgdorferi proteins [29]. Thus, with current methods, Western blot remains valuable for specificity and for assessing the duration of infection.
Acknowledgments
We thank Drs. Robert Kalish, Marcia Pellegrino, and Norma Grills for help with patient care and collection of samples; Jason Hoitt, Carolyn Suarez, and Monika Rothemich for help with serological assays; and Colleen Squires for help with preparation of the manuscript.
Financial support. The Centers for Disease Control and Prevention (cooperative agreement CCU110291); the English, Bonter, Mitchell Foundation; the Eshe Fund; and the Lyme/Arthritis Research Fund at Massachusetts General Hospital.
Footnotes
Potential conflicts of interest. A.C.S. was the recipient of a research grant from Viramed Biotech for the study of serological testing for Lyme disease. All other authors: no conflicts.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Recommendations for test performance and interpretation from the Second International Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1. [PubMed] [Google Scholar]
- 3.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol. 1999;37:3990–6. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon RM, Biggerstaff BJ, Schriefer ME, et al. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis. 2003;187:1187–99. doi: 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Case definitions for public health surveillance. MMWR Morb Mortal Wkly Rep. 1990;39(RR-13):1–43. [PubMed] [Google Scholar]
- 6.Vaz A, Glickstein L, Field JA, et al. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun. 2001;69:7437–44. doi: 10.1128/IAI.69.12.7437-7444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steere AC, McHugh G, Suarez C, Hoitt J, Damle N, Sikand VJ. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis. 2003;36:1078–81. doi: 10.1086/368187. [DOI] [PubMed] [Google Scholar]
- 8.Logigian EL, Steere AC. Clinical and electrophysiologic findings in chronic neuropathy of Lyme disease. Neurology. 1992;42:303–11. doi: 10.1212/wnl.42.2.303. [DOI] [PubMed] [Google Scholar]
- 9.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 10.Craft JE, Grodzicki RL, Steere AC. The antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149:789–95. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- 11.Peltomaa M, McHugh G, Steere AC. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis. 2003;187:1178–86. doi: 10.1086/374376. [DOI] [PubMed] [Google Scholar]
- 12.Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 13.Craven RB, Quan TJ, Bailey RE, et al. Improved serodiagnostic testing for Lyme disease: results of a multicenter serologic evaluation. Emerg Infect Dis. 1996;2:136–40. doi: 10.3201/eid0202.960211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevejo RT, Krause PJ, Schriefer ME, Dennis DT. Evaluation of a two-test serodiagnostic method for community assessment of Lyme disease in an endemic area. Am J Trop Med Hyg. 2001;65:563–6. doi: 10.4269/ajtmh.2001.65.563. [DOI] [PubMed] [Google Scholar]
- 15.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid in Lyme arthritis. N Engl J Med. 1994;330:229–34. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 16.Bradley JF, Johnson RC, Goodman JL. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann Intern Med. 1994;120:487–9. doi: 10.7326/0003-4819-120-6-199403150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Coyle PK, Goodman JL, Krupp LB, Logigian EL, Reik LJ. Continuum: lifelong learning in neurology. Philadelphia: Lippincott Williams & Wilkins; 1999. Lyme disease. [Google Scholar]
- 18.Nocton JJ, Bloom BJ, Rutledge BJ, et al. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in patients with Lyme neuroborreliosis. J Infect Dis. 1996;174:623–7. doi: 10.1093/infdis/174.3.623. [DOI] [PubMed] [Google Scholar]
- 19.Barthold SW, DeSouza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Path. 1993;143:959–71. [PMC free article] [PubMed] [Google Scholar]
- 20.Schaible UE, Gern L, Wallich R, Kramer MD, Prester M, Simon MM. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993;36:219–26. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 21.Dattwyler RJ, Volkman DJ, Luft BJ, Halperin JJ, Thomas J, Golightly MG. Seronegative Lyme disease: dissociation of the specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988;319:1441–6. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- 22.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 23.Feder HM, Jr, Johnson BJ, O’Connell S, Shapiro ED, Steere AC, Wormser GP. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–30. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- 24.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum. 2007;56:4216–25. doi: 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 25.Rose CD, Fawcett PT, Gibney KM, Doughty RA. Residual serologic reactivity in children with resolved Lyme arthritis. J Rheumatol. 1996;23:367–9. [PubMed] [Google Scholar]
- 26.Philipp MT, Bowers LC, Fawcett PT, et al. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis. 2001;184:870–8. doi: 10.1086/323392. [DOI] [PubMed] [Google Scholar]
- 27.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis. 2001;33:780–5. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]
- 28.Greer DM, Schaefer PW, Plotkin SR, Hasserjian RP, Steere AC. Case records of the Massachusetts General Hospital. Case 11–2007. A 59-year-old man with neck pain, weakness in the arms, and cranial-nerve palsies. N Engl J Med. 2007;356:1561–70. doi: 10.1056/NEJMcpc079005. [DOI] [PubMed] [Google Scholar]
- 29.Steere AC, Sikand VK, Schoen RT, Nowakowski J. Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis. 2003;37:528–32. doi: 10.1086/376914. [DOI] [PubMed] [Google Scholar]
- 30.Mogilyansky E, Loa CC, Adelson ME, Mordechai E, Tilton RC. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin Diagn Lab Immunol. 2004;11:924–9. doi: 10.1128/CDLI.11.5.924-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porwancher R. A reanalysis of IgM Western blot criteria for the diagnosis of early Lyme disease. J Infect Dis. 1999;179:1021–4. doi: 10.1086/314651. [DOI] [PubMed] [Google Scholar]