Abstract
HDLs appear to affect regulatory T cell (Treg) homeostasis, as suggested by the increased Treg counts in HDL-treated mice and by the positive correlation between Treg frequency and HDL-cholesterol levels in statin-treated healthy adults. However, the underlying mechanisms remain unclear. Herein, we show that HDLs, not LDLs, significantly decreased the apoptosis of human Tregs in vitro, whereas they did not alter naïve or memory CD4+ T cell survival. Similarly, oleic acid bound to serum albumin increased Treg survival. Tregs bound and internalized high amounts of HDL compared with other subsets, which might arise from the higher expression of the scavenger receptor class B type I by Tregs; accordingly, blocking this receptor hindered HDL-mediated Treg survival. Mechanistically, we showed that HDL increased Treg ATP concentration and mitochondrial activity, enhancing basal respiration, maximal respiration, and spare respiratory capacity. Blockade of FA oxidation by etoxomir abolished the HDL-mediated enhanced survival and mitochondrial activity. Our findings thus suggest that Tregs can specifically internalize HDLs from their microenvironment and use them as an energy source. Furthermore, a novel implication of our data is that enhanced Treg survival may contribute to HDLs’ anti-inflammatory properties.
Keywords: immunology, lipids/oxidation, lymphocytes, mitochondria
Regulatory T cells (Tregs) control both innate and adaptive immune responses and are essential to maintain immune homeostasis and curb exacerbated inflammatory processes. Tregs can be divided into two groups according to their site of origin: thymus-derived Tregs (tTregs) and peripherally derived Tregs (pTregs). The marker, CD25, and lack of the marker, CD127, used for Treg isolation do not allow for the distinction of tTregs from pTregs [reviewed in (1)]. A reciprocal interaction between Tregs and lipid metabolism was recently suggested. On one hand, Tregs seem to play an anti-atherogenic role through nonimmunological mechanisms by directly modulating lipoprotein metabolism (2). Conversely, lipid metabolism can modulate the function of Tregs because levels of cholesterol inhibit Treg migration to inflamed tissues (3). Notably, Tregs have distinct metabolic requirements compared with those of conventional CD4+ T cells (Tcons): murine Tregs (especially peripherally induced Tregs) primarily rely on lipid oxidation for energy generation (4, 5). In addition, an environment rich in FAs enhances Treg differentiation (5). These data thus suggest that Treg homeostasis may be heavily influenced by lipid metabolism.
HDLs constitute a heterogeneous group according to different isolation/separation techniques. HDLs can be separated by ultracentrifugation (HDL2 and HDL3) and gradient gel electrophoresis (HDL2b, HDL2a, HDL3a, HDL3b, and HDL3c) on the basis of density and diameter, respectively. In addition, HDLs can be separated on the basis of electrophoretic mobility (α-HDL and preβ-HDL) [reviewed in (6, 7)]. HDL is broadly considered as anti-inflammatory, notably preventing atherosclerotic disease or pulmonary hypertension (8, 9). This anti-inflammatory property could be at least partially mediated by an effect of HDL on Tregs, as splenic Treg counts in LDL receptor (LDLR) knockout mice increased after intraperitoneal injection of HDL/ApoA-I (10, 11). In addition, we recently showed that Treg frequency positively correlated with HDL-cholesterol (HDL-C) levels in healthy adults treated with statins (12). Although these data suggest that HDLs promote Treg accrual, underlying mechanisms remain unclear. It is not clear whether HDLs exert their effect indirectly or whether they can directly affect Treg homeostasis. CD4+ T cells express known HDL receptors, such as the sphingomyelin receptors, S1PR (notably S1PR1) (13, 14) or scavenger receptor class B type I (SR-BI) (15, 16). Herein, we therefore hypothesized that HDL might preferentially interact with human Tregs as an energy source, and that these interactions might promote survival by enhancing oxidative phosphorylation (OXPHOS) in the mitochondria.
MATERIALS AND METHODS
Cell isolation
Peripheral blood mononuclear cells from healthy donors were separated by centrifugation through Ficoll-Hypaque (GE, Fairfield, CT). Then, resting CD4+ T cells were purified by negative selection using the Miltenyi CD4 separation kit (Auburn, CA), per the manufacturer’s instructions. Aliquots of purified CD4+ T cells were frozen in FCS + 10% DMSO and stored in liquid nitrogen. The viability of thawed cryopreserved cells was ∼90%. In some experiments, freshly isolated CD4+ T cells that had never been cryopreserved were analyzed within 24 h of blood sample collection. To isolate Tregs (pTregs and tTregs), naïve cells, and memory cells, purified CD4+ T cells (fresh or previously cryopreserved) were stained with anti-CD8-FITC, anti-CD25-APC (BD Pharmingen, San Diego, CA), anti-CD127-PE (Beckman Coulter, Fullerton, CA), and anti-CD45RA-PB (Invitrogen, Carlsbad, CA) and sorted using a FACS Aria (BD). As shown in supplemental Fig. S1A, the populations were defined as follows: Tregs (CD8−CD25hiCD127lo cells), naïve cells (CD8−CD25loCD127hiCD45RA+), and memory cells (CD8−CD25lowCD127hiCD45RA−). Purity of the sorted populations was >90%, as determined by post-sorting analysis of FOXP3 expression (supplemental Fig. S1B). In some experiments, CD25+ CD4+ T cells were isolated by positive selection using magnetic microbeads (Miltenyi-Biotec); 80% of isolated CD25+ CD4+ T cells isolated were FOXP3+, as determined by flow cytometry.
Reagents and culture conditions
Purified Tregs, naïve T cells, and memory T cells were cultured in the serum-free medium, X-VIVO 15 (Lonza, Charleston, TN), formulated with glucose. These subsets were incubated for 1–24 h at 37°C in the presence or absence of pooled human HDL or LDL (both at 300 μg/ml unless indicated otherwise; ≥95% SDS-PAGE; Sigma-Aldrich, St. Louis, MO); human HDL-DiI or LDL-DiI (same concentration; Biotrend, Destin, FL); oleic acid bound to albumin, oleic acid, or albumin (all at 300 μg/ml; Sigma-Aldrich); etomoxir (ETX; 30 μM; Sigma-Aldrich). To confirm the data obtained with the commercial HDL pool, HDLs from seven healthy donors were isolated, pooled, and used at 300 μg/ml within 7 days of their preparation. Healthy donors were consented under a protocol approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center, Cincinnati, OH. The effect of SR-BI/II was tested by adding the rabbit polyclonal SR-BI/II blocking antibody (1:100 dilution; Novus Biological, Littleton, CO) to the cultures, starting 10 min before adding the HDL. Absolute number and cell viability were then quantified using Trypan blue staining.
Imaging flow cytometry
To measure HDL binding/uptake in vitro, purified Tregs, naïve Tcells, and memory T cells were cultured for 1–4 h with HDL-DiI or LDL-DiI in X-VIVO 15 media. Then, cells were extensively washed and fixed with 4% methanol-free formaldehyde for 30 min. Cells were stained with anti-CD4-AF700 (Biolegend, San Diego, CA) and DAPI for surface and nuclear stain, respectively. Samples were acquired with the Amnis Image Stream, using the INSPIRE software; data were analyzed using the IDEAS software (MD Millipore, Seattle, WA).
Flow cytometry
To quantify cell cycle, Tregs were stained intracellularly with Ki-67-PerCP-Cy™5.5 (clone B56; BD). Apoptosis was quantified using the Annexin V/7AAD Apoptosis Detection Kit I (BD). To estimate mitochondrial membrane potential, cells were incubated with 10 nM of MitoTracker Deep Red FM dye (Invitrogen) for 30 min at 37°C and were washed twice before analysis by flow cytometry. To evaluate the cholesteryl ester (CE) content by flow cytometry, cells were incubated for 15 min at 4°C in the dark with cholesteryl BODIPY® FL C12 (500 ng/ml; Invitrogen, Grand Island, NY). Free cholesterol content was quantified by staining with filipin III, following the manufacturer’s instructions (cholesterol assay kit; Abcam, Cambridge, MA). In addition, the rabbit blocking SR-BI/II polyclonal antibody (Novus Biological) was labeled with Zenon AF700 (Life Technology, Grand Island, NY) and used to determine levels of SR-BI/II expression. Glut1 and LDLR expression were measured using anti-Glut1-AF700 (202915) and anti-LDLR-AF488 (472413), respectively (R&D Systems, Boston, MA). Samples were acquired on an LSRII flow cytometer (BD). Flow cytometry data were analyzed using the FACS DIVA software (BD).
Confocal microscopy
T cell subsets were cultured for 1–4 h with HDL-DiI in X-VIVO 15 media. Then, cells were washed and fixed with 4% methanol-free formaldehyde for 30 min. Cells were stained with anti-CD4-AF647 (Biolegend) and DAPI for surface and nuclear stain, respectively. Cell suspension was directly applied to freshly cleaned glass slides. Following a drying time of 15–20 min, #1.5 coverslips (Fisher Scientific, Pittsburgh, PA) were mounted onto the slides using Prolong® Gold Antifade reagent (Fisher Scientific). HDL localization was visualized with an inverted Nikon A1 laser scanning confocal microscope with the 100× objective.
Analysis of Treg metabolism
Purified CD25+ CD4+ (7 × 105) T cells (three replicates per condition for each individual Treg sample) were plated in 96-well XF cell culture microplates in the serum-free XF assay medium (Agilent Technologies, Santa Clara, CA), which contains minimal substrates (such as 2 mM glutamax) and is specifically formulated for Seahorse assays. XF medium was prepared with or without glucose (2 mM). Cells were incubated for 1.5 h at 37°C without CO2 in the presence or absence of human HDL. Oxygen consumption rates (OCRs) and extracellular acidification rates were measured under basal conditions or in the presence of ETX, as well as following the addition of either oligomycin (2 μM; Sigma), an inhibitor of OXPHOS, or the uncoupler fluoro-carbonyl cyanide phenylhydrazone (2 μM, Sigma). Five measurements were taken using a 96-well extracellular flux analyzer (Agilent Technologies). Basal respiration was defined as the OCR before the addition of any additional compounds. Maximal respiration was defined as the OCR after fluoro-carbonyl cyanide phenylhydrazone treatment. Basal and maximal respiration values are the average of three experimental replicates for each Treg sample in each condition. Spare respiratory capacity (SRC) is the ratio between maximum and basal OCR and was calculated for each individual Treg sample and condition. The median (range) of these values (basal respiration, maximal respiration, and SRC) in six individual Treg samples was then calculated and displayed.
RNA and quantitative real-time PCR
Total RNA was isolated from sorted Tregs, naïve Tcells, and memory T cells using the RNeasy Mini kit (QIAGEN). RNA was used for cDNA synthesis with RT-PCR using oligo (dT) by the SuperScript® III single stranded cDNA synthesis kit (Invitrogen), per the manufacturer’s instructions. Real-time PCR reactions were performed using the FastStart TaqMan Probe Master (Roche) reagent, specific primers and results were analyzed by using the iCycler IQ real-time PCR detection system (BioRad). Expression levels were normalized by Δct using ubiquitin conjugating enzyme (UBE2D2) as housekeeping gene.
Measurement of intracellular ATP levels
Intracellular ATP was measured in isolated Treg, naïve T cell, and memory T cell subsets in the presence or absence of HDL, using a luminescence assay (ATP bioluminescent assay kit; Sigma-Aldrich) and the GloMax®-Multi detection system.
Statistical analysis
Statistical analyses were performed using Prism (GraphPad Software 5). Medians (ranges) were compared by U Mann-Whitney or Wilcoxon tests depending on the analysis. In all analyses, P values lower than 0.05 were considered to be significant.
RESULTS
HDL promotes survival of Tregs, but not of naïve or memory T cells
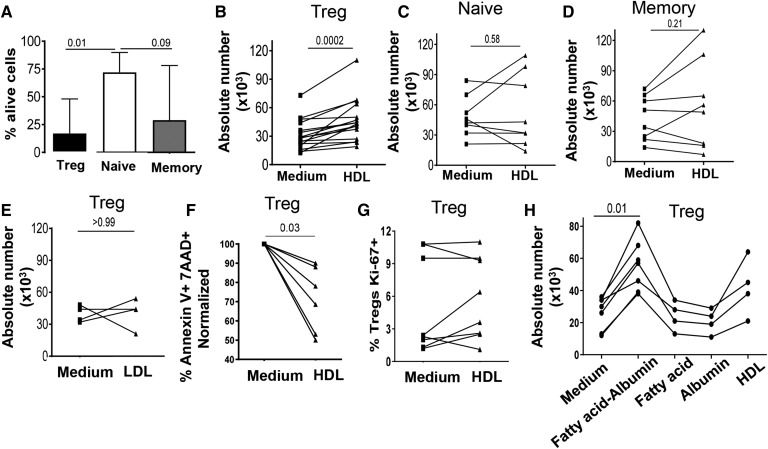
HDL increased the absolute numbers of Tregs in murine models (10). As HDL has also been shown to enhance the survival of endothelial cells in vitro (17), we thus evaluated the effect of HDL on the viability of purified Tregs (CD8−CD25+CD127−), compared with that of purified naïve (CD8−CD25−CD127+CD45RA+) and memory Tcons (CD8-CD25−CD127+CD45RA−) cells from healthy individuals. All populations were >90% pure (see supplemental Fig. S1B). As expected based on previous data (18), Tregs cultured without stimulus had reduced viability at 24 h compared with naïve and memory T cells (Fig. 1A). However, Treg counts significantly increased when they were cultured with pooled HDL used at 300 μg/ml of total protein (Fig. 1B), increasing by a median fold of 1.6. In contrast, HDL did not affect the number of naïve and memory cells (Fig. 1C, D; median fold increase: 1.0 and 1.1, both P > 0.2 compared with untreated). Importantly, the effect of HDL on Treg viability was dose dependent, increasing gradually when Tregs were cultured with HDL concentrations spanning from 75 to 600 μg/ml (supplemental Fig. S2A). Accordingly, we chose the 300 μg/ml concentration for all subsequent experiments, as it is within the range of HDL-C we measured in the plasma of normal healthy individuals (12). We confirmed these data using freshly isolated HDL prepared from the plasma of seven healthy donors, that also led to an increased number of Tregs compared with medium (P = 0.03, data not shown). In contrast to the effect exerted by HDL, LDL at the same concentration did not affect the number of Tregs (Fig. 1E), naïve T cells, or memory T cells (data not shown). Some studies have shown changes in the frequency, phenotypes, or function on Tregs after cryopreservation (19, 20); although, in our hands and in other studies (21–24), Tregs retained their suppressive capacity and phenotype after cryopreservation. Nevertheless, we validated our findings in freshly isolated unfrozen cells. As shown in Fig. 2F and supplemental Fig. S2B–D, freshly isolated cells had a similar response to HDL as cryopreserved cells.
Fig. 1.
HDLs, but not LDLs, promote Treg survival. A: Bar graphs show median and range of the baseline survival of Tregs, naïve cells, and memory cells in X-VIVO medium. B, C: Dot line graphs represent the absolute number of cells cultured for 24 h in the absence (medium) or in the presence of HDL (300 μg/ml): Tregs (B), naïveCD4+ T cells (C), and memory CD4+ T cells (D). E: Absolute number of Tregs cultured for 24 h in the presence or absence of LDL (300 μg/ml). F: Tregs were stained for apoptosis with annexin V and 7AAD. Normalized survival was calculated based on cells cultured in medium alone. Annexin V− 7AAD− cells were considered as live. G: Tregs were stained intracellularly for the cell cycle marker with Ki67. H: Absolute number of Tregs cultured for 24 h in medium or in the presence of oleic acid bound to albumin, oleic acid alone, albumin, or HDL (all at 300 μg/ml). Comparisons between groups were done with U Mann-Whitney or Wilcoxon tests. Each line represents a single donor.
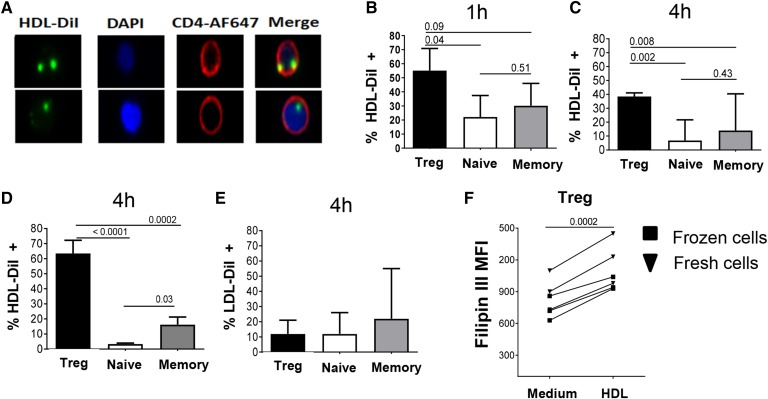
Fig. 2.
Tregs bind, uptake, and store high amounts of HDLs compared with naïve and memory Tcons. A–C: Purified Tregs, naïve cells, and memory cells from healthy controls were incubated with HDL-DiI (300 μg/ml) and analyzed by imaging flow cytometry. Representative images show HDL-DiI (green), nucleus (DAPI blue), and CD4 (red) localization (A) and summary of HDL uptake in each subset at 1 h (B) and 4 h (C) of culture. Histograms represent median (quartile) in n = 5 individuals. D: HDL uptake in the different subsets was analyzed after 4 h of culture of total CD4+ T cells with HDL-DiI. Cells were then stained extracellularly with CD25, CD127, and CD45RA to differentiate Treg (CD25+CD127−), naïve (CD25−CD45RA+), and memory (CD25−CD45−) subsets. E: LDL uptake was analyzed in each subset after 4 h of culture with LDL-DiI (300 μg/ml). F: Free cholesterol in purified Tregs cultured in the presence or absence of HDL was detected by flow cytometric filipin III stain. Each line represents an individual. Comparisons between groups were done with U Mann-Whitney or Wilcoxon tests.
As HDL has been shown to both decrease apoptosis and stimulate entry into cell cycle (25), we next evaluated these two mechanisms, analyzing the percentage of apoptotic cells by 7AAD/annexin V staining and the expression of the cell cycle marker, Ki67, respectively. After 24 h of exposure to HDL, the percentage of apoptotic Tregs significantly decreased (Fig. 1F, see representative flow cytometry data in supplemental Fig. S2E), while the frequency of Ki67+ Tregs was not changed (Fig. 1G). We observed a trend toward a negative correlation between the increased proportion of apoptotic cells and the absolute number of cells at 24 h (Pearson correlation, P = 0.078, r = −0.65).
FAs have been shown to play a vital role in Treg differentiation (5, 26). Therefore, we investigated whether free FAs or FAs bound to albumin would also promote Treg survival. As shown Fig. 1H, oleic acid bound to albumin significantly improved Treg survival, like HDL. In contrast, oleic acid alone or albumin did not affect Treg survival (Fig. 1H).
Tregs bind and uptake high amounts of HDL compared with naïve and memory CD4+ T cells
To understand the mechanisms underlying the specific effect of HDL on Treg viability, we next analyzed whether Tregs have greater affinity for HDL than the other CD4 subsets. We designed an in vitro assay to track the interaction with HDL, using labeled HDL (HDL-DiI) and visualizing HDL binding/internalization by T cells by imaging flow cytometry (Fig. 2A, and representative example in supplemental Fig. S3A). Remarkably, at both 1 h (Fig. 2B) and 4 h (Fig. 2C), the percentage of isolated Tregs labeled by HDL-DiI was very high (∼60% and ∼40%, respectively), and was always significantly higher than that of HDL-DiI+ naïve and memory Tcons. HDL-DiI intensity in the positive cells was similar in all subsets (data not shown). HDL internalization by Tregs remained specific when they were analyzed in the context of total CD4+ T cells (Fig. 2D), although Tregs represent only ∼5% of the circulating CD4+ T cell pool (27, 28). Because imaging flow cytometry does not distinguish between uptake and binding, we used confocal microscopy to study HDL-DiI localization inside Tregs. As shown in the representative supplemental Fig. S3B, HDL (red) was mainly localized in the membrane colocalizing with CD4 expression (green) in Tregs after 1 h. After 4 h, HDL staining had a punctuated intracellular pattern, reminiscent of the HDL clusters seen in CHO cells (29). Confirming the specificity of the HDL-Treg interactions, LDL-DiI uptake by Tregs or the other CD4+ subsets, was minimal (<20%; Fig. 2E).
To confirm these results, we measured the cellular content of free cholesterol and CE in Tregs or other subsets treated with HDL. Compared with the untreated condition, HDL increased the quantity of free cholesterol, as evidenced by the higher intensity of filipin staining, in both frozen and fresh Tregs (Fig. 2F and representative example of the staining shown in supplemental Fig. S3C). This increase was significant for Tregs (mean fold increase: 1.4), while it was minimal in naïve and memory Tcons (mean fold increase close to 1.0 in both subsets; supplemental Fig. S3D). Increased free cholesterol levels were not seen in presence of LDL (data not shown). In contrast, HDL did not modify the cellular content in CEs in any of the CD4 subsets, as evidenced by similar levels of BODIPY® FL C12 staining in treated or untreated cells (data not shown).
SR-BI/BII contribute to HDL-mediated survival in Tregs
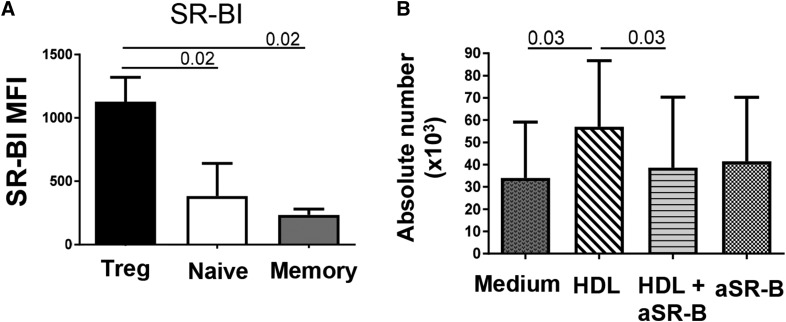
The first HDL receptor to be characterized was SR-BI, a cell surface glycoprotein that is associated with lipid rafts (30). Expression of SR-BI or its isoform, SR-BII, in human and murine T-cells had been reported (15, 16), but its differential expression by CD4 subsets had not been reported. We thus compared by flow cytometry the expression of SR-BI/BII in the different CD4+ T cell subsets using an anti-SR-BI/BII antibody. As shown in Fig. 3A, SR-BI/BII expression by Tregs was approximately twice that of the other subsets, suggesting that this difference could play a major role in the selective HDL uptake by Tregs. Accordingly, SR-BI/BII blockade abolished the HDL-mediated enhanced survival (Fig. 3B). This effect of anti-SR-BI/II was seen in both fresh and cryopreserved Tregs (data not shown). All subsets also expressed the two major receptors for sphingomyelin, SPR1 and SPR4, but in contrast to the higher expression of SRB-I/BII by Tregs, similar high levels of S1PR1 and low levels of SRP4 were observed in all subsets (supplemental Fig. S4A, B). None of the CD4 subsets expressed the main cholesterol efflux regulatory proteins, ABCA1 and ABCG1 (data not shown). Expression of LDLR was significantly higher in naïve T cells than in Tregs and memory T cells (supplemental Fig. S4C), suggesting that expression of LDLR is not related with lipoprotein (HDL or LDL) uptake in T cells.
Fig. 3.
SR-BI/BII expression by Tregs is important for their enhanced HDL-mediated survival. A: Extracellular SR-BI expression in each subset was determined by flow cytometry in total CD4 T cells. Each subset was gated as: Tregs (CD3+ CD4+ CD25+ CD127Low), naïve cells (CD3+ CD4+ CD25− CD127+ CD45RA+), and memory cells (CD3+ CD4+ CD25− CD127+ CD45RA−). Histograms represent median (quartile) in n = 5 individuals. B: Effect of SR-BI cross-linking on HDL-mediated survival. Tregs were pre-exposed or not to the rabbit SR-BI-blocking antibody (1:100 dilution) for 10 min at 37°C and then cultured with HDL. Treg absolute numbers were measured in the presence of HDL and SR-BI-blocking antibody. Comparisons between groups were done with U Mann-Whitney or Wilcoxon tests.
Increased OXPHOS and FA oxidation in Tregs contribute to the HDL-mediated survival of Tregs
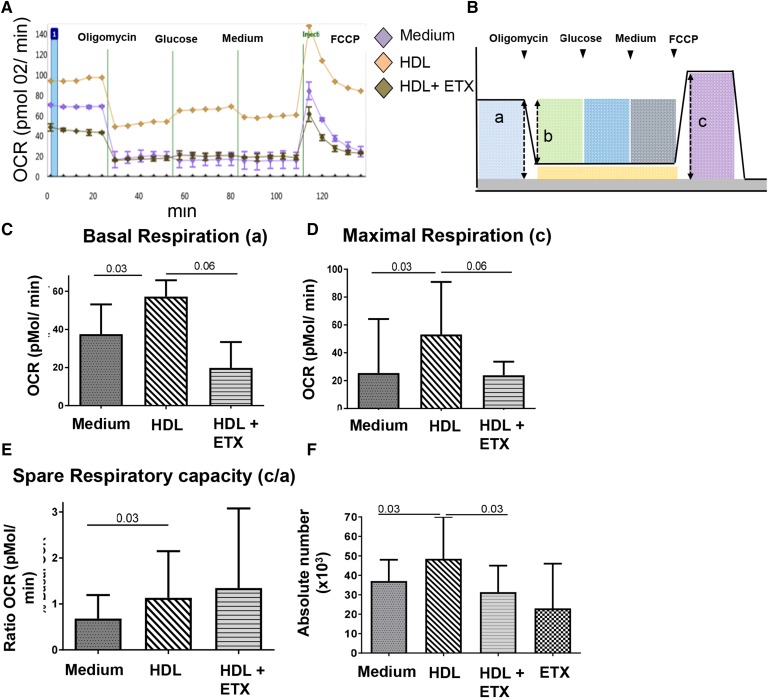
We next explored whether Tregs can use HDL as an energy substrate, which could explain the HDL-mediated increased survival. We measured the OCR as an indicator of OXPHOS in purified CD4+CD25+ cells (>80% FOXP3+) cultured in the presence or absence of HDL and in the absence of glucose. As shown in the representative Fig. 4A, Tregs exposed to HDL for 1 h showed higher OCR than Tregs cultured in medium. To determine which mitochondrial respiration process was modified by the presence of HDL, we analyzed by Seahorse Tregs’ basal respiration, ATP-coupled OCR, maximal respiration, and SRC. Tregs exposed to HDL had higher median basal and maximal respiration, (Fig. 4C, D) than untreated cells, but ATP-coupled OCR was unchanged (supplemental Fig. S5A). Extracellular acidification rate was also unchanged (supplemental Fig. S5B). OCR minimally changed in response to glucose injection, in both HDL-exposed or unexposed Tregs (Fig. 4A). In most individual Treg samples, basal respiration was equal to or higher than maximal respiration, which is consistent with previous data obtained in resting human or murine Tregs (31–33). We also determined the effect of HDL on mitochondrial SRC, because it reflects the additional capacity to produce energy under stress and appears critical for the survival of activated CD8+ T cells (34, 35). Interestingly, SRC increased in HDL-treated Tregs (Fig. 4E).
Fig. 4.
HDLs increase OXPHOS and FAO in Tregs, which contributes to enhanced Treg survival. A–E: Tregs (bead-separated CD4+CD25+) were cultured in glucose-free medium in the absence (medium) or presence of HDL alone or with ETX (100 μM). OCR was measured using a 96w-Seahorse. Each individual Treg sample was tested in three replicates for each condition, and average measurements were calculated. A: Representative experiment of one donor. B: Scheme outlining the approach to quantify mitochondrial respiration parameters: basal respiration (a); ATP-coupled respiration (b); and maximal respiration (c). C–E: Median (range) basal respiration (C); maximal respiration (D), and SRC (ratio c/a) (E). F: Treg absolute numbers were measured at 24 h in each condition. C–F: Bar graphs show median and range of six independent experiments. Comparisons between groups were done using Wilcoxon tests.
Addition of ETX, an inhibitor of carnitine palmitoyl transferase 1a (CPT1a), and thus of the transport of long-chain FAs into the mitochondria, inhibited the effect of HDL on Treg basal and maximal respiration (Fig. 4C, D), but it did not significantly affect Treg SRC (Fig. 4E). ETX alone had no effect on basal and maximal OCR (data not shown). Importantly, and consistent with the hypothesis that HDL serves as an energy substrate for Tregs, ETX abolished HDL-mediated increased Treg survival (Fig. 4F).
Because the presence of glucose has been shown to be important in supporting FA oxidation (FAO) and OXPHOS in CD8+ T cells (36), we also tested whether the effect of HDL on Treg metabolism was altered by the presence of glucose. HDL in this experimental setting also increased Treg maximal respiration and SRC (supplemental Fig. S5E, F), but it did not change Treg basal respiration or ATP-coupled mitochondrial respiration (supplemental Fig. S5C, D). Consistent with a lack of influence of glucose on Treg metabolism, Tregs expressed low levels of Glut1, one of the main glucose receptors, which is expressed by T cells with high glycolytic activity (4, 5), and HDL had no effect on its expression (data not shown).
HDL increases mitochondrial membrane potential and ATP generation in Tregs
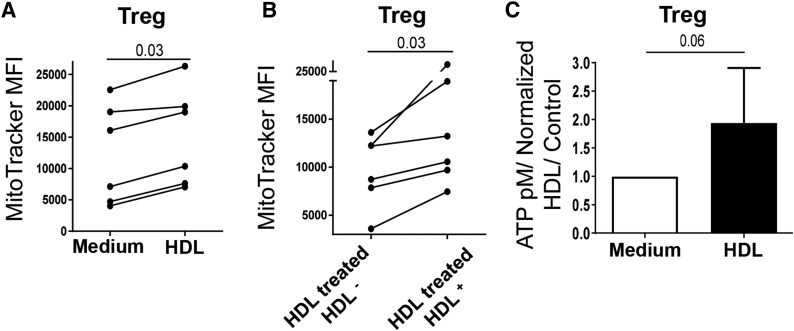
To further establish that HDL increased Treg mitochondrial activity, we measured the effect of HDL uptake on mitochondrial membrane potential that we quantified by analyzing the intensity of MitoTracker staining. HDL treatment significantly enhanced MitoTracker MFI in Tregs (Fig. 5A), whereas no change was seen in the other subsets (data not shown). One important question is whether HDL uptake is required for this effect, as opposed to a bystander effect. We compared the MitoTracker intensity of Tregs that had or had not internalized HDL (HDL-DiI+ versus HDL-DiI−) in Tregs that were all exposed to HDL-DiI for 4 h. As shown in Fig. 5B, mitochondrial membrane potential was higher in Tregs that had internalized HDL than in those who did not, suggesting that HDL uptake is needed to increase Treg mitochondrial activity.
Fig. 5.
HDLs increase mitochondrial potential membrane and ATP generation in Tregs. A, B: Treg mitochondrial membrane potential was determined by flow cytometric MitoTracker staining after 4 h of culture in X-VIVO medium. A: Comparison of untreated (medium) and HDL-treated Tregs. B: In Tregs that were all exposed to HDL-DiI, comparison of those that had (HDL-treated HDL+) or did not have (HDL-treated HDL−) internalized HDL. C: Median fold increase of ATP levels in Tregs cultured in X-VIVO medium for 4 h in the presence or absence of HDL. Comparisons between groups were done with U Mann-Whitney or Wilcoxon tests.
FAs are oxidized resulting in the synthesis of ATP by β-oxidation to generate cellular energy. We therefore quantified total ATP levels in untreated and HDL-treated CD4+ T cell subsets. Basal ATP levels were significantly lower in Tregs than in naïve and memory T cells (supplemental Fig. S6), in accordance with their lower survival (Fig. 1A). When Tregs were cultured with HDL for 4 h, total ATP levels increased by ∼2-fold (P = 0.06; Fig. 5C), whereas HDL did not affect the ATP concentration in naïve and memory cells (data not shown), suggesting that HDLs boost the Treg bioenergetic potential.
DISCUSSION
One of our main findings is that Tregs have a high capacity to bind and internalize HDL, but not LDL. In accordance, Treg survival significantly increased in the presence of HDL, but not LDL. An important question was thus to understand why Tregs preferentially interact with HDL. Our data suggest that the high expression of SR-BI/BII by Tregs could play a major role in this selective effect. SR-BI and its isoform, SR-BII, have an identical extracellular domain (37). The rabbit polyclonal anti-SR-BI we used does not distinguish between the two forms. Previously, SR-BI/BII mRNA expression was detected in total CD3+ T cells, although it was low compared with SR-B levels in hepatocytes (15). Herein, we show that Tregs exhibit a much higher level of SR-BI/BII than the other CD4 subsets. This difference could be related to Tregs’ enhanced basal level of activation compared with other Tcon subsets (38, 39). Of interest in this context, Xu and colleagues described years ago that HDL internalization could occur in activated T cells, but these reports did not identify the receptor(s) involved in this phenomenon, nor did they establish whether the different subsets might not be equally able to bind and internalize HDL (40, 41). SR-BI plasma membrane location and how it mediates HDL endocytosis have been extensively studied in cell lines with discrepant results depending on the model system and the cell line [reviewed in (42)]. In some cells, SR-BI is concentrated in plasma membrane caveolae/lipid rafts and caveolin-containing complex, although this result was not consistently found (42). SR-BI binds HDL with high affinity, and mediates cellular uptake of HDL CE (43, 44). SR-BI delivers CE to sites in the membrane where it is readily metabolized to free cholesterol by cell type-specific CE hydrolases (43, 44). Our data show that HDL increased the cellular content of free cholesterol in Tregs. However, this was not associated with enhanced CE levels. One potential explanation for this finding is that CEs are rapidly used by the enzyme, lysosomal acid lipase/CE hydrolase, which is important to the mobilization of FA to FAO and the development of T cells (36, 45). Our results also raise one intriguing question, which is why HDLs, but not LDLs, bind to Tregs; in another immune cell, macrophage’s SR-BI can bind native LDL, even if this process is much less efficient in mediating LDL internalization than the classic LDLR [reviewed in (46)]. Of note, Tregs express very low levels of LDLR. It is thus possible that HDL uptake by Tregs through a SR-BI pathway is modulated by other cellular proteins or another structural component from HDL. Indeed, in hepatocytes, caveolin expression increases HDL-CE selective uptake by SR-BI, while it decreases LDL-CE selective uptake (47). Similarly, the presence of ApoE in HDL facilitates HDL-CE selective uptake through binding to SR-BI and inducing conformational changes (48). Further studies will thus be needed to completely elucidate how Tregs uptake HDL.
Importantly, similar to mice with FoxP3 mutation or with selective depletion of Tregs (49, 50), SR-BI-null mice developed systemic autoimmune disorders characterized by splenomegaly and high T cell activation/proliferation (15). Treg frequencies and numbers in SR-BI-null mice were not diminished compared with the wild-type mice (15), which could be related to the compensatory expansion of Tregs during inflammation. In addition, Treg functionality on a per cell basis was not analyzed in these mice. The role of metabolic programming on Treg functionality has not been thoroughly studied, but impaired mitochondrial activity in murine Tregs is associated with poor suppressive activity, likely due to decreased levels of CTLA4, ICOS, and CD71 (51). Similarly, key OXPHOS regulators were recently shown to be required for optimal Treg function (32). Therefore, the role of SR-B-mediated uptake of HDL in Treg functionality will need to be further ascertained.
Downstream of HDL binding, the decreased frequency of annexin V-positive Tregs and the unchanged expression of Ki-67 indicate that HDL increased Treg survival, but did not affect Treg cell cycle. These data are in agreement with the effect of the HDL on endothelial cells (52, 53). Importantly, FAs derive from extrinsic sources, and both short-chain FAs (propionate, butyrate, and acetate) and exogenous long-chain FAs (oleate/palmitate) can be used as fuel and are important metabolites for Treg homeostasis (5, 26). Importantly, unlike memory CD8+ T cells that synthesize FAs from extracellular glucose, Tregs take up externally derived FAs to support their high rates of FAO, but the involved molecules and pathways are not known [reviewed in (54, 55)]. Our in vitro assay suggests that Tregs can use HDL from their microenvironment as a source of energy, activating FA OXPHOS and increasing their basal and maximal respiration. This hypothesis is supported by the fact that oleate bound to serum albumin had a similar pro-survival effect for Tregs as HDL. Increased SRC was reported to promote the survival of memory CD8+ T cells treated by IL-15 through a FAO-dependent pathway (34). Our data are partially in concordance with this scenario, as HDL also enhanced Treg SRC, but this increase was not dependent on FAO because it was insensitive to ETX. However, ETX abolished HDL-mediated increased Treg survival and Treg basal and maximal respiration, suggesting that SRC is not the only parameter associated with Treg survival.
Tregs’ metabolic dependency on FAO or glycolysis remains disputed. Indeed, activated Tregs appear to require both glycolysis and FAO (31, 32), while Tregs generated through in vitro polarization of CD4+ T cells preferentially use lipid metabolism (4, 5, 56). A recent study reported that freshly isolated human Tregs have higher glycolysis and OXPHOS, but lower FAO, in comparison with Tcons (31). These discrepancies raise the question of whether the presence of glucose in culture medium might modify how Tregs use extracellular sources of lipids for their metabolism. One of these modifications could be the enhanced synthesis of FA, as has been shown in memory CD8+ T cells (36). Our results do not support such a model for several reasons. First, Tregs expressed very low levels of Glut1, and these levels were not modified by HDL. Second, consistent with these data, Treg glycolysis did not augment in response to glucose injection. Finally, the presence of glucose had only a very modest influence on the effect of HDL on Treg metabolism.
An important parameter related to cellular survival is the synthesis of intracellular ATP. Basal levels of intracellular ATP were much lower in Tregs than in the other T cell subsets, which is in agreement with a previous study showing that high levels of adenylyl cyclase 9 degrade Treg intracellular ATP (57). These low levels of total ATP are likely causative to their low survival. Total ATP levels generated by OXPHOS or another metabolic process were upregulated by HDL. This robust enhancement of ATP generation appears to require some time to occur, as it was only apparent after 4 h, whereas enhanced Treg respiration was seen after 1 h. These differences might be related to the time the cells require to completely uptake HDL, as internalization was more pronounced at 4 h than at 1 h (Fig. 2), and this may influence the kinetics by which HDLs affect different aspects of Treg metabolism. Of interest, mitochondrial activity was mostly increased in the Tregs that internalized HDL, and not in the exposed “bystander” Tregs, which reinforces the hypothesis that the main mechanism underlying increased survival is through providing additional “fuel.” Alternatively and nonexclusively, Tregs have been shown to use exogenous FA to produce phospholipids for cellular membranes, while other CD4 subsets predominantly use de novo FA synthesis (58), and this mechanism could also contribute to the HDL- or FA-mediated increased Treg survival. More studies are thus needed to determine the mechanism(s) by which HDLs promote Treg survival. Another area for future studies is to better understand the relationship between HDL composition and its biological effect on Tregs. It is a highly significant question due to the existence of numerous subpopulations of HDLs with distinct protein/lipid composition (59). HDL composition influences HDL anti-apoptotic capacities for endothelial cells or macrophages (60, 61), and might also dictate HDL anti-apoptotic effect on Treg survival.
Altogether, our results show for the first time that human resting Tregs internalize HDL, which leads to their increased survival. The recent failure of HDL-C-raising agents to provide clinical benefits has created a controversy on the mechanisms underlying HDLs’ atheroprotective properties (62) and has highlighted the need for a better understanding of the biology of HDL particles and how they exert their pleiotropic effects on inflammation (63). Our data, by providing a novel understanding of the effect of HDLs on Treg homeostasis, could thus open a new metric by which HDL-C-raising agents could be evaluated.
Supplementary Material
Acknowledgments
The authors thank J. Matthew Kofron for his invaluable help with the confocal microscopy experiments.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- ETX
- etomoxir
- FAO
- FA oxidation
- HDL-C
- HDL-cholesterol
- LDLR
- LDL receptor
- OCR
- oxygen consumption rate
- OXPHOS
- oxidative phosphorylation
- pTreg
- peripherally derived regulatory T cell
- SR-BI
- scavenger receptor class B type I
- SRC
- spare respiratory capacity
- Tcon
- conventional CD4+ T cell
- Treg
- regulatory T cell
- tTreg
- thymus-derived regulatory T cell
This work was partially supported by a Cincinnati Children’s Hospital Medical Center Research Innovation/Pilot Funding Program grant and National Institutes of Health Grants R21 AI128218 (to C.A.C.) and R01 HL60793 (to W.S.D.). Additional partial support was provided by the Universidad de Antioquia and Departamento Administrativo de Ciencia, Tecnología e Innovación Grant 111551928730 (to A.L.R-P.). Partial support of the flow cytometry experiments done in the Research Flow Cytometry Core at Cincinnati Children’s Hospital Medical Center was provided by Center for Excellence in Molecular Hematology Grant 1P30DK090971-01 and Digestive Health Center Grant AR47363. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Rodríguez-Perea A. L., Arcia E. D., Rueda C. M., and Velilla P. A.. 2016. Phenotypical characterization of regulatory T cells in humans and rodents. Clin. Exp. Immunol. 185: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingenberg R., Gerdes N., Badeau R. M., Gistera A., Strodthoff D., Ketelhuth D. F., Lundberg A. M., Rudling M., Nilsson S. K., Olivecrona G., et al. 2013. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 123: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maganto-García E., Tarrio M. L., Grabie N., Bu D. X., and Lichtman A. H.. 2011. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 124: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L. Z., Wang R., Huang G., Vogel P., Neale G., Green D. R., and Chi H.. 2011. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208: 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalek R. D., Gerriets V. A., Jacobs S. R., Macintyre A. N., MacIver N. J., Mason E. F., Sullivan S. A., Nichols A. G., and Rathmell J. C.. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186: 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothblat G. H., and Phillips M. C.. 2010. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 21: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontush A., Lindahl M., Lhomme M., Calabresi L., Chapman M. J., and Davidson W. S.. 2015. Structure of HDL: particle subclasses and molecular components. Handb. Exp. Pharmacol. 224: 3–51. [DOI] [PubMed] [Google Scholar]
- 8.Tamosiuniene R., Tian W., Dhillon G., Wang L., Sung Y. K., Gera L., Patterson A. J., Agrawal R., Rabinovitch M., Ambler K., et al. 2011. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ. Res. 109: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffrin E. L. 2014. Immune mechanisms in hypertension and vascular injury. Clin. Sci. (Lond.). 126: 267–274. [DOI] [PubMed] [Google Scholar]
- 10.Ru D., Zhiqing H., Lin Z., Feng W., Feng Z., Jiayou Z., Yusheng R., Min F., Chun L., and Zonggui W.. 2015. Oxidized high-density lipoprotein accelerates atherosclerosis progression by inducing the imbalance between treg and teff in LDLR knockout mice. APMIS. 123: 410–421. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm A. J., Zabalawi M., Owen J. S., Shah D., Grayson J. M., Major A. S., Bhat S., Gibbs D. P. Jr., Thomas M. J., and Sorci-Thomas M. G.. 2010. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr-/-, ApoA-I-/- mice. J. Biol. Chem. 285: 36158–36169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Perea A. L., Montoya C. J., Olek S., Chougnet C. A., and Velilla P. A.. 2015. Statins increase the frequency of circulating CD4+ FOXP3+ regulatory T cells in healthy individuals. J. Immunol. Res. 2015: 762506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H. Y., Da W. M., Gao C. J., Li M., Chen W. H., Yu L., and Huang W. R.. 2010. Impact of rhG-CSF on Sphingosine 1-phosphate receptor 1 expression in CD4+ T cells of donor peripheral blood [article in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18: 427–430. [PubMed] [Google Scholar]
- 14.Nomachi A., Yoshinaga M., Liu J., Kanchanawong P., Tohyama K., Thumkeo D., Watanabe T., Narumiya S., and Hirata T.. 2013. Moesin controls clathrin-mediated S1PR1 internalization in T cells. PLoS One. 8: e82590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H., Guo L., Wang D., Gao H., Hou G., Zheng Z., Ai J., Foreman O., Daugherty A., and Li X. A.. 2011. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler. Thromb. Vasc. Biol. 31: 2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larbi A., Fortin C., Dupuis G., Berrougui H., Khalil A., and Fulop T.. 2014. Immunomodulatory role of high-density lipoproteins: impact on immunosenescence. Age (Dordr). 36: 9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nofer J. R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., and Assmann G.. 2001. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J. Biol. Chem. 276: 34480–34485. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., Shen S., Gorentla B. K., Gao J., and Zhong X. P.. 2012. Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression. J. Immunol. 188: 1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sattui S., de la Flor C., Sanchez C., Lewis D., Lopez G., Rizo-Patron E., White A. C. Jr., and Montes M.. 2012. Cryopreservation modulates the detection of regulatory T cell markers. Cytometry B Clin. Cytom. 82: 54–58. [DOI] [PubMed] [Google Scholar]
- 20.Golab K., Leveson-Gower D., Wang X. J., Grzanka J., Marek-Trzonkowska N., Krzystyniak A., Millis J. M., Trzonkowski P., and Witkowski P.. 2013. Challenges in cryopreservation of regulatory T cells (Tregs) for clinical therapeutic applications. Int. Immunopharmacol. 16: 371–375. [DOI] [PubMed] [Google Scholar]
- 21.Mavin E., Dickinson A., and Wang X. N.. 2013. Do cryopreserved regulatory T cells retain their suppressive potency? Transplantation. 95: e68–e70. [DOI] [PubMed] [Google Scholar]
- 22.Rydén A., and Faresjö M.. 2011. Efficient expansion of cryopreserved CD4(+)CD25(+)CD127(lo/-) cells in Type 1 diabetes. Results Immunol. 1: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venet F., Malcus C., Ferry T., Poitevin F., and Monneret G.. 2010. Percentage of regulatory T cells CD4+CD25+CD127- in HIV-infected patients is not reduced after cryopreservation. J. Immunol. Methods. 357: 55–58. [DOI] [PubMed] [Google Scholar]
- 24.Nettenstrom L., Alderson K., Raschke E. E., Evans M. D., Sondel P. M., Olek S., and Seroogy C. M.. 2013. An optimized multi-parameter flow cytometry protocol for human T regulatory cell analysis on fresh and viably frozen cells, correlation with epigenetic analysis, and comparison of cord and adult blood. J. Immunol. Methods. 387: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norata G. D., and Catapano A. L.. 2005. Molecular mechanisms responsible for the antiinflammatory and protective effect of HDL on the endothelium. Vasc. Health Risk Manag. 1: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith P. M., Howitt M. R., Panikov N., Michaud M., Gallini C. A., Bohlooly Y. M., Glickman J. N., and Garrett W. S.. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rueda C. M., Moreno-Fernandez M. E., Jackson C. M., Kallapur S. G., Jobe A. H., and Chougnet C. A.. 2015. Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur. J. Immunol. 45: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rueda C. M., Velilla P. A., Chougnet C. A., and Rugeles M. T.. 2013. Incomplete normalization of regulatory t-cell frequency in the gut mucosa of Colombian HIV-infected patients receiving long-term antiretroviral treatment. PLoS One. 8: e71062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barth H., Schnober E. K., Neumann-Haefelin C., Thumann C., Zeisel M. B., Diepolder H. M., Hu Z., Liang T. J., Blum H. E., Thimme R., et al. 2008. Scavenger receptor class B is required for hepatitis C virus uptake and cross-presentation by human dendritic cells. J. Virol. 82: 3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhainds D., Bourgeois P., Bourret G., Huard K., Falstrault L., and Brissette L.. 2004. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J. Cell Sci. 117: 3095–3105. [DOI] [PubMed] [Google Scholar]
- 31.Procaccini C., Carbone F., Di Silvestre D., Brambilla F., De Rosa V., Galgani M., Faicchia D., Marone G., Tramontano D., Corona M., et al. 2016. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity. 44: 406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier U. H., Angelin A., Akimova T., Wang L., Liu Y., Xiao H., Koike M. A., Hancock S. A., Bhatti T. R., Han R., et al. 2015. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J. 29: 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis G. I., Zhi L., Akundi R., Bueler H., and Marti F.. 2013. Mitochondrial and cytosolic roles of PINK1 shape induced regulatory T-cell development and function. Eur. J. Immunol. 43: 3355–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Windt G. J., Everts B., Chang C. H., Curtis J. D., Freitas T. C., Amiel E., Pearce E. J., and Pearce E. L.. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls D. G. 2009. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem. Soc. Trans. 37: 1385–1388. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan D., van der Windt G. J., Huang S. C., Curtis J. D., Chang C. H., Buck M. D., Qiu J., Smith A. M., Lam W. Y., DiPlato L. M., et al. 2014. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 41: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb N. R., Connell P. M., Graf G. A., Smart E. J., de Villiers W. J., de Beer F. C., and van der Westhuyzen D. R.. 1998. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J. Biol. Chem. 273: 15241–15248. [DOI] [PubMed] [Google Scholar]
- 38.Rueda C. M., Wells C. B., Gisslen T., Jobe A. H., Kallapur S. G., and Chougnet C. A.. 2015. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum. Immunol. 76: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presicce P., Orsborn K., King E., Pratt J., Fichtenbaum C. J., and Chougnet C. A.. 2011. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One. 6: e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Q., Buhler E., Steinmetz A., Schonitzer D., Bock G., Jurgens G., and Wick G.. 1992. A high-density-lipoprotein receptor appears to mediate the transfer of essential fatty acids from high-density lipoprotein to lymphocytes. Biochem. J. 287: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Q., Jurgens G., Huber L. A., Bock G., Wolf H., and Wick G.. 1992. Lipid utilization by human lymphocytes is correlated with high-density-lipoprotein binding site activity. Biochem. J. 285: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trigatti B. L., Krieger M., and Rigotti A.. 2003. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 1732–1738. [DOI] [PubMed] [Google Scholar]
- 43.Connelly M. A., and Williams D. L.. 2004. SR-BI and HDL cholesteryl ester metabolism. Endocr. Res. 30: 697–703. [DOI] [PubMed] [Google Scholar]
- 44.Connelly M. A. 2009. SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Mol. Cell. Endocrinol. 300: 83–88. [DOI] [PubMed] [Google Scholar]
- 45.Qu P., Du H., Wilkes D. S., and Yan C.. 2009. Critical roles of lysosomal acid lipase in T cell development and function. Am. J. Pathol. 174: 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krieger M. 1999. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 68: 523–558. [DOI] [PubMed] [Google Scholar]
- 47.Truong T. Q., Aubin D., Bourgeois P., Falstrault L., and Brissette L.. 2006. Opposite effect of caveolin-1 in the metabolism of high-density and low-density lipoproteins. Biochim. Biophys. Acta. 1761: 24–36. [DOI] [PubMed] [Google Scholar]
- 48.Bultel-Brienne S., Lestavel S., Pilon A., Laffont I., Tailleux A., Fruchart J. C., Siest G., and Clavey V.. 2002. Lipid free apolipoprotein E binds to the class B Type I scavenger receptor I (SR-BI) and enhances cholesteryl ester uptake from lipoproteins. J. Biol. Chem. 277: 36092–36099. [DOI] [PubMed] [Google Scholar]
- 49.Fontenot J. D., Gavin M. A., and Rudensky A. Y.. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 50.Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., and Sparwasser T.. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng H., Yang K., Cloer C., Neale G., Vogel P., and Chi H.. 2013. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 499: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutter I., Velagapudi S., Othman A., Riwanto M., Manz J., Rohrer L., Rentsch K., Hornemann T., Landmesser U., and von Eckardstein A.. 2015. Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis. 241: 539–546. [DOI] [PubMed] [Google Scholar]
- 53.de Souza J. A., Vindis C., Negre-Salvayre A., Rye K. A., Couturier M., Therond P., Chantepie S., Salvayre R., Chapman M. J., and Kontush A.. 2010. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J. Cell. Mol. Med. 14: 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerriets V. A., and Rathmell J. C.. 2012. Metabolic pathways in T cell fate and function. Trends Immunol. 33: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lochner M., Berod L., and Sparwasser T.. 2015. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36: 81–91. [DOI] [PubMed] [Google Scholar]
- 56.Gerriets V. A., Kishton R. J., Nichols A. G., Macintyre A. N., Inoue M., Ilkayeva O., Winter P. S., Liu X., Priyadharshini B., Slawinska M. E., et al. 2015. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125: 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J., Cao Y., Lei Z., Yang Z., Zhang B., and Huang B.. 2010. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 70: 4850–4858. [DOI] [PubMed] [Google Scholar]
- 58.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C. N., Bahre H., et al. 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 59.Shah A. S., Tan L., Long J. L., and Davidson W. S.. 2013. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 54: 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Souza J. A., Vindis C., Hansel B., Negre-Salvayre A., Therond P., Serrano C. V. Jr., Chantepie S., Salvayre R., Bruckert E., Chapman M. J., et al. 2008. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis. 197: 84–94. [DOI] [PubMed] [Google Scholar]
- 61.Brodeur M. R., Brissette L., Falstrault L., and Moreau R.. 2008. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: a protection against cell death. J. Cell. Biochem. 105: 1374–1385. [DOI] [PubMed] [Google Scholar]
- 62.Kingwell B. A., Chapman M. J., Kontush A., and Miller N. E.. 2014. HDL-targeted therapies: progress, failures and future. Nat. Rev. Drug Discov. 13: 445–464. [DOI] [PubMed] [Google Scholar]
- 63.Bauerfeld C. P., Rastogi R., Pirockinaite G., Lee I., Huttemann M., Monks B., Birnbaum M. J., Franchi L., Nunez G., and Samavati L.. 2012. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 188: 2847–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.