Abstract
GPR40 and GPR120 are fatty acid sensors that play important roles in glucose and energy homeostasis. GPR40 potentiates glucose-dependent insulin secretion and demonstrated in clinical studies robust glucose lowering in type 2 diabetes. GPR120 improves insulin sensitivity in rodents, albeit its mechanism of action is not fully understood. Here, we postulated that the antidiabetic efficacy of GPR40 could be enhanced by coactivating GPR120. A combination of GPR40 and GPR120 agonists in db/db mice, as well as a single molecule with dual agonist activities, achieved superior glycemic control compared with either monotherapy. Compared with a GPR40 selective agonist, the dual agonist improved insulin sensitivity in ob/ob mice measured by hyperinsulinemic-euglycemic clamp, preserved islet morphology, and increased expression of several key lipolytic genes in adipose tissue of Zucker diabetic fatty rats. Novel insights into the mechanism of action for GPR120 were obtained. Selective GPR120 activation suppressed lipolysis in primary white adipocytes, although this effect was attenuated in adipocytes from obese rats and obese rhesus, and sensitized the antilipolytic effect of insulin in rat and rhesus primary adipocytes. In conclusion, GPR120 agonism enhances insulin action in adipose tissue and yields a synergistic efficacy when combined with GPR40 agonism.
Keywords: lipolysis and fatty acid metabolism, insulin resistance, diabetes, fatty acid
Type 2 diabetes mellitus (T2DM) represents a global health threat, and it is well recognized that insulin resistance and β-cell failure are core metabolic impairments in the pathobiology of T2DM. Discovery of novel pharmacological approaches to alleviate insulin resistance has proven especially challenging. Thiazolidinediones (TZDs) are generally acknowledged as the only class of antidiabetic drugs that work directly as insulin sensitizers in the clinic. However, adverse effects such as edema, weight gain, and increased cardiovascular and bone fracture risk have limited the use of this class of drugs in the treatment of T2DM (1).
GPR40 and GPR120 have each emerged as a potential target for T2DM in recent years (2, 3). They are both fatty acid-sensing G protein-coupled receptors (GPCRs) that play important roles in glucose and energy homeostasis. GPR120 is primarily expressed in adipocytes, macrophages, and gut enteroendocrine cells (4), and its activation was reported to promote insulin sensitization in diet-induced obese (DIO) mice (5). It has been postulated that one underlying mechanism by which GPR120 improves insulin resistance is through an amelioration of chronic inflammation in adipose tissue that often arises in a context of obesity (5, 6). More recently, branched fatty acid esters of hydroxy fatty acids were found to signal through GPR120 to enhance insulin-stimulated glucose uptake in adipocytes (7). Clinical translation of GPR120 remains to be tested. Recent human genetics findings might offer some insights. A nonsynonymous mutation, p.R270H, was identified in GPR120 that partially inhibits the Gq signaling activity, and this variant was reported to increase the risk of obesity in European populations (8), although a significant association with risk for T2DM was not observed (9). More recently, the p.R270H variant was reported to affect different signaling pathways (i.e., Gq-, Gi-, or β-arrestin-mediated pathways), although it was not associated with increased risk of obesity or fasting plasma glucose levels in a Danish adult cohort that was studied (10). One of the goals of the current study was to further investigate the underlying mechanisms by which GPR120 activation modulates insulin sensitivity and glucose homeostasis.
GPR40 is preferentially and highly expressed in pancreatic β-cells (11). Activation of GPR40 potentiates glucose-dependent insulin secretion, lowering fasting plasma glucose and achieving sustained improvement in hyperglycemia in preclinical models of diabetes (11, 12). Clinical translation of GPR40 has been demonstrated as a small molecule agonist fasiglifam achieved impressive glucose lowering with minimal risk of hypoglycemia in phase 2 and 3 clinical trials in T2DM (13, 14). Further development of this compound was halted due to the occurrence of hepatic toxicity (13), but regardless of whether the finding was compound-related or tied to the GPR40 mechanism of action, GPR40 can be considered as a clinically validated target for glucose lowering in T2DM. Certain GPR40 agonists such as AM-1638 and AM-5262, in contrast to the endogenous fatty acid ligands and synthetic agonists such as fasiglifam, signal through both the Gq/IP3 and Gs/cAMP signaling pathways, rendering more robust incretin secretion and efficacy in rodents (15). Whether treatment with these “full” agonists can translate to superior clinical benefits is yet to be demonstrated.
Medium- and long-chain fatty acids (LCFAs) appear to be the endogenous ligands for both GPR40 and GPR120, although the receptors share just 15% sequence homology (16). It has been reported in the literature that it is feasible to design small-molecule dual agonist compounds, potent for both GPR40 and GPR120 (17). In the present study, we postulated that an effective strategy to enhance the antidiabetic efficacy of GPR40 would be to target both GPR40 and GPR120, activating both insulin sensitization and insulin secretion. We tested this hypothesis by comparing chronic and subchronic treatment in several diabetic rodent models with a combination of a GPR40 agonist and a GPR120 agonist, or with a single-molecule dual agonist, versus a GPR40 selective agonist. In subsequent studies, we explored the mechanism of action underlying GPR120-mediated insulin sensitization, and special attention was placed on studies that examined lipolysis in adipocytes.
MATERIALS AND METHODS
Reagents
The GPR40 selective agonists MK-2305 (15) and cpd X, the GPR120 selective agonists cpd A (5) and cpd B (18), and the GPR40 and GPR120 dual agonist cpd C (MK-1642) (19) were synthesized by Merck Research Laboratories (Rahway, NJ). The LCFA analog MEDICA16, rosiglitazone (Rosi), and pioglitazone (Pio) were synthesized by WuXi Pharma. Niacin was purchased from Sigma-Aldrich (St. Louis, MO). Insulin (Humulin R) was obtained from Eli Lilly (Indianapolis, IN).
The KRB HEPES buffer used for preparation and treatment of primary adipocytes was composed of 0.13 M NaCl, 4.7 mM KCl, 1.24 mM MgSO4, 2.47 mM CaCl2, 100 mM HEPES, 24.8 mM NaH2PO4, 246 mM NaHCO3, and 11.1 mM KH2PO4 (pH 7.4) supplemented with 2% fatty acid-free BSA fraction V (CalBiochem, Billerica, MA). All of the reagents, except for BSA, were obtained from Sigma-Aldrich.
GPR40 IP1 accumulation assay
Stable cell lines expressing mouse GPR40 (mGPR40/CHO-K1) or rat GPR40 (rGPR40/CHO-K1) were cultured in DMEM supplemented with 10% FBS, glutamine, nonessential amino acids, and penicillin/streptomycin (Life Technologies). mGPR40/CHO-K1 cell media was supplemented with 500 mg/ml G418 (Life Technologies), while rGPR40/CHO-K1 cells were grown in 10 mg/ml blasticidin plus 200 mg/ml hygromycin (Life Technologies). mGPR40 and rGPR40 IP1 potencies were determined by using the CisBio IP-one Tb HTRF kit as previously described (20).
GPR120 IP1 accumulation assay
Agonist-mediated GPR120 IP1 accumulation (Gq signaling) at mouse and rat GPR120 was determined with the CisBio IP-one Tb HTRF kit as previously described (20).
GPR120 β-arrestin assay
Agonist-mediated β-arrestin recruitment was determined by using mouse and rat GPR120 PathHunter® β-Arrestin 2 cell lines (DiscoveRx). Potencies were determined as previously described (20).
GPR120 cAMP (Gi) Assay
Potency of GPR120 Gi-mediated signaling was determined by measuring inhibition of forskolin stimulated cAMP accumulation in the same recombinant cell lines used to determine IP1 accumulation potency. Cells were harvested with nonenzymatic cell dissociation buffer and resuspended in HBSS (Life Technologies) containing 0.1% BSA (Sigma-Aldrich) and 1.2 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich) at a concentration of 0.83 × 106 per ml for mouse GPR120 stably expressed cells (1.66 × 106 for rat GPR120 cells). Test compounds dissolved in DMSO were serially diluted in 1/2 log increments starting from 2 or 0.2 mM, and 120 nl of the compound dilution was acoustically added to each well of a 384-well white OptiPlate (PerkinElmer no. 6007299). The final starting concentration in the assay was 10 or 1 μM. Forskolin (Sigma-Aldrich) was diluted in HBSS assay buffer at a concentration of 60 μM, and 12 μl was added to each well of the assay plate (final concentration in the assay was 30 μM). Twelve microliters of the cell solution was then added to each well of the assay plate, and the plates were then incubated for 30 min (45 min for rat GPR120) at room temperature. cAMP detection reagent (CisBio cAMP Kit) was prepared as per the manufacturer’s directions, and 12 μl was added to each well. The plates were then incubated one additional hour at room temperature. After the final incubation, the plates were read in a Perkin Elmer Envision reader with a method designed for HTRF assays (320 nm excitation, dual emission 615 and 655 nm). For each assay, a standard curve of cAMP titration was also included. All fluorescent readings (using the 655/615 nm ratio) were back-calculated to a concentration of cAMP by using the cAMP standard curve, and the percent activity at each concentration of test compound was determined by using 0% activation (basal activity) determined in those wells that contained DMSO and forskolin alone, whereas 100% activity was determined in wells that contained forskolin and a concentration of a standard GPR120 agonist known to maximally activate the receptor.
In vivo rodent studies
All animal procedures were carried out in a facility accredited by AAALAC International and were approved by the Merck Research Laboratories Kenilworth IACUC.
Intervention treatment in db/db mice
Male 6-week-old db/db mice (Jackson Laboratories) were housed five mice per cage and fed with rodent chow diet (Research Diet D5053) and water ad libitum. At 12 weeks old, the db/db mice were randomized based on nonfasting blood glucose, plasma insulin, and body weight into five treatment groups (n = 8 per group), and the following compounds were dosed orally every morning around 9 AM for 10 days: 1) vehicle: 0.5% methylcellulose (Sigma-Aldrich), 2) GPR40 agonist MK-2305 at 10 mg/kg, 3) GPR120 agonist cpd A at 30 mg/kg, 4) coadministration of MK-2305 (10 mg/kg) and cpd A (30 mg/kg), and 5) Rosi at 3 mg/kg. The dose selection for each compound was determined in pilot studies, and the maximally efficacious doses without observable adverse effects were chosen. Morning nonfasting blood glucose levels were measured daily, and body weight was monitored on study days 4, 7, and 11. On study day 11, mice were fasted at the beginning of light phase for 5.5 h before administration of compounds. One hour after dosing, the mice received intraperitoneal injection of 3 U/kg insulin, and blood glucose levels were measured by tail sampling using a hand-held glucometer (Lifescan, Milpitas, CA) immediately before and at 30, 60, 90, and 120 min after insulin injection.
Prevention treatment in db/db mice
Six-week-old male db/db mice (Jackson Laboratories) were randomized and grouped according to nonfasting blood glucose and body weight, and a group of age-matched db/+ lean male mice without treatment was included as a comparator. The compounds were administered in feed for 5 weeks, and the treatment groups (n = 8 per group) included 1) vehicle (regular diet D5053), 2) Rosi at 3 mg/kg/day, 3) GPR40 agonist MK-2305 at 10 mg/kg/day, 4) GPR120 agonist cpd A at 30 mg/kg/day, 5) combination of MK-2305 (10 mg/kg) and cpd A (30 mg/kg), and 6) GPR40/120 dual agonist cpd C at 30 mg/kg/day. Body weight, food intake, and blood glucose were measured weekly. Six-hour fasting blood glucose and insulin were measured on study day 35.
Oral glucose tolerance test (oGTT) was performed on study day 32 with 6 h fasting in the morning starting from the beginning of light phase. At the time point of t = 0 min, 1 g of dextrose per kg of body weight was administered by oral gavage. Blood glucose levels were determined from tail vein blood sampling by using a hand-held glucometer (Lifescan) immediately before and at 20, 40, 60, 90, and 120 min after glucose challenge. Fasting plasma insulin levels were measured by using the Rat Insulin ELISA kit (Mercodia, Sweden). Plasma triglycerides (TGs) and NEFAs were measured by using the Triglyceride Assay kit (Roche, Indianapolis, IN) and the FFA kit (Roche).
Treatment in ob/ob mice and hyperinsulinemic-euglycemic clamp
Five-week-old ob/ob male mice on the C57Bl/6J background (Jackson Laboratories) were acclimatized for 1 week, randomized into four groups, and fed with diet alone (Research Diet 5053), or the same diet containing a GPR40 agonist (cpd X, 100 mg/kg), a GPR40/120 dual agonist (cpd C, 30 mg/kg), or Pio (30 mg/kg) for 4 weeks. Body weight, blood glucose, and plasma insulin levels were measured at 21 days after treatment. After 4 weeks of treatment, the mice were surgically implanted with jugular vein and carotid artery catheters and allowed to recover for 1 week. On day 8 after surgery, mice with greater than 10% body weight loss were excluded from the following clamp study.
Insulin sensitivity was determined by a hyperinsulinemic-euglycemic clamp procedure as previously described (21). Briefly, on the day of the procedure, mice were fasted for ∼5 h and at t = −90 min, mice were administered a primed continuous infusion of [U-13C6]glucose (∼50 µmol/ml at 2.5 µl/min), and blood samples were collected at t = −15 and −5 min to measure basal glucose turnover. At t = 0 min, the clamp procedure was initiated by continuous infusion of insulin (25 mU/kg/min). Blood glucose levels were measured at 10 min intervals and maintained at euglycemia (∼120 mg/dl) by variable infusion rates of dextrose (30%) mixed with [U-13C6]glucose (∼50 µmol/ml). Blood samples were collected during the steady state (t = 90–120 min) at every 10 min to determine endogenous glucose appearance rate (Ra) and glucose disposal rate (Rd). At t = 120 min, a bolus of 2-deoxy glucose (2-DG, 50 mg/kg) was administered to assess tissue-specific glucose uptake. For 30 min, blood glucose levels were maintained at euglycemia, and at t = 150 min mice were euthanized, and skeletal muscle tissues were collected and frozen until further analysis. The insulin sensitivity index (M/I ratio) was calculated by dividing M [the glucose infusion rate (GIR) during the steady state] by the mean insulin concentration during the same period of the clamp.
Isotopomer analysis of plasma glucose
Plasma samples were derivatized by using hydroxylamine and acetylated with acetic anhydride to yield aldonitirile-penta acetate derivatives of glucose. Samples were analyzed in Agilent 6890N GC coupled with MS (5973), an HP 7673 autosampler, with a 6890N GC (HP 5 column, 175°C initial temperature, and hold for 0 min and ramp to 300°C at 35°C/min, hold for 1 min). Helium flowing was used as the carrier gas. The 1 μl injection (15:1 split) was used to maximize the amount of analyte on the column. Data were acquired by using selected ion monitoring under electron impact ionization (m/z 314–319, 10 ms dwell per ion). Relative quantities of glucose types were determined by comparing single-ion response ratios between each analyte with MassHunter software (EnviroQuant module; Agilent) (22).
Tissue glucose uptake
Skeletal muscle samples were weighed and homogenized in a 0.1% formic acid in acetonitrile: water (75:25 v/v) containing [U-13C6]2-deoxyglucose-6-phosphate as an internal standard to obtain 100 mg of tissue per 1 ml of homogenization solution. The resulting homogenate was centrifuged, and analytes were extracted from supernatant by using solid phase extraction. The extracts were analyzed by Waters UPLC coupled with Waters Xevo TQ mass spectrometer, 2-deoxy-glucose 6-phosphate, and [U-13C6]2-deoxyglucose-6-phosphate were monitored in a multiple reaction monitoring mode.
Chronic prevention treatment in female Zucker diabetic fatty rats
Female Zucker diabetic fatty [fZDF (fa/fa)] rats and age- and gender-matched genetic control Zucker lean (ZL) rats were purchased at 6 weeks of age from the Charles River Laboratories (Wilmington, MA). The fZDF rats were fed a high-fat, high-carbohydrate diet (Research Diets D10111701, 48% kcal from fat and 34% kcal from carbohydrate, Research Diets, New Brunswick, NJ) for 3–4 weeks to induce hyperglycemia (23). The ZL rats were fed a chow diet (Research Diets D7012). Compound treatment in fZDF rats started at 7 weeks of age, at the same time diet was switched to D10111701. There were three treatment groups with n = 8 per group: 1) vehicle (0.5% methylcellulose), 2) GPR40 agonist cpd X at 30 mg per kg of body weight (mpk), and 3) GPR40/120 dual agonist cpd C at 30 mpk. Compounds were administered orally once a day, and the treatment lasted for 28 days. An oGTT was performed on study day 29. Animals were fasted for 16 h, followed by oral dosing of 3 g per kg of body weight of dextrose. Blood glucose levels were determined from tail vein blood sampling by using a hand-held glucometer (Lifescan) immediately before and at 20, 40, 60, 90, and 120 min after glucose administration. Fasting and oGTT plasma insulin levels were measured by using the Rat Insulin ELISA kit (Mercodia).
Immunohistochemistry
Pancreatic tissue was evaluated immunohistochemically. After animals were euthanized, pancreas was excised and immediately fixed in 10% neutral buffered formalin for 48 h, embedded en bloc in paraffin. After deparaffinization and rehydration, three levels of tissue sections (4 μm each section, and 150 μm apart) were processed to identify cells containing insulin and glucagon by using immunohistochemistry double-staining procedures. The following primary antibodies were used in this study: guinea pig anti-insulin antibody (Dako, Inc., Carpinteria, CA) diluted 1:900 and rabbit anti-glucagon antibody (Vector Laboratories, Burlingame, CA) at 1:500 dilution. Bound antibodies were detected by immunoperoxidase microscopy using secondary anti-IgG antibodies of appropriate species and the DAKO Envision method. Peroxidase reaction products were developed with a glucose oxidase–diaminobenzidine method (UltraVision Detection System, Thermo Sciences, Pittsburgh, PA) for detecting glucagon-positive stain and with the ImmPACT SG Substrate method (Vector Laboratories) for detecting insulin-positive stain. Sections were counterstained with fast red. Whole slide image analysis was performed by using the Aperio system (Leica, IL). Briefly, whole digital images were captured by using a 20× objective and the ScanScope XT slide scanner system and managed by using Spectrum. The Genie (Genetic Imagery Exploration) algorithm was utilized to automatically identify islets in pancreas sections. Color deconvolution was used to separate the stain contributions, followed by area quantification to determine the relative percentage of α and β cells present in each islet. Cells containing insulin were considered to be β-cells, and cells with glucagon were regarded as α-cells.
Acute treatment with a GPR120 agonist in Wistar Hans rats
Wistar Hans (WH) rats were purchased at 6–7 weeks of age from the Charles River Laboratories. The rats were grouped based on body weight and were fasted overnight. Plasma samples were collected from tail vein blood before and at 0.5, 2, and 4 h after oral dosing of either vehicle (0.5% methyl cellulose) or a GPR120 agonist cpd B at 30 mg/kg. Plasma FFA, glycerol, and insulin levels were measured with the FFAs Half-Micro Test Kit from Roche, the Glycerol Reagent and Standard from Sigma-Aldrich, and the Rat Insulin ELISA kit (Mercodia), respectively.
Taqman quantitative PCR analysis of selective mRNA from adipose tissues
Fluorogenic Taqman probes and primer sets for selected genes were purchased from Applied Biosystems (Foster City, CA). Relative mRNA expression levels were determined by real-time quantitative PCR using the ABI PRISM 7900 Sequence Detection System from Applied Biosystems. The β-actin gene was used as the normalization control to determine the relative abundance of each mRNA species in different samples.
RNA-sequencing profiling of adipose tissue
Gene expression analysis for the adipose tissue obtained from the fZDF rat study was performed by RNA sequencing (Labcorp, Seattle, WA) as described previously (24). Briefly, sequencing was performed by using the Truseq stranded total RNA RiboZero library preparation kit (catalog no. RS-122-2201) according to the manufacturer’s instructions (Illumina, San Diego, CA). The resulting cDNA libraries were sequenced on an Illumina (HiSeqTM 4000) by using a 50 base paired-end run. Alignment and differential gene expression analysis was performed in Omicsoft Array Studio version 9.0.3.90. Briefly, cleaned reads were aligned to the rat B6.0 genome references by using the Omicsoft Aligner, with a maximum of two allowed mismatches. Gene level counts were determined by the OSA algorithm as implemented in Omicsoft Array Studio and using Ensembl.R82 gene models. At least 90% of reads across all samples mapped to the rat genome (corresponding to 40–70 million reads). Differential gene expression analysis was performed by the DESeq2 algorithm as implemented in Omicsoft Array Studio with the samples from the vehicle-treated animals serving as reference.
Lipolysis in primary adipocytes
Primary adipocytes were isolated from white adipose tissue (WAT) as described (25). Briefly, the epididymal fat pads (∼0.5 g) from a 6–8 week old mouse or rat were harvested and rinsed with PBS (pH 7.4) at room temperature. The fat pads were blotted dry, weighed, and minced thoroughly (to ∼2–3 mm pieces in diameter) in a Petri dish. The minced fat pieces were transferred into a 50 ml tube with KRB HEPES buffer with 4% fatty acid-free BSA fortified with 1 mg/ml type I collagenase (Worthington Biomedical Corp., Lakewood, NJ) solution (3 ml/g of adipose tissue) and incubated at 37°C with constant shaking at 220 rpm for 45–60 min. The mixture was filtered through a 250 μm gauze mesh into a 50 ml conical polypropylene tube and allowed to stand for 2–3 min. The infranatant containing the collagenase solution was carefully removed by using a long needle and syringe. The floating layer of adipocytes was washed three times with 10 ml of washing buffer (KRB HEPES buffer with 2% fatty acid-free BSA). The adipocytes were resuspended into 10 ml of Assay Buffer (KRB HEPES buffer with 5 mM glucose and 1 U/ml adenosine deaminase). After brief agitation of the suspension to obtain a homogeneous mixture of adipocytes, 50 μl was added to each well of a 96-well plate. Compound treatment as described in Results and figure legends was performed for 1 h at 37°C. After incubation, 10 μl of infranatant was pipetted into a 96-well plate to measure the glycerol concentration by using Glycerol Reagent and Standard from Sigma-Aldrich or FFA concentration by using the FFAs Half-Micro Test Kit from Roche.
Rhesus WAT biopsy and primary adipocyte preparation
Adipose biopsy samples were obtained from a cohort of rhesus monkeys (Macaca mulatta) that were housed at either Merck & Co., Inc. (West Point, PA) or the Crown Bioscience (Kannapolis, NC). The monkeys were maintained on a low-fat, high complex carbohydrate diet (Labdiet 5037, Jumbo monkey diet) provided ad lib. Overnight fasted animals were sedated with ketamine [10 mg/kg, intramuscularly (IM)] and supplemented with diazepam (0.5–1 mg/kg, IM) or dexmedetomidine (Domitor, 7.5–15 μg/kg, IM) if necessary. Buprenorphine was provided presurgically. A small incision (1–2 cm) was made in the region of the lower ventral abdomen (lateral to the umbilicus on right and left side), through which a small amount of subcutaneous fat (∼4 mg) was excised via an excisional method. The excisional adipose tissue samples were stored and shipped overnight in PBS buffer at room temperature. The primary adipocytes were prepared within 24 h following a similar protocol as the mouse/rat adipocyte preparation protocol except that 1.5 mg/ml type I collagenase was used in the digestion buffer. Human adipose tissue was obtained from Zenbio (Research Triangle Park, NC). The donor was a 46-year-old Caucasian female with BMI of 27.4.
Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was conducted by using either one-way ANOVA followed by Dunnett’s post hoc test in Prism (Version 4.0.3, GraphPad, La Jolla, CA) or Student’s t-test, as appropriate. Statistical significance was defined as two-tailed P < 0.05.
RESULTS
Combination treatment with GPR40 and GPR120 agonists in diabetic db/db mice
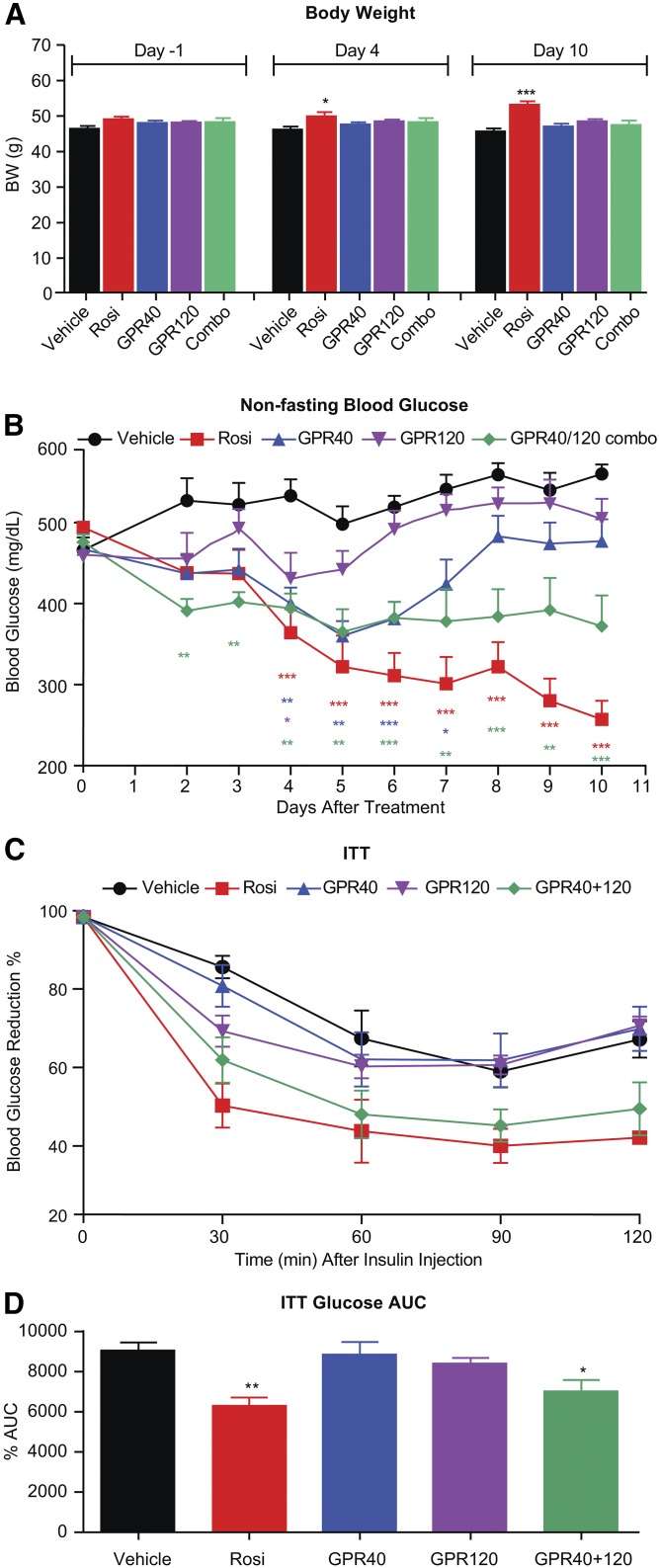
To compare the glucose-lowering efficacy of a combination of GPR40 and GPR120 against either monotherapy, 12-week-old db/db male mice were treated for 10 days with a GPR40 selective agonist MK-2305 (15), a GPR120 selective agonist cpd A (5), or the combination. The structures of these tool compounds were previously reported (5, 15), and their potency, selectivity, and pharmacokinetic profiles are summarized in Tables 1–3; the dose that was selected for each compound was determined in pilot studies as the maximal efficacious dose. Rosi treatment was included in the study as a comparator group. Cpd A was chosen for chronic studies in mice because it has a relatively long half-life in circulation (Table 2), which is suitable for once daily dosing, Before treatment, the average nonfasting blood glucose was approximately 480 mg/dl (Fig. 1B). Consistent with prior literature, Rosi treatment increased body weight, but none of the other treatments had a significant effect on body weight compared with vehicle (Fig. 1A). Treatment with the GPR40 agonist MK-2305 (alone) resulted in a significant reduction of blood glucose, as evident on study days 4–7, although the effect seemed to attenuate from treatment days 7–10, whereas treatment with the GPR120 agonist cpd A (alone) showed an initial trend for glucose lowering on study days 4–5, but this efficacy was not sustained (Fig. 1B). In contrast to either monotherapy, the coadministration of GPR40 and GPR120 agonists yielded a persistent lowering of blood glucose (Fig. 1B). An insulin tolerance test, conducted after 10 days of treatment, was significantly improved by the combination therapy and by Rosi, but not by either GPR40 agonist or GPR120 agonist given as respective monotherapies (Fig. 1C, D).
TABLE 1.
In vitro potencies of compounds
| Mouse/Rat | ||||
| Compound | GPR40 IP1 EC50 | GPR120 IP1 EC50 | GPR120 β-arrestin EC50 | GPR120 Gi cAMP EC50 |
| nM | ||||
| GPR40 Selective | ||||
| MK-2305 | 2.6/5.3 | >10,000/>10,000 | >10,000/>10,000 | >10,000/ND |
| Cpd X | 6.4/10 | >10,000/>10,000 | >10,000/>10,000 | >10,000/ND |
| GPR120 Selective | ||||
| Cpd A | >10,000/>10,000 | 33/106 | 54/42 | 630/380 |
| Cpd B | >10,000/>10,000 | 2.2/5.8 | 2.9/ND | 21/18 |
| GPR40/GPR120 dual | ||||
| Cpd C | 4.6/11 | 13/36 | 46/24 | 310/220 |
Values are the geometric mean of N ≥ 2. ND, not determined.
TABLE 3.
Pharmacokinetic profiles of compounds in rat
| Property | Cpd A | Cpd B | Cpd C | MK-2305 | Cpd X |
| AUC iv (μM·h) | 17.5 | 6.05 | 95.9 | 52.66 | 39.7 |
| Clp (ml/min/kg) | 2.3 | 7.25 | 0.43 | 0.72 | 0.77 |
| Vdss (l/kg) | 0.4 | 0.618 | 0.16 | 0.58 | 0.15 |
| t1/2 (h) | 1.4 | 1.36 | 4.62 | 9.5 | 4.47 |
| MRT (h) | 3 | 1.45 | 6.31 | 13.4 | 3.27 |
| Foral (%) | 95 | 77.2 | 100 | 100 | 75.8 |
| PPB (% bound) | 99.87 | 99.86 | 99.5 | 99.98 | 99.69 |
| Unbound Cl (Clp/funbe) | 1,769 | 5,179 | 86 | 3,600 | 248 |
iv, intravenous; MRT, mean residence time; PPB, plasma protein binding.
TABLE 2.
Pharmacokinetic profiles of compounds in mouse
| Property | Cpd A | Cpd B | Cpd C | MK-2305 | Cpd X |
| AUC iv (μM·h) | 14.6 | 0.182 | 8.99 | 17.1 | 1.76 |
| Clp (ml/min/kg) | 8.4 | 222 | 4.74 | 2.33 | 18 |
| Vdss (l/kg) | 8.9 | 5.08 | 1.71 | 1.38 | 3.94 |
| t1/2 (h) | 18.1 | 0.2 | 5.42 | 7.16 | 4.96 |
| MRT (h) | 26.5 | 0.4 | 6.15 | 10.5 | 3.88 |
| Foral (%) | 100 | 79 | 100 | 86 | 82 |
| PPB (% bound) | 99.79 | 99.65 | 99.5 | 99.97 | 99.91 |
| Unbound Cl (Clp/funbe) | 4,000 | 62,900 | 948 | 7,767 | 20,000 |
iv, intravenous; MRT, mean residence time; PPB, plasma protein binding.
Fig. 1.
Combination treatment with GPR40 and GPR120 agonists in diabetic db/db mice provided better glycemic control than either monotherapy. A: Body weight changes. B: Nonfasting blood glucose changes. C: Insulin tolerance test (ITT) glucose. D: Glucose AUC (0–120 min) during ITT. Data are mean ± SEM. Combo, MK-2305 at 10 mpk and Cpd A at 30 mpk were codosed (n = 8); GPR40, GPR40 selective agonist MK-2305 dosed at 10 mpk (n = 8); GPR120, GPR120 selective agonist Cpd A dosed at 30 mpk (n = 8); Rosi, Rosi dosed at 3 mpk (n = 8); vehicle, 0.5% methylcellulose (n = 8). One-way ANOVA test. * P < 0.05, ** P < 0.01, *** P < 0.001.
Chronic prevention treatment with combination therapy
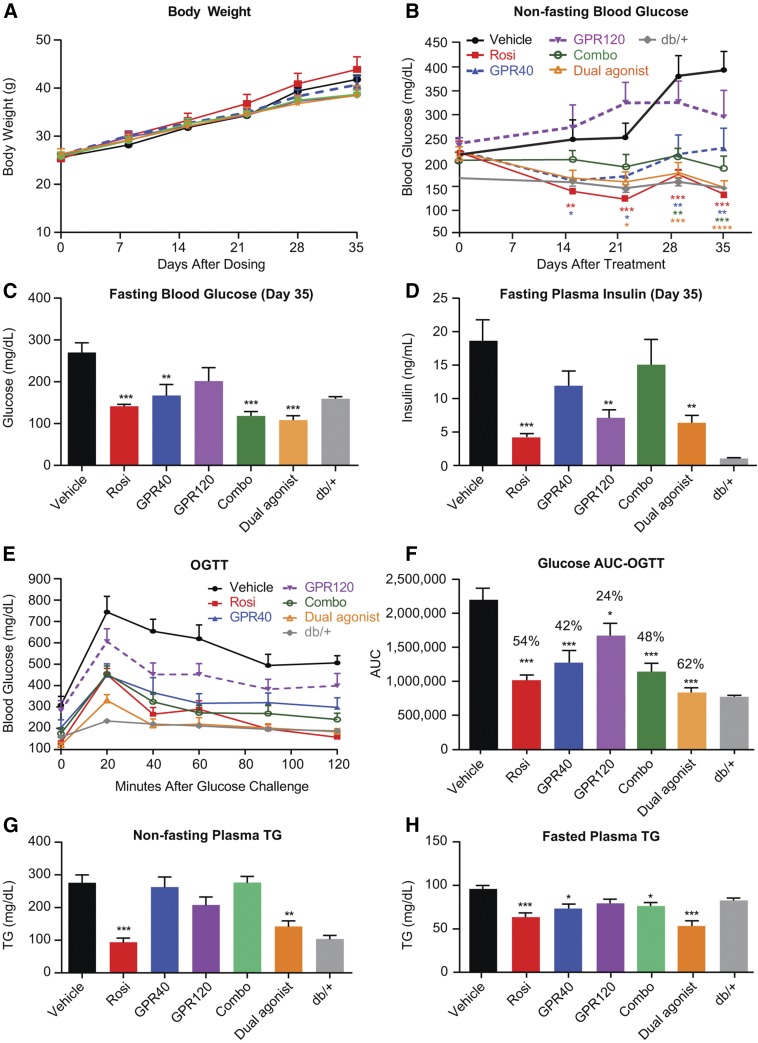
We next assessed the metabolic benefits of chronic combination therapy of GPR40 and GPR120 in a preventive paradigm in young db/db mice, and as compared with respective monotherapies and Rosi, as described above. Treatments were started in 6-week-old db/db mice, which exhibit mild hyperglycemia at this age but progressively and rapidly develop severe hyperglycemia over the subsequent 5 weeks. None of the treatments had a significant effect on body weight compared with the vehicle group (Fig. 2A). During the first 3 weeks of treatment, the GPR40 selective agonist MK-2305 (alone) showed a robust effect to attenuate development of hyperglycemia; however, the effect was not sustained during the 4th and 5th weeks of treatment, whereas the GPR120 selective agonist cpd A (alone) did not achieve a significant effect to prevent the emergence of hyperglycemia (Fig. 2B). The combination of GPR40 and GPR120 agonists did, however, markedly attenuate development of hyperglycemia throughout the 5 week treatment period (Fig. 2B). Corroborating this observation, a single molecule with potent and balanced dual agonist activity (cpd C) also prevented the emergence of hyperglycemia, with an efficacy similar to that of Rosi and much more effective than the GPR40 agonist or the GPR120 agonist given as monotherapies (Fig. 2B). On study day 35, fasting blood glucose levels were significantly lower than in the vehicle group for all treatments except GPR120 agonist (Fig. 2C). Fasting insulin levels were significantly lowered in the GPR120 and dual agonist groups, suggesting that GPR120 activation improved insulin sensitivity (Fig. 2D). Oral glucose tolerance measured after 28 days of treatment was significantly improved by all treatments, with the dual agonist being the most efficacious (Fig. 2E, F). Plasma nonfasting TG levels were markedly increased in the db/db mice compared with age- and gender-matched db/+ mice (Fig. 2G). The dual agonist treatment exerted a significant effect to reduce plasma TG in both the fasted and fed states (Fig. 2G, H). In a paradigm of preventing diabetes progression, these results indicate that a single-molecule dual agonist for GPR40 and GPR120 can achieve efficacy at least equal to a combination of selective agents in improving glycemic control and superior to either agent given as monotherapy. Furthermore, the dual agonist had a robust effect to lower plasma TG.
Fig. 2.
Five-week chronic treatment in db/db mice with combination therapy and a single-molecule dual agonist of GPR40 and GPR120 resulted in metabolic benefits better than monotherapy. A: Body weight changes. B: Nonfasting blood glucose changes. C: Fasting blood glucose levels on study day 35. D: Fasting plasma insulin levels on study day 35. E: oGTT performed on study day 28. F: Glucose AUC (0–120 min) during oGTT. G: Nonfasting plasma TG on study day 35. H: Fasting plasma TG on study day 35. Data are mean ± SEM. Combo, MK-2305 at 10 mpk and Cpd A at 30 mpk were codosed (n = 8); Dual, GPR40/GPR120 dual agonist Cpd C dosed at 30 mpk (n = 8). db/+: age-matched genetic control (n = 8); GPR40, GPR40 selective agonist MK-2305 dosed at 10 mpk (n = 8); GPR120, GPR120 selective agonist Cpd A dosed at 30 mpk (n = 8); Rosi, Rosi dosed at 3 mpk (n = 8); vehicle, regular diet D5053 (n = 8). . One-way ANOVA test was applied to db/db mice with treatment (the db/+ group was excluded from statistical analysis). * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Chronic treatment with a GPR40/GPR120 dual agonist improves insulin sensitivity
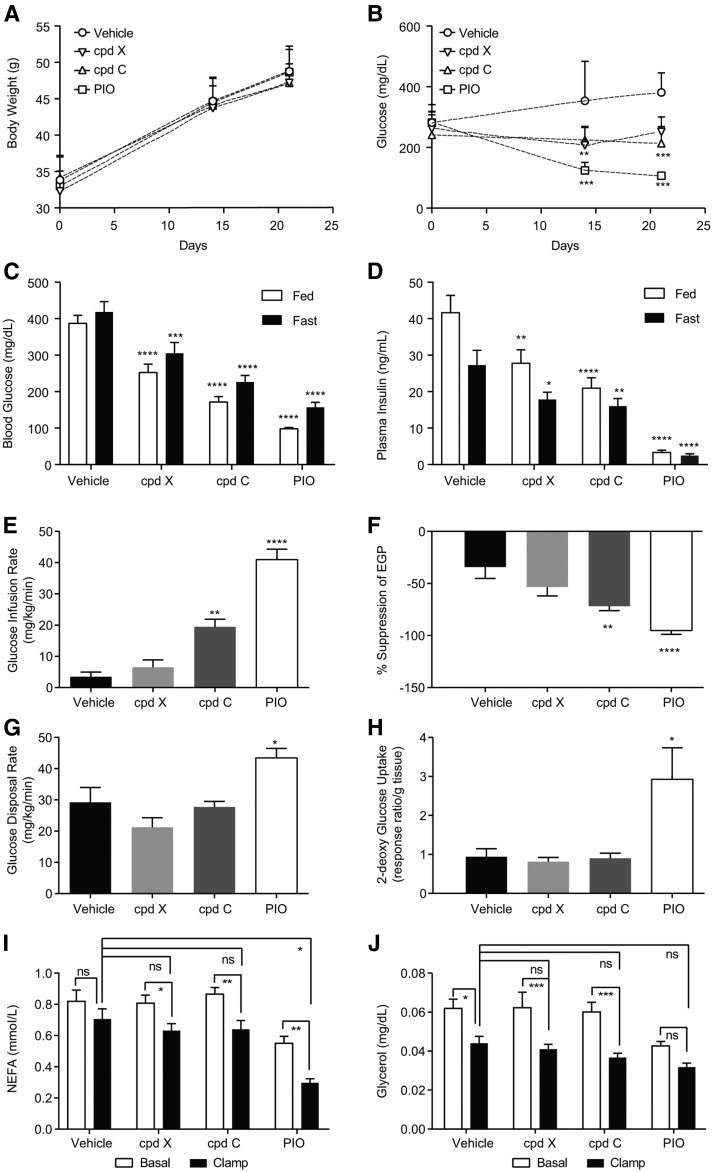
Previously, the GPR120 selective agonist cpd A was reported to improve insulin sensitivity in DIO mice, a model of insulin resistance with mild hyperglycemia (5). To address whether simultaneous activation of GPR120 and GPR40 by a dual agonist can improve insulin sensitivity in a diabetic animal model that exhibits hyperglycemia and hyperinsulinemia, we compared chronic treatment with a dual agonist (cpd C) against a GPR40 selective agonist (cpd X) in ob/ob mice. Cpd X and cpd C have similar pharmacokinetic profiles in mouse, in particular, similar half-lives (Table 2). Pio was included as a positive control for insulin sensitization.
Chronic treatment with cpd X, cpd C, or Pio had no significant effect on body weight when compared with vehicle-treated mice during the 5 week treatment period (Fig. 3A). Hyperglycemia was prevented in all treated groups compared with vehicle (Fig. 3B). Blood glucose measured on day 21 of treatment with cpd X and cpd C was significantly decreased in both the fed state (by 1.5- and 2.2-fold, respectively) and the fasted state (by 1.3- and 1.8-fold, respectively) when compared with vehicle-treated mice (Fig. 3C). Further, both cpd X and cpd C significantly decreased hyperinsulinemia in both a fed (by 1.5- and 2-fold, respectively) and fasted (by 1.5-fold for both compounds) state as compared with vehicle-treated mice (Fig. 3D).
Fig. 3.
Treatment with GPR40/GPR120 dual agonist, but not GPR40 selective agonist alone, significantly improved insulin sensitivity during hyperinsulinemic-euglycemic clamps in ob/ob mice. A: Body weight changes. B: Nonfasting blood glucose changes. C: Fasted and fed blood glucose levels on study day 21. D: Fasted and fed plasma insulin levels on study day 21. E: Glucose infusion rate. F: Suppression of EGP. G: Glucose disposal rate during hyperinsulinemic-euglycemic clamps on study day 28. H: 2-DG uptake in skeletal muscle at the end of clamps. Plasma NEFA (I) and plasma glycerol (J) during hyperinsulinemic-euglycemic clamps. Cpd C, GPR40 and GPR120 dual agonist dosed at 30 mpk (n = 8); Cpd X, GPR40 selective agonist dosed at 100 mpk (n = 8); PIO, Pio dosed at 30 mpk (n = 8); vehicle, regular diet D5053. One-way ANOVA test. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. ns, not significant.
Insulin sensitivity was assessed by hyperinsulinemic-euglycemic clamp after 28 days of treatment. Blood glucose levels during clamps are shown in supplemental Fig. S1A. Consistent with prior literature, Pio treatment markedly increased the GIR required during the clamp, by ∼40-fold compared with vehicle-treated mice, and improved suppression of endogenous glucose production (EGP; ∼2.8-fold), and the glucose disposal rate (GDR; ∼1.5-fold), as shown in Fig. 3E–G. GIR was also significantly increased by the dual agonist (∼5.5-fold), but not by the GPR40 selective agonist treatment, suggesting that the improvement in insulin sensitivity of the dual agonist is mediated by GPR120 (Fig. 3E). The insulin sensitivity index (M/I ratio) was calculated and shown in supplemental Fig. S1B. There was a marked enhancement in the ability of insulin to suppress EGP (∼2.2-fold), but not to stimulate GDR, by the dual agonist cpd C (Fig. 3F, G), suggesting that increased insulin sensitivity in the liver by the dual agonist constitutes the key component of improved whole body insulin sensitivity. To further assess the pharmacological effects on peripheral tissue insulin sensitivity, 2-DG uptake was measured in skeletal muscle at the end of the clamp. Pio treatment significantly increased glucose uptake in skeletal muscle (∼3-fold) when compared with vehicle-treated mice (Fig. 3H). However, neither the GPR40 selective agonist nor the dual agonist affected glucose uptake in skeletal muscle (Fig. 3H).
Insulin-mediated suppression of lipolysis is a key metabolic pathway indicative of insulin sensitivity in adipose tissue. To examine potential treatment effects on adipose tissue, we assessed circulating NEFAs and glycerol levels under basal (fasting) conditions and during hyperinsulinemic-euglycemic clamps. Under basal conditions, treatment with Pio resulted in markedly decreased plasma NEFA and glycerol. However, neither the GPR40 selective agonist nor the GPR40/120 dual agonist had a similar effect on fasting levels of NEFA and glycerol (Fig. 3I, J). Under conditions of the euglycemic hyperinsulinemic clamp, glycerol was suppressed by 25 ± 9% (P < 0.05) and NEFA by 12 ± 8% (nonsignificant) in vehicle-treated mice, reflecting severe adipose tissue insulin resistance in ob/ob mice (Fig. 3I, J). Pio significantly decreased NEFA by 41% during clamp, consistent with its effect to improve insulin action (Fig. 3I). Chronic treatment with both cpd X and cpd C decreased NEFA during clamp conditions by 19% and 26%, respectively, although neither was statistically significant compared with vehicle (Fig. 3I). None of the treatments yielded a significant change relative to vehicle in suppression of plasma glycerol (Fig. 3J). Taking together the results of glucose flux determination, assessments of 2-DG uptake into muscle, and measurements of plasma NEFA and glycerol during clamps, it appears that the significant improvement of insulin sensitivity by a GPR40/120 dual agonist is largely mediated by improved insulin action in the liver.
GPR40/120 dual agonist chronic treatment preserved islet function and morphology in diabetic fZDF rats
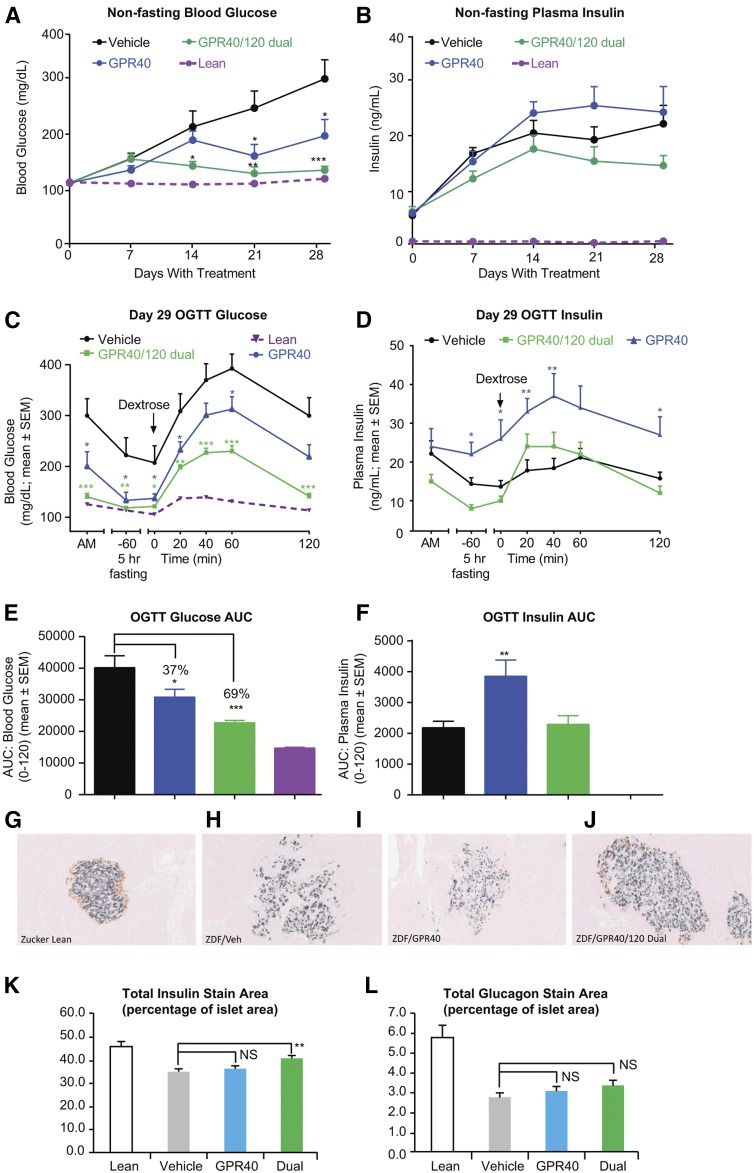
To determine whether the GPR40/120 dual agonist treatment would have metabolic benefit in a second species, we compared chronic treatment of the GPR40/120 dual agonist cpd C with the GPR40 selective agonist cpd X in diabetic fZDF rats, a model of “diabesity” with both genetic and dietary components. The fZDF rats were placed on a high-fat and high-carbohydrate diet to induce hyperglycemia and hyperinsulinemia (23). Compounds were administered via feed, and treatment was initiated at the start of the diet challenge. The two compounds, cpd X and cpd C, have comparable in vitro rat GPR40 IP1 potencies and PK profiles (Tables 1 and 3). Three treatment groups were included in this study: the GPR40 selective agonist cpd X (30 mg/kg/day), the GPR40/120 dual agonist cpd C (30 mg/kg/day), and a vehicle control group (no compound in the diet). Four-week treatment with cpd X or cpd C had no significant effect on body weight compared with the vehicle-treated group (data not shown). Treatment with either compound led to marked attenuation in the development of hyperglycemia under the dietary challenge; the dual agonist cpd C achieved a nearly complete prevention of hyperglycemia, and this was accompanied by a trend for decreased plasma insulin (Fig. 4A, B). During an oGTT conducted after 28 days of treatment, the GPR40 selective agonist and the dual agonist lowered fasting glucose by approximately the same amount (Fig. 4C). Despite the difference in fasting glucose, after glucose challenge, the overall shape of glucose excursion was similar for the GPR40 agonist and the vehicle groups (Fig. 4C). In contrast, the shape of the glucose excursion was flattened by the dual agonist treatment (Fig. 4C), resulting in a lower glucose area under the curve (AUC) than with the selective GPR40 agonist (Fig. 4E). Both fasting and postchallenge plasma insulin were significantly elevated under GPR40 agonist treatment, consistent with GPR40’s effect as an insulin secretagogue (Fig. 4D, F). Fasting insulin was similar between the dual agonist and vehicle-treated groups, but, notably, insulin release in response to glucose challenge increased more rapidly and returned to basal levels more quickly in the dual agonist treatment than the vehicle group (Fig. 4C, F). These observations suggest an improved glucose responsiveness of β-cell function in the dual agonist-treated rats. Immunohistochemistry of pancreatic sections taken at the end of the study revealed severe degranulation and a markedly reduced insulin content in islets of the vehicle-treated fZDF rats compared with healthy (lean) Zucker control rats (Fig. 4G, H). The morphology of islets taken from GPR40/120 dual agonist-treated rats (Fig. 4J), but not islets from selective GPR40 agonist-treated rats (Fig. 4I), exhibited a better preservation of islet structure and a significantly higher percentage of insulin-positive β-cells (Fig. 4K). The percentage of glucagon-positive α-cells was not significantly changed by either treatment (Fig. 4L).
Fig. 4.
GPR40/120 dual agonist chronic treatment in diabetic fZDF rats. A: Nonfasting blood glucose changes. B: Nonfasting plasma insulin changes. C: Glucose excursion during an oGTT on study day 29. D: Plasma insulin profiles during an oGTT on study day 29. E: oGTT glucose AUC (0–120 min). F: oGTT insulin AUC (0–120 min). Immunohistochemistry with anti-insulin and anti-glucagon antibodies in pancreatic sections of ZL rat (G), vehicle-treated fZDF rat (H), GPR40 agonist-treated fZDF rat (I), and GPR40/GPR120 dual agonist treated fZDF rat (J). Quantification of total insulin staining area (K) and glucagon staining area (L) of pancreatic immunohistochemistry sections. Cpd C, a GPR40 and GPR120 dual agonist dosed at 30 mpk (n = 8); Cpd X, a GPR40 selective agonist dosed at 30 mpk (n = 8); vehicle, 0.5% methylcellulose (n = 8). One-way ANOVA test. * P < 0.05, ** P < 0.01, *** P < 0.001. NS, not significant.
Effect of GPR40/120 dual agonist on lipolysis-related adipose gene expression
Our results in the ob/ob mouse study indicated that GPR40/120 dual agonism leads to an improvement of hepatic insulin sensitivity. We postulated that this effect can be attributed to GPR120 agonism because GPR40 agonism alone did not yield a similar metabolic benefit (Fig. 3). However, GPR120 is known to have quite limited expression in the liver, whereas it has abundant expression in adipose tissue (6, 26, 27). Because it is well recognized that insulin regulates FFA release from adipose tissue, and circulating FFAs can play an important role in governing hepatic glucose production (28), we focused in subsequent studies on understanding GPR120 expression and its function in adipose tissue.
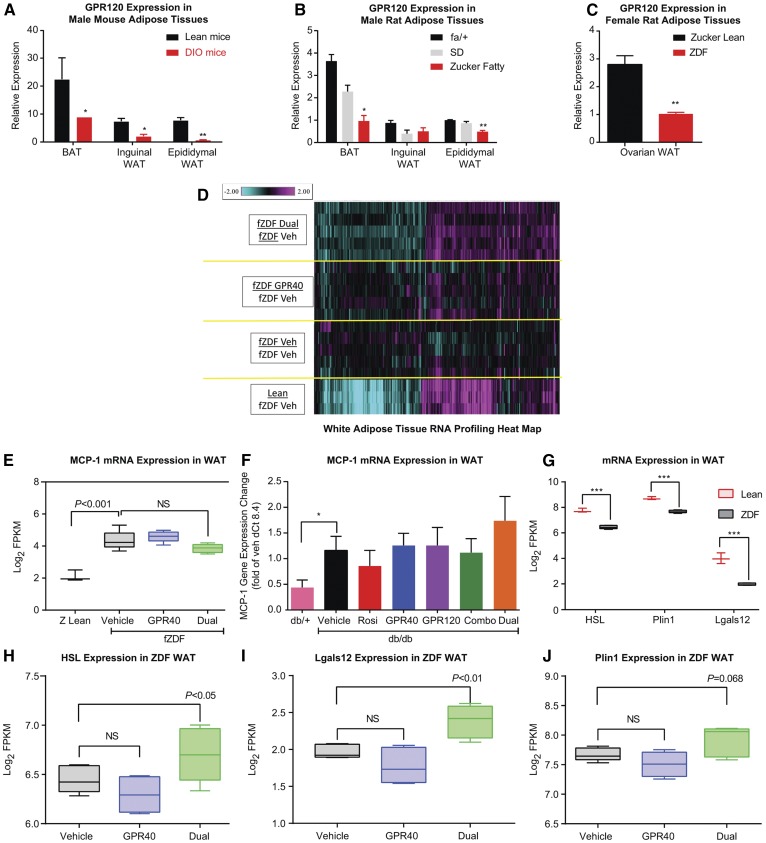
We examined GPR120 gene expression in adipose tissues from several insulin-resistant and diabetic rodent models. GPR120 mRNA levels were abundant in all three fat depots examined, including gonadal white adipose, inguinal white adipose, and interscapular brown adipose, in male lean C57Bl/6J mice (Fig. 5A) and male lean Sprague-Dawley (SD) and fa/+ rats (Fig. 5B). However, in obese and insulin-resistant DIO mice and Zucker fatty rats, GPR120 gene expression was markedly downregulated in adipose tissues (Fig. 5A, B). The downregulation of GPR120 in WAT was also observed in fZDF rats compared with age-matched female ZL rats (Fig. 5C).
Fig. 5.
GPR40/120 dual agonist treatment reversed the downregulation of lipolysis gene expression in WAT. GPR120 gene expression in adipose tissues of lean and DIO male mice (A); SD, Zucker Fatty, and lean (fa/+) male rats (B); and fZDF and ZL female rats (C). BAT, brown adipose tissue. D: Heatmap of the WAT gene signature by the GPR40 and GPR120 dual agonist in fZDF rats. fZDF Dual, the GPR40/120 dual agonist-treated group (n = 5); fZDF GPR40, the GPR40 agonist-treated group (n = 5); fZDF Veh: the vehicle-treated fZDF rats (n = 5); Lean: the ZL rats (n = 3). Shown are the 341 genes that were significantly regulated by the dual agonist versus vehicle (with greater than 1.2-fold, P < 0.05, and expression was above detection limits by RNA sequencing). The color gradient represents fold change compared with vehicle-treated fZDF rats (−2.0- to 2.0-fold). E: MCP-1 (Ccl2) gene expression in the WAT of fZDF rats with the treatments as indicated and of the lean (Z Lean) rats. F: MCP-1 gene expression in WAT of db/db mice with the treatments as indicated and in db/+ mice. G: Lipolysis genes, HSL (Lipe), Plin1, and Lgals12, were downregulated in WAT of fZDF rats compared with ZL rats. The expression of HSL (H), Lgals12 (I), and Plin1 (J) were upregulated by the GPR40/GPR120 dual agonist treatment, but not by the GPR40 selective agonist treatment, in WAT of fZDF rats. Dual, Cpd C which is a GPR40 and GPR120 dual agonist was dosed at 30 mpk for 4 weeks in fZDF rats (n = 8); GPR40, Cpd X was dosed at 30 mpk for 4 weeks in fZDF rats (n = 8); vehicle: 0.5% methylcellulose. One-way ANOVA test. * P < 0.05, ** P < 0.01, *** P < 0.001. NS, not significant.
Because GPR120 gene expression was consistently lower in adipose tissue of insulin-resistant and diabetic rodents, we sought to identify the metabolic pathways that are potentially regulated by the GPR40/120 dual agonist in WAT. RNA profiling was performed by using WAT samples prepared from the fZDF rats after 4 week treatment with a GPR40/120 dual agonist, a GPR40 selective agonist, or vehicle, as well as in WAT from age-matched ZL rats. A set of signature genes (n = 341) was identified that was significantly regulated by the dual agonist versus vehicle (with >1.2-fold, P < 0.05), as shown in Fig. 5D. The directionality of the gene expression changes was largely toward normalization, as delineated by expression patterns in ZL rats (Fig. 5D).
GPR120 was reported to have an antiinflammatory effect in WAT of DIO mice (5). In our studies, the proinflammatory marker MCP-1 (Ccl2) was found to be upregulated in WAT of fZDF rats compared with that in ZL rats (Fig. 5E). Treatment with the GPR40/120 dual agonist for 4 weeks in fZDF rats did not influence the baseline upregulation of MCP-1 expression in WAT of fZDF rats (Fig. 5E). Similar observations were obtained in the aforementioned treatment in db/db mice, in which MCP-1 mRNA expression in WAT was upregulated in db/db mice compared with db/+ control mice (Fig. 5F). Neither therapy with the combination of GPR40 and GPR120 agonists nor with the dual agonist had a significant effect to normalize (decrease) MCP-1 gene expression in WAT in male db/db mice (Fig. 5F), despite significant lowering of blood glucose levels in vivo with both treatments (Fig. 3B). Several other proinflammatory marker genes, such as IL-6 and TNFα, exhibited a similar pattern of expression in WAT in response to the dual agonist treatment in fZDF rats and db/db mice (data not shown). The expression of key genes governing lipolysis, such as hormone-sensitive lipase (HSL/Lipe), perilipin 1 (Plin1), and galectin-12 (Lgals12), was significantly downregulated in WAT of fZDF rats versus lean rats (Fig. 5G), and the expression was upregulated by the dual agonist, but not by the GPR40 selective agonist, after a 4 week treatment in fZDF rats (Fig. 5H–J). These findings prompted us to ask whether GPR120 has a direct effect on lipolysis in adipose tissue.
GPR120 activation exerts an antilipolytic effect in adipocytes
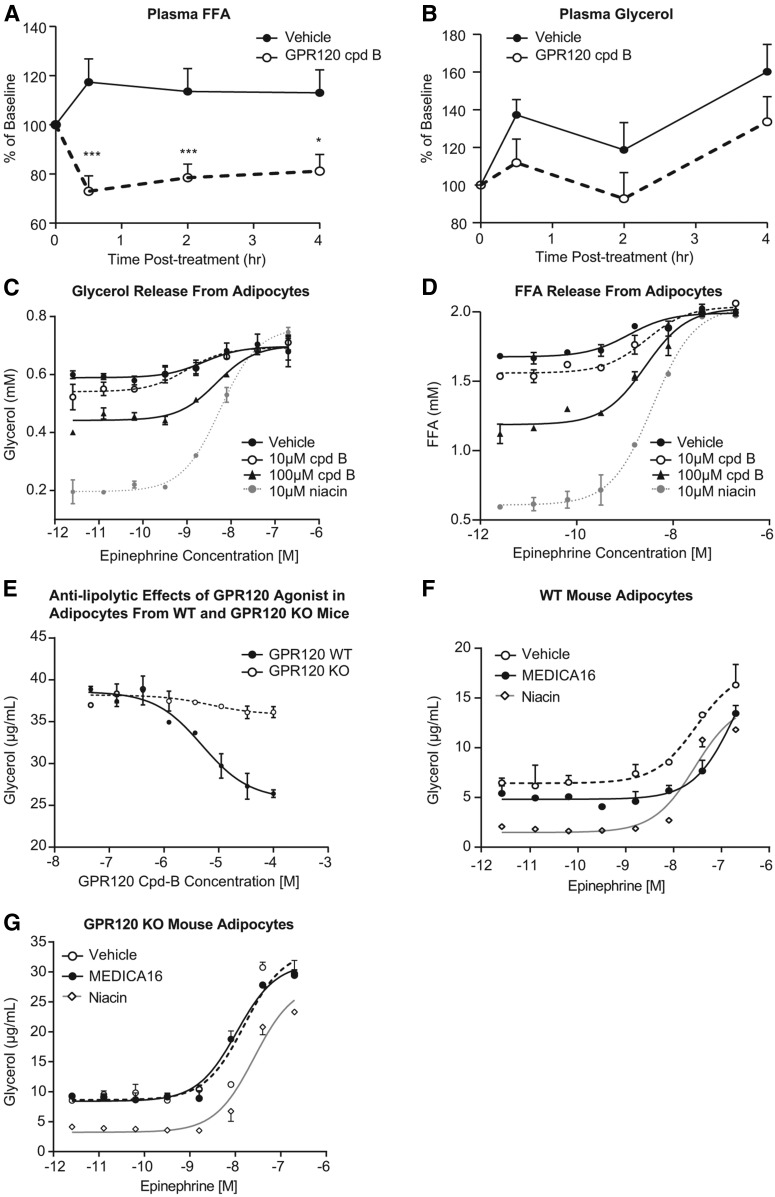
To test the hypothesis that GPR120 activation regulates lipolysis in vivo, a GPR120 selective agonist cpd B (18) was orally administered to lean rats, and plasma FFA and glycerol levels were measured. Cpd B was selected for this acute in vivo study and subsequent ex vivo studies using primary adipocytes because it is highly potent on GPR120 activation in cell-based in vitro assays (it has even better potency than cpd A, as shown in Table 1). Compared with vehicle-treated rats, plasma FFA levels were significantly decreased at 0.5, 2, and 4 h after dosing of the GPR120 agonist; treatment differences for plasma glycerol levels were not significant (Fig. 6A, B). The acute effects of the GPR120 agonist on plasma FFA occurred in the absence of a change in plasma insulin (data not shown), suggesting that GPR120 activation directly suppresses lipolysis. Therefore, we next examined whether GPR120 activation can suppress adipocyte lipolysis in a cell-autonomous manner. Primary cultures of mature rat white adipocytes were prepared and treated with cpd B, without or with increasing concentrations of epinephrine to stimulate lipolysis. Treatment with cpd B dose-dependently suppressed the release of glycerol and FFA into culture medium both in the presence and absence of concomitant epinephrine, indicating that GPR120 activation suppresses lipolysis in rat adipocytes in a cell-autonomous manner (Fig. 6C, D). Niacin, a well-known potent inhibitor of lipolysis, was included as a positive control in this experiment and demonstrated the expected robust antilipolytic effect (Fig. 6C, D). Because of the similarity of response of FFA and glycerol, in subsequent experiments in primary adipocyte cultures, only glycerol was measured. To confirm that the suppression of lipolysis by cpd B is mediated by GPR120, the response in primary adipocytes isolated from GPR120 KO mice versus age- and gender-matched WT mice was compared. Dose-dependent suppression of glycerol release by cpd B was observed in adipocytes from WT mice, but the effect was substantially blunted in adipocytes from GPR120 KO mice (Fig. 6D, E), affirming that cpd B acts via activation of GPR120 to exert its antilipolytic effect.
Fig. 6.
GPR120 activation exerts an antilipolytic effect in vivo and in vitro. Plasma NEFA (A) and glycerol (B) levels in lean rats after a single dose of a GPR120 selective agonist cpd B at 30 mpk (n = 8) or vehicle (n = 8). Suppression of glycerol (C) and FFA (D) release in rat primary white adipocytes with increased concentration of epinephrine and GPR120 agonist cpd B. E: The effects of GPR120 agonist cpd B on suppressing glycerol release in primary adipocytes from WT mice and from GPR120 KO mice. Primary adipocytes from WT mice (F) and the GPR120 KO mice (G) were treated with 350 μM MEDICA16 or 100 μM niacin in the presence of increasing concentrations of epinephrine, and glycerol release was measured in the culture medium.
MEDICA16 is a nonmetabolizable LCFA analog that has previously been reported to function as an insulin sensitizer in obese Zucker fatty rats; its mechanism of action was attributed to suppression of lipolysis in adipocytes (29). We asked whether the effect of MEDICA16 on lipolysis might be mediated by GPR120. Primary adipocytes from the GPR120 KO mice and WT mice were treated with 350 μM MEDICA16 in the presence of increasing concentrations of epinephrine and with comparison to niacin as a positive control. Niacin suppressed glycerol release from both WT and GPR120 KO mouse adipocytes (Fig. 6F, G). In contrast, suppression of glycerol release by MEDICA16 was only observed in WT adipocytes, but not from GPR120 KO adipocytes, suggesting that MEDICA16’s effect on lipolysis is mediated by GPR120 (Fig. 6F, G).
GPR120 sensitizes insulin action in rat and rhesus primary adipocytes
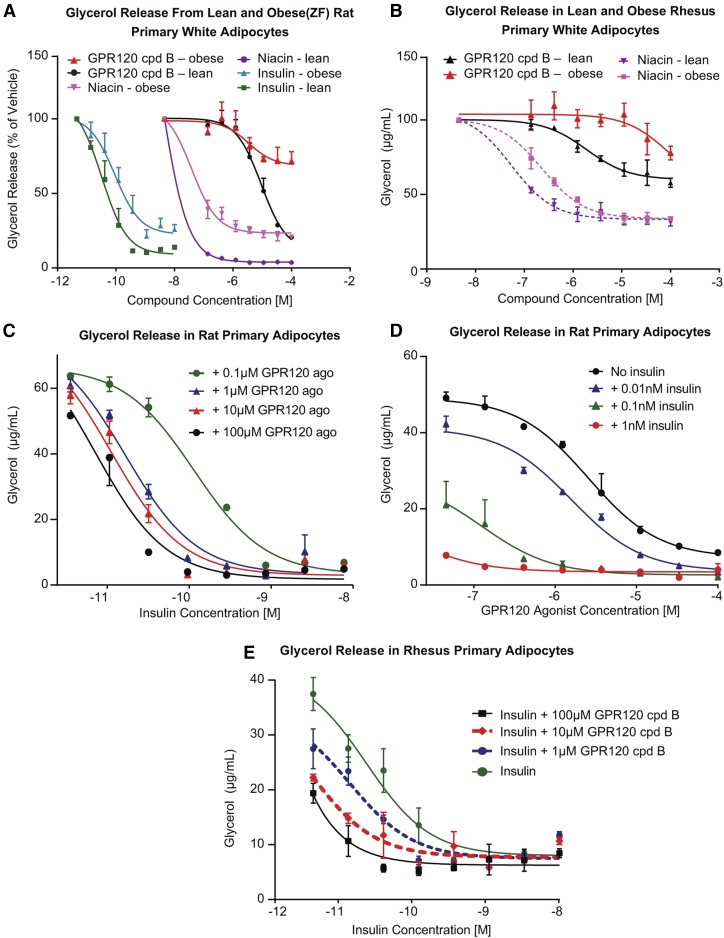
Insulin exerts a potent antilipolytic effect in adipocytes; this is obviously blunted in insulin resistant conditions (30–32). Indeed, we confirmed that lipolysis in primary adipocytes from insulin-resistant Zucker fatty rats was less sensitive to insulin suppression than in primary adipocytes from lean rats (Fig. 7A). The GPR120 agonist cpd B displayed an attenuated antilipolytic effect in adipocytes from obese compared with lean rats (Fig. 7A). Niacin also had a blunted antilipolytic effect in obese rat adipocytes (Fig. 7A). Similar insulin-resistant phenotypes were observed in the adipocytes prepared from subcutaneous WAT from obese rhesus compared with that from lean rhesus, and curves for suppression of glycerol release for both the GPR120 agonist and niacin were right-shifted in the obese rhesus adipocytes (Fig. 7B). Additionally, the GPR120 agonist exerts an antilipolytic effect in primary human adipocytes (supplemental Fig. S2).
Fig. 7.
GPR120 agonist sensitizes insulin action in rat and rhesus primary adipocytes. A: Dose-response curves of GPR120 agonist cpd B, niacin, and insulin on suppression of glycerol release from adipocytes from lean (SD) and obese (ZF) rats. B: Dose-response curves of GPR120 agonist cpd B and niacin on suppression of glycerol release from the primary adipocytes of lean and obese rhesus monkeys. C: GPR120 agonist cpd B was titrated in rat primary adipocytes in the presence of increasing concentrations of insulin. D: Insulin was titrated in rat primary adipocytes in the presence of increasing concentrations of cpd B. E: Primary rhesus adipocytes were treated with increasing amount of insulin in the absence or presence of three doses of the GPR120 agonist.
To examine whether a GPR120 agonist can sensitize insulin action in adipocytes, cpd B was up-titrated in the presence of increasing concentrations of insulin in rat primary adipocytes. The GPR120 agonist dose-dependently improved (left-shifted response plots) the suppression of glycerol release by insulin, indicating that GPR120 agonism enhances insulin action in adipocytes (Fig. 7C). Furthermore, the GPR120 agonist itself displayed a dose-dependent effect to suppress lipolysis, and in the presence of increasing insulin exposure, the response to cpd B was left shifted, indicating interaction of GPR120 and insulin to enhance suppression of lipolysis (Fig. 7D). To extend this finding to a higher species, primary rhesus adipocytes were treated with increasing insulin concentrations, in the absence or presence of three doses of cpd B. The findings in rhesus adipocytes were similar to those observed in rat adipocytes: GPR120 agonism enhanced the action of insulin to suppress lipolysis (Fig. 7E).
DISCUSSION
Insulin resistance and impaired insulin secretion are two principal components for the pathophysiology of T2DM. From this mechanistic perspective, an ideal pharmacologic therapy for T2DM would address both aspects, yet a practical challenge is the few available options for treating insulin resistance, especially in view of safety concerns associated with TZDs (1). In the present studies, we tested the hypothesis that coactivation of two novel targets, GPR40 and GPR120, would enhance antidiabetic efficacy relative to that of GPR40 monotherapy in rodent models of diabetes that are associated with insulin resistance and impaired insulin secretion.
The first set of studies reported here was based on 10 day treatment with the combination of a GPR40 selective agonist and a GPR120 selective agonist in severely hyperglycemic db/db mice, an aggressive model of rapid disease progression. Neither of these two agonists when given alone achieved sufficient traction to sustain glucose lowering in db/db mice, yet substantial and persistent glucose lowering was observed when the two agonists were combined. The combination improved the response during an insulin tolerance test, denoting improvement of insulin action. Indeed, the overall response to combined GPR40 and GPR120 agonism was nearly equivalent to the effect of Rosi, and, as a comparative benchmark, it has been recognized that TZD achieves remarkable efficacy in db/db mice.
The next study examined the combination of GPR40 and GPR120 in a “prevention mode” in young db/db mice, testing whether disease modification could be achieved. During a 5 week treatment, GPR40 and GPR120 combination therapy improved glucose tolerance and overall glycemic control that was superior to the effect of GPR40 monotherapy. A single chemical entity, a dual-agonist agent that engaged both receptors, was equally successful in this prevention mode. Identifying a small molecule that functions as a potent dual agonist for GPR40 and GPR120 represents a potentially valuable medicinal chemistry achievement. As noted earlier, the respective receptors have only modest (15%) homology, yet the putative respective endogenous ligand(s) for each are long-chain and medium-chain fatty acids. Thus, the notion was entertained that it might be feasible to synthesize a small molecule that could potently engage both receptors, and the current studies establish the feasibility of such an approach.
Another rodent model that was employed to test efficacy was the fZDF rats, a model of disease progression with twin impairments of insulin secretion and action. Treatment of fZDF rats with a GPR40/GPR120 dual agonist showed better glycemic control than the response to single agent agonizing GPR40 and was, again, nearly as effective as the response to TZD. Efficacy with the GPR40/GPR120 dual agonist was associated with evidence of preservation of islet function and healthier islet morphology, indicative of favorable disease modification. Taken together, these results indicate that targeting both GPR40 and GPR120 improves the metabolic benefits compared with targeting GPR40 alone and that this effect is attained by adding in a component of insulin sensitization mediated by GPR120. The finding that alleviating insulin resistance can potentiate efficacy attained by improving insulin secretion is not surprising as such; rather, this confirms fundamental concepts of the disease process of T2DM and how to best intervene. Raising insulin secretion (e.g., GPR40) can reduce hyperglycemia, but is less than ideal if the insulin-responsive tissues remain resistant to insulin action. Alleviating insulin resistance (e.g., GPR120) addresses a core defect, but may not effectively improve hyperglycemia in the face of deficient insulin secretion. Doing both together can have a compounding effect, a concept captured in a parameter like the disposition index that is calculated as the product of insulin secretion and insulin action and that is substantially reduced in T2DM (33). However, beyond affirming the value of improving both insulin secretion and insulin action, the current findings are important because we focused on testing two novel targets. Furthermore, the current studies begin to demonstrate that it can be technically feasible through medicinal chemistry efforts to identify small-molecule compounds that can simultaneously engage both GPR40 and GPR120.
The effect of a small-molecule GPR40/120 dual agonist on insulin action was tested in ob/ob mice, a model of moderate hyperglycemic but severe insulin resistance. Improvement of insulin sensitivity was demonstrated during a hyperglycemic-euglycemic clamp with a GPR40/120 dual agonist, but not with a GPR40 agonist alone. Based on the glucose flux determinations carried out in conjunction with the clamp, our interpretation is that the key site for improved insulin sensitization is amelioration of hepatic insulin resistance, more specifically, improved insulin action in suppressing EGP. Interestingly, this was not achieved by a GPR40 selective agonist despite lowering of fasting glucose similar to that achieved with dual agonist treatment. The implication is that the addition of GPR120 improved hepatic insulin resistance. Although this finding appears consistent with an earlier report (5), it is noteworthy that there is little expression of GPR120 in liver. Instead, the predominant sites of GPR120 expression are in adipocytes and macrophages (4). We postulated that GPR120 mechanism of action to improve hepatic insulin resistance might therefore entail adipose tissue-liver cross-talk. Our findings on the mechanism of action of GPR120 in adipose tissue certainly support the concept of potential hepatic-adipose tissue cross-talk, but, unlike the earlier report, our findings discount that the fulcrum of GPR120 action is antiinflammation and instead point to a cell-autonomous action to govern lipolysis in adipocytes, as will be discussed further below.
In the subsequent studies, we aimed to gain a better understanding of how GPR120 agonism improves insulin sensitivity in these diabetic rodent models. Because GPR120 was previously shown to have an antiinflammatory effect in adipose tissue (6), we first looked at the inflammatory marker gene expression in adipose tissue. Consistent with prior literature, expression of proinflammatory marker genes such as MCP-1 was significantly elevated in untreated fZDF rats and db/db mice compared with respective controls. Chronic treatment with a GPR40/120 dual agonist, although significantly improving glycemic control, did not alter their expression, suggesting that antiinflammation was not a major component of the mechanism to improve insulin resistance in these diabetic rodent models. The reasons for the differences from the earlier report are uncertain. It is noted that different disease models were used in the present study versus the earlier report. The present studies were conducted in db/db and ob/ob mice, as well as fZDF rats; these are more severely hyperglycemic than DIO mice, which were used in the earlier report. It is well recognized that the level of local inflammation in adipose tissue can vary considerably in rodent models of obesity and diabetes (34).
GPR120 gene expression was found to be downregulated in different fat depots in the insulin-resistant and diabetic state (Fig. 5A–C). These expression changes are consistent with the findings from human visceral adipose tissue from morbidly obese subjects (35, 36). It has been reported that morbidly obese human subjects had lower GPR120 expression levels in adipose tissue than in nonobese subjects (36). In addition, it has been reported that GPR120 expression was upregulated during adipocyte differentiation similarly in human preadipocytes and in mouse 3T3L1 cells (37). We further found that GPR120 has a direct and cell-autonomous effect to suppress lipolysis in primary white adipocytes. In humans with obesity and T2DM, suppression of lipolysis by insulin is impaired (30–32). In our rodent studies, impaired suppression of lipolysis by insulin in adipocytes from obese, insulin-resistant rats and rhesus was also observed. GPR120 activation was demonstrated to sensitize insulin action on lipolysis in the insulin-resistant state. We postulated that this is one mechanism by which activation of GPR120 in adipose could improve insulin resistance. However, in the hyperinsulinemic-euglycemic clamp study in ob/ob mice, the dual agonist treatment did not result in a significant change in lipolysis, as measured by plasma NEFA. The reason for the apparent discrepancy between the in vitro and in vivo data is not clear. One possible explanation could be that GPR120 activation changes FFA levels in the local environment of visceral fat, which affects the portal vein FFA and impacts hepatic glucose production. In our clamp study, we only measured peripheral FFA. Further research will need to be done to deepen mechanistic insights on how the novel GPR120 actions in adipose tissue identified in these studies lead to improved insulin action in the liver as well as amelioration of lipotoxicity in pancreatic islets. There is one published study that directly demonstrates that lowering of fat cell lipolysis improves glucose metabolism and insulin sensitivity in man and rodents (38). We asked whether our findings in rodents on GPR120-mediated antilipolytic effect translate to higher species. We recently demonstrated that insulin-mediated control of lipolysis is altered in nonhuman primates with obesity in vivo (39); the studies performed herein extend that observation to the in vitro setting. We were able to show that GPR120 exhibits a similar effect in primary adipocytes from adipose biopsy samples from rhesus monkey in that GPR120 sensitizes insulin action on lipolysis, and obesity is associated with an impaired antilipolysis by GPR120 in rhesus. This finding was further extended herein by using primary adipocytes from an overweight human donor (with BMI of 27); our data indicated that a GPR120 agonist partially inhibits lipolysis (supplemental Fig. S2). Although this is a promising translational observation, additional studies with human adipocytes, including from subjects of normal weight and of obesity, are needed to confirm this initial finding.
It should be noted that receptor desensitization is a potential concern for pharmacological agonism of GPCR targets. In particular, it has been shown that a single dose of niacin can effectively lower FFA, but repetitive or continuous dosing of niacin resulted in a FFA rebound in both rodents (40) and in clinical studies (41). It is worth mentioning that a recent study showed that dosing regimen has a critical impact on whether niacin can achieve sustained metabolic control (42). In obese Zucker rats, timing of nicotinic acid delivery with feeding, but not with fasting, was shown to deliver durable FFA lowering and improve glucose control. This can, in theory, be an effective means of reversing lipid overload-induced insulin resistance. It is interesting that GPR120 expression appears to be upregulated after a high-fat meal in nonobese human subjects, but this regulation is blunted in morbidly obese subjects (36). Further investigation on the persistence of antilipolytic effect mediated by a GPR120 agonist side by side with niacin treatment in a preclinical model is warranted to better understand the durability of GPR120 agonism as a potential therapy for FFA lowering and insulin sensitization.
In summary, GPR40 and GPR120 dual agonism addresses the two fundamental pathological defects of T2DM, insulin resistance and β-cell failure, and leads to efficacious and durable glycemic improvement in four rodent models of T2DM. Our results in preclinical disease models suggest that GPR40 and GPR120 dual agonism represents a potentially promising treatment for T2DM.
Note added in proof
In the version of this article that was published as a Paper in Press on June 5, 2017, the designation indicating that Santhosh Santapati and Ying Qian contributed equally to this work was inadvertently omitted. This error has now been corrected.
Supplementary Material
Acknowledgments
The authors thank Kenneth Lodge at Merck & Co., Inc. and Keefe Chng and Yixin Wang at CrownBio (Kannapolis, NC) for kindly providing rhesus adipose tissue biopsy samples; Wenyu Li, George Eiermann, Dan Metzger, and Sandra Souza for their excellent technical assistance and help; and Jennifer Cho for help with the RNA-sequencing study.
Footnotes
Abbreviations:
- 2-DG
- 2-deoxy glucose
- AUC
- area under the curve
- DIO
- diet-induced obese
- EGP
- endogenous glucose production
- fZDF
- female Zucker diabetic fatty
- GIR
- glucose infusion rate
- GPCR
- G protein-coupled receptor
- HSL
- hormone-sensitive lipase
- IM
- intramuscularly
- LCFA
- long-chain fatty acid
- Lgals12
- galectin-12
- oGTT
- oral glucose tolerance test
- Pio
- pioglitazone
- Plin1
- perilipin 1
- Rosi
- rosiglitazone
- SD
- Sprague-Dawley
- T2DM
- type 2 diabetes mellitus
- TG
- triglyceride
- TZD
- thiazolidinedione
- WAT
- white adipose tissue
- WH
- Wistar Hans
- ZL
- Zucker lean
This work was supported by Merck.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Ciudin A., Hernandez C., and Simo R.. 2012. Update on cardiovascular safety of PPARgamma agonists and relevance to medicinal chemistry and clinical pharmacology. Curr. Top. Med. Chem. 12: 585–604. [DOI] [PubMed] [Google Scholar]
- 2.Ichimura A., Hasegawa S., Kasubuchi M., and Kimura I.. 2014. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front. Pharmacol. 5: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran B. M., Flatt P. R., and McKillop A. M.. 2016. G protein-coupled receptors: signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 53: 177–188. [DOI] [PubMed] [Google Scholar]
- 4.Miyauchi S., Hirasawa A., Iga T., Liu N., Itsubo C., Sadakane K., Hara T., and Tsujimoto G.. 2009. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 379: 427–434. [DOI] [PubMed] [Google Scholar]
- 5.Oh D. Y., Walenta E., Akiyama T. E., Lagakos W. S., Lackey D., Pessentheiner A. R., Sasik R., Hah N., Chi T. J., Cox J. M., et al. . 2014. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 20: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M.. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yore M. M., Syed I., Moraes-Vieira P. M., Zhang T., Herman M. A., Homan E. A., Patel R. T., Lee J., Chen S., Peroni O. D., et al. . 2014. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 159: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. . 2012. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 483: 350–354. [DOI] [PubMed] [Google Scholar]
- 9.Bonnefond A., Lamri A., Leloire A., Vaillant E., Roussel R., Levy-Marchal C., Weill J., Galan P., Hercberg S., Ragot S., et al. . 2015. Contribution of the low-frequency, loss-of-function p.R270H mutation in FFAR4 (GPR120) to increased fasting plasma glucose levels. J. Med. Genet. 52: 595–598. [DOI] [PubMed] [Google Scholar]
- 10.Vestmar M. A., Andersson E. A., Christensen C. R., Hauge M., Glumer C., Linneberg A., Witte D. R., Jorgensen M. E., Christensen C., Brandslund I., et al. . 2016. Functional and genetic epidemiological characterisation of the FFAR4 (GPR120) p.R270H variant in the Danish population. J. Med. Genet. 53: 616–623. [DOI] [PubMed] [Google Scholar]
- 11.Tan C. P., Feng Y., Zhou Y. P., Eiermann G. J., Petrov A., Zhou C., Lin S., Salituro G., Meinke P., Mosley R., et al. . 2008. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes. 57: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yashiro H., Tsujihata Y., Takeuchi K., Hazama M., Johnson P. R., and Rorsman P.. 2012. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J. Pharmacol. Exp. Ther. 340: 483–489. [DOI] [PubMed] [Google Scholar]
- 13.Kaku K., Enya K., Nakaya R., Ohira T., and Matsuno R.. 2015. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes. Metab. 17: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaku K., Enya K., Nakaya R., Ohira T., and Matsuno R.. 2016. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: a 52-week open-label phase III study. Diabetes Obes. Metab. 18: 925–929. [DOI] [PubMed] [Google Scholar]
- 15.Hauge M., Vestmar M. A., Husted A. S., Ekberg J. P., Wright M. J., Di Salvo J., Weinglass A. B., Engelstoft M. S., Madsen A. N., Luckmann M., et al. . 2014. GPR40 (FFAR1) - combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 4: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan N. G., and Dhayal S.. 2009. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem. Pharmacol. 78: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen E., Watterson K. R., Stocker C. J., Sokol E., Jenkins L., Simon K., Grundmann M., Petersen R. K., Wargent E. T., Hudson B. D., et al. . 2015. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br. J. Nutr. 113: 1677–1688. [DOI] [PubMed] [Google Scholar]
- 18.Egerod K. L., Engelstoft M. S., Lund M. L., Grunddal K. V., Zhao M., Barir-Jensen D., Nygaard E. B., Petersen N., Holst J. J., and Schwartz T. W.. 2015. Transcriptional and functional characterization of the G protein-coupled receptor repertoire of gastric somatostatin cells. Endocrinology. 156: 3909–3923. [DOI] [PubMed] [Google Scholar]
- 19.Nargund R. P. 2016. Design, synthesis and biological profile of the FFA 1 receptor (GPR40) agonist MK-8666. 6th Royal Society of Chemistry/Society of Chemical Industry Symposium on GPCRs in Medicinal Chemistry in Verona, Italy, June 13–15, 2016.
- 20.Cox J. M., Chu H. D., Chelliah M. V., Debenham J. S., Eagen K., Lan P., Lombardo M., London C., Plotkin M. A., Shah U., et al. . 2016. Design, synthesis, and evaluation of novel and selective G-protein coupled receptor 120 (GPR120) spirocyclic agonists. ACS Med. Chem. Lett. 8: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala J. E., Bracy D. P., Malabanan C., James F. D., Ansari T., Fueger P. T., McGuinness O. P., and Wasserman D. H.. 2011. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J. Vis. Exp. 57: e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S. P., Zhou D., Yao Z., Satapati S., Chen Y., Daurio N. A., Petrov A., Shen X., Metzger D., Yin W., et al. . 2016. Quantifying rates of glucose production in vivo following an intraperitoneal tracer bolus. Am. J. Physiol. Endocrinol. Metab. 311: E911–E921. [DOI] [PubMed] [Google Scholar]
- 23.Shang J., Chen Z., Wang M., Li Q., Feng W., Wu Y., Wu W., Graziano M. P., and Chintala M.. 2014. Zucker diabetic fatty rats exhibit hypercoagulability and accelerated thrombus formation in the arterio-venous shunt model of thrombosis. Thromb. Res. 134: 433–439. [DOI] [PubMed] [Google Scholar]
- 24.Schlessinger K., Li W., Tan Y., Liu F., Souza S. C., Tozzo E., Liu K., Thompson J. R., Wang L., and Muise E. S.. 2015. Gene expression in WAT from healthy humans and monkeys correlates with FGF21-induced browning of WAT in mice. Obesity (Silver Spring). 23: 1818–1829. [DOI] [PubMed] [Google Scholar]
- 25.Viswanadha S., and Londos C.. 2006. Optimized conditions for measuring lipolysis in murine primary adipocytes. J. Lipid Res. 47: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 26.Ichimura A., Hirasawa A., Hara T., and Tsujimoto G.. 2009. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 89: 82–88. [DOI] [PubMed] [Google Scholar]
- 27.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., and Tsujimoto G.. 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11: 90–94. [DOI] [PubMed] [Google Scholar]
- 28.Bradley D. C., Poulin R. A., and Bergman R. N.. 1993. Dynamics of hepatic and peripheral insulin effects suggest common rate-limiting step in vivo. Diabetes. 42: 296–306. [DOI] [PubMed] [Google Scholar]
- 29.Kalderon B., Azazmeh N., Azulay N., Vissler N., Valitsky M., and Bar-Tana J.. 2012. Suppression of adipose lipolysis by long-chain fatty acid analogs. J. Lipid Res. 53: 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell P. J., Carlson M. G., and Nurjhan N.. 1994. Fat metabolism in human obesity. Am. J. Physiol. 266: E600–E605. [DOI] [PubMed] [Google Scholar]
- 31.Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., and DeFronzo R. A.. 1989. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groop L. C., Bonadonna R. C., Simonson D. C., Petrides A. S., Shank M., and DeFronzo R. A.. 1992. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. Am. J. Physiol. 263: E79–E84. [DOI] [PubMed] [Google Scholar]
- 33.Bergman R. N., Ader M., Huecking K., and Van Citters G.. 2002. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 51 (Suppl. 1): S212–S220. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery M. K., Hallahan N. L., Brown S. H., Liu M., Mitchell T. W., Cooney G. J., and Turner N.. 2013. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 56: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 35.Trayhurn P., and Denyer G.. 2012. Mining microarray datasets in nutrition: expression of the GPR120 (n-3 fatty acid receptor/sensor) gene is down-regulated in human adipocytes by macrophage secretions. J. Nutr. Sci. 1: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Pacheco F., Garcia-Serrano S., Garcia-Escobar E., Gutierrez-Repiso C., Garcia-Arnes J., Valdes S., Gonzalo M., Soriguer F., Moreno-Ruiz F. J., Rodriguez-Canete A., et al. . 2014. Effects of obesity/fatty acids on the expression of GPR120. Mol. Nutr. Food Res. 58: 1852–1860. [DOI] [PubMed] [Google Scholar]
- 37.Gotoh C., Hong Y. H., Iga T., Hishikawa D., Suzuki Y., Song S. H., Choi K. C., Adachi T., Hirasawa A., Tsujimoto G., et al. . 2007. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 354: 591–597. [DOI] [PubMed] [Google Scholar]
- 38.Girousse A., Tavernier G., Valle C., Moro C., Mejhert N., Dinel A. L., Houssier M., Roussel B., Besse-Patin A., Combes M., et al. . 2013. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 11: e1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang J., Previs S. F., Conarello S., Chng K., Zhu Y., Souza S. C., Staup M., Chen Y., Xie D., Zycband E., et al. . 2017. Phenotyping of adipose, liver, and skeletal muscle insulin resistance and response to pioglitazone in spontaneously obese rhesus monkeys. Am. J. Physiol. Endocrinol. Metab. 312: E235–E243. [DOI] [PubMed] [Google Scholar]
- 40.Pereira J. N. 1967. The plasma free fatty acid rebound induced by nicotinic acid. J. Lipid Res. 8: 239–244. [PubMed] [Google Scholar]
- 41.Wang W., Basinger A., Neese R. A., Christiansen M., and Hellerstein M. K.. 2000. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am. J. Physiol. Endocrinol. Metab. 279: E50–E59. [DOI] [PubMed] [Google Scholar]
- 42.Kroon T., Baccega T., Olsen A., Gabrielsson J., and Oakes N. D.. 2017. Nicotinic acid timed to feeding reverses tissue lipid accumulation and improves glucose control in obese Zucker rats[S]. J. Lipid Res. 58: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.