Abstract
A vast literature on fatty acids in mammals exists, but comparable compositional data on oxylipins is lacking. Weanling Sprague-Dawley rats were therefore provided control diets or diets with higher linoleic acid (LA) or with higher LA and α-linolenic acid (LA+ALA) for 6 weeks. Kidneys, livers, and serum were analyzed for oxylipins and fatty acids. The proportion of tissue oxylipins derived from LA was greater than the relative proportion of LA itself, whereas arachidonic acid (AA) oxylipins were overrepresented in serum. Higher dietary LA increased kidney LA and AA oxylipins, despite not altering LA or AA. In liver, both LA and AA and their oxylipins were higher, whereas in serum only LA oxylipins were higher with higher dietary LA. Higher LA resulted in a higher ratio of n-6/n-3 PUFA-derived oxylipins; adding ALA to the LA diet mitigated this and many, but not all, effects of the LA diet. Approximately 40% of oxylipins detected were influenced by sex and, unlike their PUFA precursors, most (>90%) of these were higher in males. These differences in dietary LA and sex on oxylipin and fatty acid profiles further our understanding of the effects of fatty acids and may have implications for dietary LA recommendations.
Keywords: arachidonic acid, diet and dietary lipids, eicosanoids, lipid mediators, targeted lipidomics, nutrition, lipids, linoleic acid, polyunsaturated fatty acids
There is a vast and growing literature on fatty acids in mammals, but basic compositional data on the bioactive lipids derived from these fatty acids is currently lacking. One important set of bioactive lipids that mediate many of the effects of PUFAs are known as oxylipins. These oxygenated products of PUFAs are produced via the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) pathways to produce oxylipins, such as prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and many more, including the more recently described resolvins and protectins (1). With the advancement of MS methods in tandem with LC (2), profiling of these oxylipins is now feasible. Several recent studies in renal disease suggest that PUFA composition may not necessarily provide an accurate reflection of oxylipins in tissues (3, 4). Because fatty acid composition is often interpreted under the assumption that many of its effects are mediated via the oxylipins that it produces, it is imperative to determine the actual oxylipin profile and how it is associated with the PUFA profile under normal conditions.
Oxylipin compared with fatty acid profiling may have relevance to dietary recommendations for PUFAs. For example, linoleic acid (LA) intake has increased over the last half century in North America (5, 6), but a consensus on optimal intake has not been reached. Recommendations for dietary LA still vary considerably around the world, ranging from 1–2% of energy, the level required to prevent essential fatty acid deficiency, to 5–10% of energy, the level thought to reduce the risk of chronic disease [reviewed in (7)]. The dietary reference intake report for fatty acids and the Dietary Guidelines for Americans 2015–2020 support 5–10% of energy from n-6 PUFAs (predominantly LA) for optimal health (8, 9), while the American Heart Association suggests that even more than 10% energy from n-6 PUFAs may confer additional benefits (10). This remains highly controversial because a higher n-6/n-3 PUFA ratio is thought to increase inflammation via its effect on altering the n-6/n-3 oxylipin profile (11–13). However, although it has recently been shown that large changes in LA alter the levels of LA oxylipins (4, 14, 15), whether changes in dietary LA in the normal range of current intake affect LA oxylipins is not known. The effect of dietary LA on LA oxylipins is also likely important because LA oxylipins can make up more than half of all oxylipins by mass (e.g., rat kidney) (4). Further, it is not known how normal dietary LA affects oxylipins derived from other n-6 or n-3 PUFA-derived oxylipins. The arachidonic acid (AA)-derived oxylipins are of particular interest, as they are the most well-characterized and have predominantly pro-inflammatory effects (1, 13). Large changes in dietary LA do not alter blood AA levels (16), so it has been assumed that AA oxylipins are also not affected, but whether this tight regulation of AA levels in blood is reflected in AA oxylipins or whether blood oxylipin levels accurately reflect tissue levels is also not known.
Oxylipin profiling may also be relevant to further understanding sex differences in health and disease (17). Sex effects on fatty acid metabolism have been documented (18–20), but effects in oxylipins have largely been restricted to studies of one or two eicosanoids derived from AA (21, 22) and to in vitro and ex vivo studies of sex hormone effects on oxylipin synthetic and degradative enzymes (23–25). Results from these studies appear to be conflicting, with no pattern being apparent. Examination of the overall oxylipin profile in tissues may reveal such patterns and provide further understanding of sex differences in health and metabolism.
In the current study, we report the oxylipin profiles in rat kidney, liver, and serum and examine the effect of dietary LA and sex differences on these profiles. These compositional data, in comparison to PUFA data, reveal that the oxylipin profile differs significantly from the profile of the precursor PUFA, and that dietary LA and sex effects on the oxylipin profile also do not mimic the PUFA profile. The serum oxylipin profile also does not reflect tissue profiles. This foundational data has implications for further understanding of fatty acids and their effects on mammalian physiology, including dietary and sex effects.
MATERIALS AND METHODS
Diets and rat procedures
Six male and six female weanling Sprague-Dawley rats were provided three different diets, for a total of 36 rats. The rats were provided diets based on the AIN93G diet, except that the diets contained 10 g oil per100 g diet instead of 7 g oil per 100 g diet, and the source of oil varied between diets, as outlined below and in the diet composition table (Table 1). The control diet had adequate levels of LA and α-linolenic acid (ALA). In comparison, the LA diet had 3 g more LA per 100 g diet and the same amount of ALA. The LA+ALA diet also had 3 g more LA per 100 g diet than the control diet, plus a higher ALA level to match the LA/ALA ratio of the control diet. The higher LA and ALA in the latter diets were primarily at the expense of MUFAs, so all three diets had similar saturated and unsaturated fatty acid compositions. All diet ingredients were obtained from Dyets Inc. (Bethlehem, PA), except for tert-butylhydroquinone, which was from Sigma-Aldrich (Oakville, ON, Canada).
TABLE 1.
Formulation of experimental diets
| Control | LA | LA+ALA | |
| g/100 g diet | |||
| Ingredients | |||
| Cornstarch | 34.9 | 34.9 | 34.9 |
| Casein (87% protein) | 20.7 | 20.7 | 20.7 |
| Dextrinized cornstarch | 13.7 | 13.7 | 13.7 |
| Sucrose | 10.3 | 10.3 | 10.3 |
| Fiber | 5.17 | 5.17 | 5.17 |
| Mineral mix (AIN93G) | 3.62 | 3.62 | 3.62 |
| Vitamin mix (AIN 93) | 1.03 | 1.03 | 1.03 |
| L-cystine | 0.31 | 0.31 | 0.31 |
| Choline bitartrate | 0.259 | 0.259 | 0.259 |
| tert-Butylhydroquinone | 0.002 | 0.002 | 0.002 |
| Safflower oil | — | 4.3 | — |
| Olive oil | 7 | — | — |
| Soy oil | 2.2 | 3.8 | 10 |
| Coconut oil | 0.65 | 1.9 | — |
| Flax oil | 0.15 | — | — |
| Total | 100 | 100 | 100 |
| Fatty acids | |||
| LA | 2.13 | 5.21 | 5.35 |
| ALA | 0.27 | 0.28 | 0.71 |
| Saturated fatty acids | 1.93 | 2.37 | 1.56 |
| Unsaturated fatty acids | 6.63 | 7.16 | 8.15 |
| MUFAs | 4.22 | 1.66 | 2.07 |
| PUFAs | 2.41 | 5.5 | 6.08 |
| LA/ALA | 7.76 | 18.31 | 7.50 |
| n-6/n-3 fatty acid ratio | 7.74 | 18.11 | 7.45 |
Rats were weighed bi-weekly and after 6 weeks of feeding were anesthetized with isofluorane and euthanized via decapitation to collect trunk blood to obtain serum, which was stored at −80°C until analysis. The right kidney and a portion of the liver were subsequently removed, immediately frozen in liquid nitrogen, and also stored at −80°C until analysis. All procedures were performed in accordance with the Canadian Council for Animal Care guidelines and approved by the University of Manitoba Animal Care Committee.
Oxylipin analysis
Kidneys and livers were lyophilized, a representative portion was homogenized in Tyrode’s salt solution, and samples for oxylipin analysis were prepared and analyzed by HPLC/MS/MS multiple-reaction monitoring, as described (4, 26). Briefly, 200 μl of tissue homogenate or 400 ul of serum containing antioxidant were used for oxylipin analysis. After adding deuterated internal standards (Cayman Chemical, Ann Arbor, MI), samples were adjusted to pH <3. Solid phase extraction was with Strata-X SPE columns (Phenomenex, Torrance, CA) that were preconditioned with methanol and pH 3 water, loaded with sample, rinsed with 10% methanol in pH 3 water, and eluted with methanol. Samples were dried down and resuspended in the starting mobile phase (water/acetonitrile/acetic acid, 70/30/0.02, v/v/v) for analysis by HPLC/MS/MS (QTRAP 6500; Sciex, ON, Canada). Quantification of oxylipins was determined using the stable isotope dilution method (27). Dose response curves were used to determine response factors, which were applied to all oxylipins, unless otherwise noted when primary standards were unavailable. Further details of oxylipins scanned for, but below the limit of detection (<3 times above baseline) or below the limit of quantitation (<5 times above baseline), as well as mass transitions, internal standards, and retention times, are provided in supplemental Tables S1 and S2.
Fatty acid analysis
For fatty acid analysis, 250 μl aliquots of the tissue homogenates and serum containing antioxidants were used and analyzed as described (28). Briefly, lipids were extracted via solvent-solvent extraction and purified by TLC (heptane/isopropyl/acetic acid, 60/40/3, v/v/v) to isolate the phospholipid fraction from tissues, while the whole lipid extract from serum was used for fatty acid analysis. The phospholipid fraction was analyzed for the tissues because PUFAs in phospholipid are the source of oxylipins in tissue (29, 30). Oxylipins in blood, however, are secreted from multiple tissues, so the total fatty acids in serum were analyzed as representative of overall fatty acid composition. Fatty acids were methylated using methanolic HCl and quantified by GC.
Statistical analyses
Data were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC). The Shapiro-Wilk test was used to test normality. Data were analyzed by using two-way ANOVA to test the main effects or were analyzed using the Kruskal-Wallis test when data could not be normalized by logarithmic transformation. The protected least squares means test was used to detect differences between the three diets. Outliers were removed if they were outside of the mean ± 3SD for the diet and sex group.
RESULTS
Differences in oxylipin and fatty acid profiles in rat kidney, liver, and serum
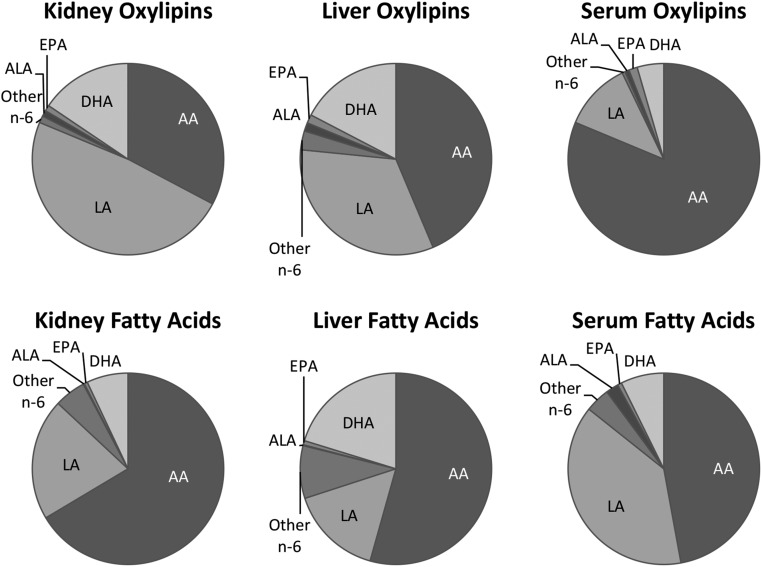
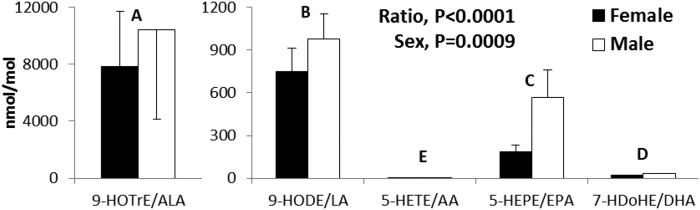
All rats grew well, with males having higher body weights throughout the study (supplemental Table S3). Of the 163 oxylipins scanned, 69 were quantified in the kidney, 71 in liver, and 60 in serum. As illustrated in the example in Fig. 1 and in detail in supplemental Table S4, ∼80–90% of oxylipin mass in kidney and liver was derived from n-6 PUFAs, particularly from LA (33–61%) and AA (22–44%). Similarly, >90% of oxylipin mass in serum was derived from n-6 PUFAs, but in serum most (73–81%) was derived from AA rather than LA. In comparison to the mass amount of LA, the oxylipins in kidney and liver derived from LA were proportionately higher than LA itself, and the opposite was true for AA and the other n-6 oxylipins. In comparison, in serum, it was the AA oxylipins that were proportionately higher than the level of their AA precursor (Fig. 1, supplemental Table S4).
Fig. 1.
Distribution of oxylipin and fatty acid mass in kidney, liver, and serum of rats provided the control diet. Data are from female and male rats combined. For separate female and male data for all diets in kidney, liver, and serum, see supplemental Table S4.
Higher dietary LA results in higher levels of n-6 PUFA-derived oxylipins, even when n-6 PUFAs are not higher
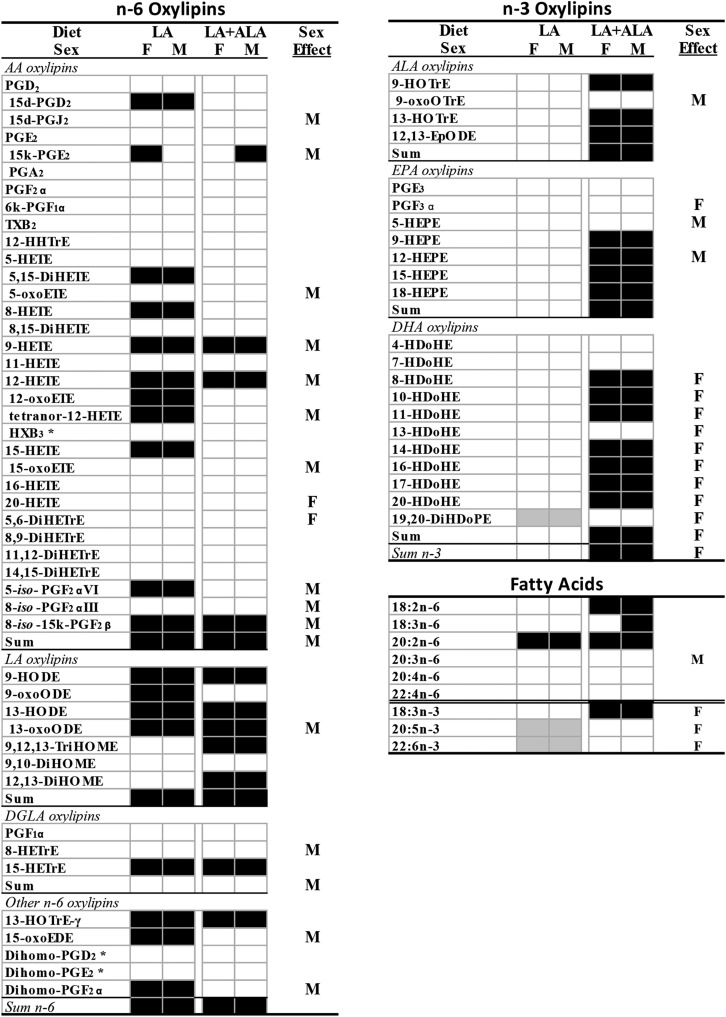
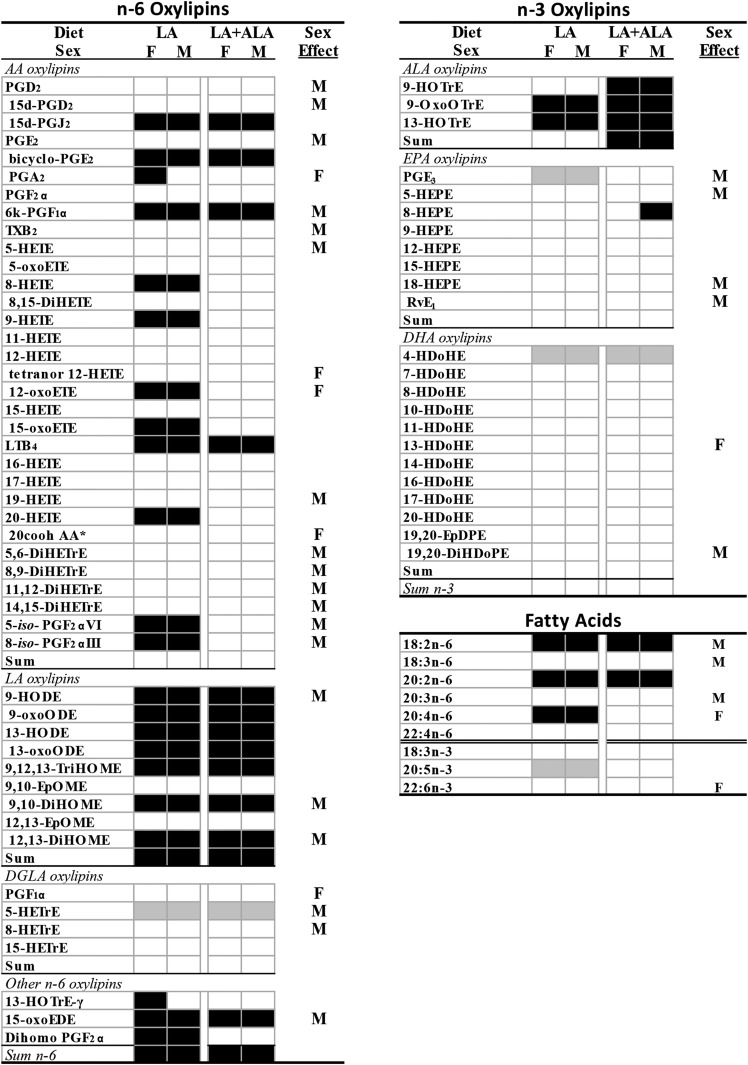
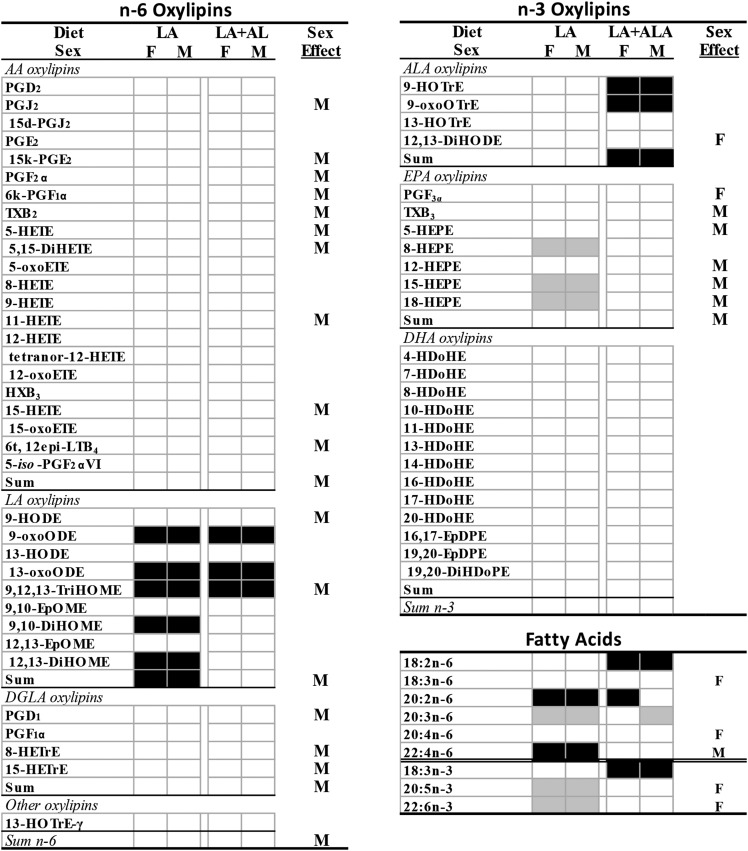
Increasing the level of dietary LA from 2 to 5 g per 100 g diet resulted in higher n-6 oxylipins, even when the precursor PUFA level was not increased. This was most clearly exhibited in the kidney, where eicosadienoic acid (EDA) was the only renal n-6 PUFA that was higher in the rats given the LA diet, yet oxylipins derived from LA (4), AA (11), and one each from γ-linolenic acid (GLA), dihomo-GLA acid (DGLA), EDA, and adrenic acid (AdA) were higher (Fig. 2; complete data and statistics are in supplemental Tables S5a, S8). Other n-6-derived oxylipins that were not significantly elevated followed the same trend, as evidenced by the higher levels of total LA (84% higher), total AA (35% higher), and total n-6-derived oxylipins (64% higher) in the LA compared with the control group. In liver, the LA diet increased the levels of LA, EDA, and AA, but not GLA, DGLA, or AdA. This was accompanied by higher levels of oxylipins from LA (7), AA (12), GLA (1), EDA (1), and AdA (1), as well as higher levels of total LA (175% higher) and total n-6 PUFA-derived oxylipins (82% higher) (Fig. 3; complete data and statistics are in supplemental Tables S6a, S9). In comparison, serum exhibited only elevated levels of EDA and AdA in LA compared with control-fed rats, and only exhibited higher levels of oxylipins derived from LA (5) and total LA oxylipins (75% higher) (Fig. 4; complete data and statistics are in supplemental Tables S7a, S10).
Fig. 2.
Differences in kidney oxylipins and fatty acids in rats given LA and LA+ALA compared with control diets for 6 weeks. Oxylipins that are higher (black boxes) or lower (gray boxes) than the control diet are denoted by shading. Sex effects in at least one of the diets are denoted by M (higher in males) or F (higher in females). Means, SEs, and P values are shown in supplemental Tables S5 and S8. *Denotes no primary standard so not quantified and not included in the sums. d, deoxy; DiHDoPE, dihydroxy-docosapentaenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHOME, dihydroxy-octadecenoic acid; EpODE, epoxy-eicosadienoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETrE, hydroxy-eicosatrienoic acid; HHTrE, hydroxy-heptadecatrienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HX, hepoxilin; k, keto; oxoEDE, oxo-eicosadienoic acid; oxoETE, oxo-eicosatetraenoic acid; oxoODE, oxo-octadecadienoic acid; oxoOTrE, oxo-octadecatrienoic acid; PG, prostaglandin; TriHOME, trihydroxy-octadecenoic acid; TX, thromboxane.
Fig. 3.
Differences in liver oxylipins and fatty acids in rats given LA and LA+ALA compared with control diets for 6 weeks. Oxylipins that are higher (black boxes) or lower (gray boxes) than control diet are denoted by shading. Sex effects in at least one of the diets are denoted by M (higher in males) or F (higher in females). Means, SEs, and P values are shown in supplemental Tables S6 and S9. *Denotes no primary standard so not quantified and not included in the sums. cooh, carboxy; d, deoxy; DiHDoPE, dihydroxy-docosapentaenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHOME, dihydroxy-octadecenoic acid; EpDPE, epoxy-eicosadocosapentaenoic acid; EpOME, epoxy-octadecenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETrE, hydroxy-eicosatrienoic acid; HOTrE, hydroxy-octadecatrienoic acid; k, keto; LT, leukotriene; oxoEDE, oxo-eicosadienoic acid; oxoETE, oxo-eicosatetraenoic acid; oxoODE, oxo-octadecadienoic acid; oxoOTrE, oxo-octadecatrienoic acid; PG, prostaglandin; Rv, resolvin; TriHOME, trihydroxy-octadecenoic acid; TX, thromboxane.
Fig. 4.
Differences in serum oxylipins and fatty acids in rats given LA and La+ALA compared with control diets for 6 weeks. Oxylipins that are higher (black boxes) or lower (gray boxes) than control diet are denoted by shading. Sex effects in at least one of the diets are denoted by M (higher in males) or F (higher in females). Means, SEs, and P values are shown in supplemental Tables S7 and S10. d, deoxy; DiHETE, dihydroxy-eicosatetraenoic acid; DiHDoPE, dihydroxy-docosapentaenoic acid; DiHOME, dihydroxy-octadecenoic acid; EpDPE, epoxy-eicosadocosapentaenoic acid; EpOME, epoxy-octadecenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETrE, hydroxy-eicosatrienoic acid; HOTrE, hydroxy-octadecatrienoic acid; HX, hepoxilin; k, keto; LT, leukotriene; oxoETE, oxo-eicosatetraenoic acid; oxoODE, oxo-octadecadienoic acid; oxoOTrE, oxo-octadecatrienoic acid; PG, prostaglandin; t, trans; TriHOME, trihydroxy-octadecenoic acid; TX, thromboxane.
Higher dietary LA results in lower levels of n-3 PUFA-derived oxylipins
A higher level of dietary LA had fewer effects on n-3 PUFA-derived oxylipins that also differed in kidney, liver, and serum. In kidney, the LA compared with the control diet reduced EPA and DHA levels, but not ALA. Yet, only one oxylipin derived from DHA was lower in kidneys of LA fed rats (Fig. 2; supplemental Tables S5b, S8). In the liver, EPA was lower, but two ALA oxylipins were higher in the LA group, and one EPA oxylipin and one DHA oxylipin were lower (Fig. 3; supplemental Tables S6a, S9). In serum, EPA and DHA, but not ALA, were lower in the LA group, but only three EPA oxylipins were lower (Fig. 4; supplemental Tables S7a, S10). As a result, in kidney, liver, and serum, both the fatty acid and the oxylipin n-6/n-3 ratios were higher in the LA compared with the control group (Table 2).
TABLE 2.
The n-6/n-3 oxylipin and fatty acid ratios in kidney, liver, and serum of rats given control, LA and LA+ALA diets for 6 weeks
| Diet | Control | LA | LA+ALA | Diet | Sex | |||
| Female | Male | Female | Male | Female | Male | |||
| Oxylipin ratios | ||||||||
| Kidney | 3.81 ± 0.45B | 6.09 ± 0.60 | 7.71 ± 0.80A | 9.72 ± 0.77 | 4.53 ± 0.60B | 7.54 ± 0.77 | <0.0001 | 0.0002 |
| Liver | 4.13 ± 0.40c | 4.2 ± 0.15c | 6.61 ± 0.49b | 9.72 ± 0.66a | 5.31 ± 0.59bc | 5.23 ± 0.28c | 0.0022a | |
| Serum | 13.5 ± 2.34B | 14.4 ± 0.92 | 18.1 ± 1.16A | 20 ± 2.63 | 8.93 ± 0.62C | 12.2 ± 1.07 | <0.0001 | |
| Fatty acid ratios | ||||||||
| Kidney | 8.59 ± 0.246C | 16.2 ± 0.545 | 12.7 ± 0.897A | 22 ± 0.745 | 9.73 ± 0.493B | 16.9 ± 0.293 | <0.0001 | <0.0001 |
| Liver | 3.28 ± 0.072d | 4.55 ± 0.08bc | 4.32 ± 0.264c | 6.82 ± 0.532a | 3.34 ± 0.093d | 5.31 ± 0.252b | <0.0001 | |
| Serum | 7.35 ± 0.515B | 9.50 ± 0.581 | 11.9 ± 0.461A | 14.9 ± 0.811 | 7.48 ± 0.221B | 8.94 ± 0.717 | <0.0001 | 0.0001 |
Differing uppercase superscript letters shown on the female values within a row indicate differences between diets. Differing lowercase superscript letters within a row indicate differences between means.
P value for diet × sex interaction or for Wilcoxin’s test P value if the data were not normally distributed.
Higher LA with ALA, together, mitigates some, but not all, effects on n-6 oxylipins
When both LA and ALA were increased in the diet to maintain a similar LA/ALA ratio, the effect of elevating n-6 oxylipins followed the same pattern as higher dietary LA alone, although fewer n-6 PUFA-derived oxylipins were elevated. In the kidney, LA, EDA, and GLA (in males) were higher in the LA+ALA compared with control-fed rats; this treatment resulted in higher levels of oxylipins derived from LA (5), AA (4), and one each from GLA and DGLA, as well as higher levels of total LA (121% higher), total AA (24% higher), and total n-6 PUFA-derived oxylipins (82% higher) (Fig. 2; supplemental Tables S5a, S8). In liver, the LA+ALA diet increased only LA and EDA, but increased n-6 PUFA oxylipins derived from LA (7), AA (5), and EDA (1), as well as resulting in higher levels of total LA (150% higher) and total n-6 PUFA-derived oxylipins (59% higher) (Fig. 3; supplemental Tables S6a, S9). Similar to the fewer effects of the LA diet on n-6 PUFA-derived oxylipins in serum compared with kidney and liver, the effects of LA+ALA in serum on these oxylipins were also fewer: only LA and three LA-derived oxylipins were higher in serum of rats given the high LA+ALA diet compared with the control diet (Fig. 3; supplemental Tables S7a, S10).
Higher LA with ALA, together, results in higher n-3 oxylipins, even when n-3 PUFAs are not higher
The LA+ALA diet had a much greater effect on n-3 PUFA-derived oxylipins than the LA diet when both were compared with the control diet, particularly in the kidney. In the kidney, the LA+ALA diet increased three ALA, four EPA, and seven DHA oxylipins, as well as total ALA (186% higher), total EPA (46% higher), total DHA (38% higher), and total n-3 PUFA-derived oxylipins (55% higher) when compared with the control group. These higher levels of EPA and DHA oxylipins were present even though there were no differences in EPA or DHA levels between the LA+ALA and control groups (Fig. 2; supplemental Tables S5b, S8). In the liver and serum, the LA+ALA diet also affected n-3 PUFA-derived oxylipins, but the differences were fewer than in the kidney. In the liver, only three ALA oxylipins and one EPA oxylipin, as well as total ALA-derived oxylipins were higher (by 278%) when compared with the control diet. Again, these were higher despite a lack of any differences in the levels of ALA or EPA between these two groups (Fig. 3; supplemental Tables S6b, S9). In serum, ALA was increased by the LA+ALA diet, and so were two ALA as well as total ALA oxylipins (202% higher), while neither EPA nor DHA, nor their oxylipin levels, were different in rats given the LA+ALA compared with the control diet (Fig. 4; supplemental Tables S7b, S10). Therefore, in contrast to the higher n-6/n-3 fatty acid and oxylipin ratios in the LA compared with control groups, in the LA+ALA group, these ratios were more similar to the control group (Table 2).
Oxylipins with sex effects are higher in males
Sex differences were observed in ∼40% (30/69 oxylipins in kidney, 32/71 in liver, and 23/60 in serum) of oxylipins (Figs. 2–4, supplemental Tables S5–S7). Out of these, almost all were higher in males, with the following exceptions: in kidney, 20-HETE, 5,6-dihydroxy-eicosatrienoic acid (DiHETrE) in the LA+ALA group, and all DHA-derived oxylipins with sex effect were higher in females; in liver, PGA2 in the LA group, tetranor-12-HETE, 12-oxoETE, 20-carboxy-AA, PGF1α, and 13-hydroxy-docosahexaenoic acid (HDoHE) were higher in females; in serum, 12,13-DiHODE and PGF3α were higher in females. In comparison, the only PUFAs that were higher in males were DGLA in kidney, LA, GLA, and DGLA in liver, and AdA in serum, while in females all three n-3 PUFAs were higher in kidney, AA and DHA were higher in liver, and GLA, AA, EPA, and DHA were higher in serum (Figs. 2–4, supplemental Tables S7–S10).
To account for the differing PUFA levels on oxylipin levels, the ratios of oxylipins to parent PUFAs were determined for oxylipins formed from multiple PUFAs via the following pathways: COX/PGE synthase, 5-LOX, 12-LOX, 15-LOX, CYP hydroxylase (PUFA hydroxylated at n-2 selected), and CYP epoxygenase. These analyses revealed that oxylipin/PUFA ratios in males were greater than or similar to females for all oxylipins for all PUFA substrates (except for the serum 12,13-DiHODE/ALA ratio). This was also the case for DHA-derived oxylipins in kidneys, which were higher in females despite having greater than or similar oxylipin/DHA levels for males. Oxylipin/PUFAs for liver 5-LOX in rats provided control diets is shown in Fig. 5 as an example; data for other enzymes for kidney, liver, and serum and all diet groups are in supplemental Table S11.
Fig. 5.
Oxylipin/PUFA ratios for 5-LOX in liver in rats given control diets for 6 weeks. Differing letters above each set of bars indicate differences between oxylipin/PUFA ratios (P < 0.05). For all ratios [COX/PGE synthase, 5- LOX, 12- LOX, 15-LOX, CYP hydroxylase (PUFA hydroxylated at n-2 selected), and CYP epoxygenase] for all control, LA, and LA+ALA diets in kidney, liver and serum, see supplemental Table S11.
Oxylipin to PUFA ratios are n-3 > n-6 and 18-carbon ≥ 20-carbon ≥ 22-carbon
The oxylipin to PUFA ratios were also used to examine the relative levels of oxylipins compared with their precursor PUFAs. The pattern was similar in all pathways, with relative oxylipin levels being higher in oxylipins derived from n-3 compared with n-6 PUFAs. In addition, the order of oxylipin/PUFA ratios was 18-carbon ≥ 20-carbon ≥ 22-carbon PUFAs, with the exception of the 15-LOX pathway, where 20-HDoHE/DHA was higher than 18-hydroxy-eicosapentaenoic acid (HEPE)/EPA in kidney and serum (Fig. 5, supplemental Table S11).
DISCUSSION
The oxylipin profiles of rat kidney, liver, and serum presented herein provide fundamental data on these bioactive lipids. These compositional data complement the vast fatty acid literature that currently exists and provides an expanded perspective of the physiological effects of lipids based on fatty acid profiles. It also points to the imperative for greater understanding of the functions of oxylipins individually and in combination in the context of tissue-specific profiles, as our understanding of the functions and relative bioactivities of many oxylipins is still limited. While the rat tissue oxylipin profiles generally were similar to the PUFA profiles, notable differences were observed that have implications for how fatty acid data are interpreted.
Although large changes in dietary LA have been shown to alter LA oxylipin production, the current study demonstrates that more moderate differences in dietary LA also affect LA oxylipins. Prior studies that have shown increased LA oxylipins with increased dietary LA have compared diets ranging from 4% to 40% of energy from LA in obese rats (4), from 0.4% to 10.5% in normal rats (31), and from 2.4% to 6.7% in humans (32). In the current study, dietary LA levels were 4.7% in the adequate diet and 11.4–11.8% in the LA and LA+ALA diets, similar to the 5–10% of energy range of dietary LA that is recommended by the Institute of Medicine (8). Thus dietary LA changes around the normal range of intake levels influences LA oxylipins, even when tissue LA levels may not be altered.
Because only extreme differences in LA intake have previously been documented to alter a small number of tissue AA oxylipins (4, 14, 15, 33), and because there is strong evidence that dietary LA does not alter blood AA levels (16), it has been concluded that dietary LA has minimal effects on AA oxylipins. The current findings, however, demonstrate that increased dietary LA within the usual dietary range resulted in higher levels of 10–12 AA oxylipins in kidney and liver, as well as many LA, GLA, EDA, DGLA, and AdA oxylipins in these tissues, and LA oxylipins in serum. This may have implications for the current controversy on whether dietary LA levels should be increased in the Western diet. Over the past half century, dietary LA consumption in the US has more than doubled (5, 6), and current recommendations sanctioned by the American Heart Association suggest that increasing these levels to over 10% of energy may confer a cardiovascular benefit (10). On the other hand, n-6 (compared with n-3) PUFAs are generally considered to have pro-inflammatory characteristics and caution in increasing the level of these PUFAs also has been suggested (34–36). However, attempts to find a pro-inflammatory effect of dietary LA using classical cytokine and chemokine inflammatory markers have largely failed to demonstrate an increase in these biomarkers (37). The current study reveals that AA and other n-6 PUFA-derived oxylipins with generally pro-inflammatory effects are elevated in tissues of normal rats provided a higher LA diet.
Further, higher dietary LA also resulted in lower levels of a small number of n-3 PUFA-derived oxylipins, as previously shown with more extreme changes in dietary LA (14, 31). Thus, the increase in n-6 PUFA oxylipins resulted in an increase (by 37–96%) in the ratio of oxylipins derived from n-6 to n-3 PUFAs, a change that would also be expected to increase inflammation, as n-3 oxylipins tend to have anti-inflammatory properties (38). Increasing the ALA content of the diet in the LA+ALA diet to maintain the same LA/ALA ratio as in the control diet mitigated some of the higher n-6 PUFA oxylipin levels even though tissue LA levels were not different between the LA and LA+ALA diets. This change in n-6 PUFA oxylipins in combination with the higher levels of many n-3 PUFA oxylipins in tissues and serum from rats given the LA+ALA diet resulted in a lower or similar ratio of n-6/n-3 oxylipins to that of the control diet, indicating that a small amount of ALA may counteract the effects of a high LA diet.
The mechanism by which oxylipins are altered without a corresponding change in their precursor PUFA levels in tissue phospholipid remains to be explored. It may be that the PUFA pool that provides precursors for oxylipin synthesis is not reflected in tissue phospholipid or serum total PUFA content. In addition, the PUFA amount is two to three orders of magnitude greater than the oxylipin level in tissues, so only a very small portion of PUFA is converted to oxylipins. There is also some evidence that phospholipid is not the only possible source of tissue oxylipins (39). If other lipid classes are also direct sources of oxylipins, or are indirect sources via flux of fatty acids through phospholipids, then the phospholipid fatty acid composition may not necessarily reflect oxylipin levels. The conversion of PUFAs to oxylipins and/or their degradation also may be altered differently with different dietary PUFAs, as indicated by the differences in the oxylipin/PUFA ratios for different PUFAs metabolized by the same enzymes. Other metabolic pathways, including β-oxidation of fatty acids and conversion of fatty acids to other fatty acids and lipid mediators, also possibly contribute to differences in the levels of the products and precursors (40–42).
Interestingly, the higher n-3-derived oxylipins in kidneys from rats given the LA+ALA diets compared with both the control and the LA groups resulted in higher levels of not only ALA, but also EPA and DHA oxylipins (despite no differences in EPA or DHA). This is consistent with our studies in models of renal disease that have demonstrated increased DHA and EPA oxylipins with flax oil feeding (3, 4). Although the conversion of ALA to DHA is considered to be very limited under normal conditions, DHA levels can be increased by dietary ALA up to a plateau (43). A similar phenomenon may be occurring at the oxylipin production level and whether DHA oxylipins also reach a plateau remains to be determined. The relative efficacy of dietary ALA compared with EPA or DHA in increasing their oxylipins also needs to be elucidated, as studies suggest that the change in EPA and DHA oxylipins with dietary fish oil is much greater than the relatively small differences observed in the current study (44–46).
The effect of sex on oxylipin levels in tissues is largely unexplored and the reports that are in the literature have examined only individual or small numbers of oxylipins in a number of tissues, with no general pattern being apparent. Approximately 40% of all oxylipins detected in the current analysis displayed sex effects and, of these, ∼90% were higher in males. Further, when the level of oxylipins was standardized relative to the precursor PUFA level (i.e., oxylipin/PUFA ratios), almost all oxylipins that had a sex effect were higher in males.
Because sex differences in oxylipin profiles have not previously been reported, the rationale for this has not been explored, but may be due to several factors. One reason may be the level of substrate PUFAs, as was the case with higher DHA oxylipins in female kidneys. In this case, the much higher renal DHA levels in female kidneys was associated with higher DHA oxylipins, even though renal oxylipin/DHA were either higher or not different in males compared with females. Estrogen increases delta-6 desaturase expression and liver and plasma DHA levels in rats (20), likely providing some explanation for the higher DHA and subsequent formation of DHA oxylipins in female kidneys. However, in many cases, sex differences in PUFA and oxylipin levels were not correlated. Sex hormone effects on enzymes involved in oxylipin synthesis or metabolism have been observed, and some examples may explain some of the findings herein: increased renal 5-LOX in ovariectomized rats with metabolic syndrome (23); inhibition of liver COX-2 activity (24); and increased TxA2 excretion in spontaneously hypertensive female rats (21). On the other hand, there are also examples in the literature that appear to conflict with the current findings: estrogen induces 12- and 15-LOX activity in vascular smooth muscle cells and upregulates COX-1 expression in pulmonary artery endothelium (25); peritoneal exudates of female mice have higher levels of LTs (47).
One of the exceptions to the finding of higher oxylipin levels in males was the higher level of 20-HETE or its metabolite (20-carboxy-AA) in females. This finding also requires further exploration because, while renal 20-HETE levels have been reported to increase differentially during different phases of pregnancy in rats (48), another report demonstrated that androgen treatment in male CYP 4a14 knockout mice increases 20-HETE levels (49). Thus, while the current data provides original data on sex differences in oxylipins in the rat, much work remains to understand and elucidate these differences.
The oxylipin/PUFA ratios also provide information on in vivo PUFA selectivity of enzymes involved in the synthesis and/or degradation of oxylipins. The n-3/n-6 oxylipin ratios were higher than the precursor n-3/n-6 PUFA ratios, indicating increased conversion and/or decreased degradation of n-3 compared with n-6 oxylipins, relative to their precursor PUFAs. Similar findings were shown in kidneys of obese rats (4) and in CYP metabolites in the blood and urine of normal volunteers supplemented with fish oil (50). While the selectivity of CYP products by n-3 over n-6 PUFAs has been supported by in vitro analysis of various CYP isoforms (44), opposite selectivity has been observed in vitro in other studies of LOX and COX enzymes (51–54), indicating the complexity of the regulation of these pathways. The discrepancy may also be due to the fact that oxylipin/PUFA ratios not only reflect synthesis but also may reflect degradation. Additionally, the oxylipin/PUFA ratios for tissue C18 PUFAs compared with their longer-chain PUFA counterparts were higher, also consistent with previous findings in obese rat kidneys (4). As a result, C18-derived oxylipins are present in much higher amounts than would be predicted from their fatty acid levels, suggesting that they may have a greater impact on physiology. Although much less is known about the physiological effects of C18 compared with C20- and C22-derived oxylipins, they have been associated with varied effects that are either protective or harmful in a number of states, including inflammation in processes associated with atherosclerosis, mitogenic effects associated with cancer, pain sensitivity, and with obesity and the metabolic syndrome (55, 56).
Although the data presented herein provide fundamental data on oxylipins, several important limitations exist. Over 160 oxylipins were scanned for, but there are more metabolites that are known, but for which standards are unavailable, as well as potentially many other oxylipins that are yet to be discovered. This also has implications for the n-6/n-3 oxylipin ratios calculated herein, and it is possible that unknown oxylipins not measured could impact these ratios. In addition, the oxylipins analyzed are those that are present in free form and therefore presumably the bioactive forms, but it does not include those esterified to tissue lipids. Hydrolysis procedures that are currently used to cleave these bound oxylipins result in degradation of certain classes of oxylipins and potential formation of artifacts, thus limiting the use of this approach (57). Furthermore, solid phase extraction methods for oxylipins vary in their efficiency, with some favoring some types of oxylipins over others (58). The method used herein is one of the most efficient overall, but there are some oxylipins (e.g., some of the AA-derived CYP products) that are not extracted, as well as by other procedures (58).
In conclusion, these data provide the first comprehensive profile of oxylipins in rat kidney, liver, and serum, and demonstrate that PUFA and oxylipin profiles can be highly divergent. Dietary LA increases many LA and AA oxylipins and reduces some n-3 PUFA-derived oxylipins, and dietary ALA mitigates many of these effects. Further, higher levels of oxylipins in male rats may have important physiological effects that remain to be elucidated. Tissue n-3 compared with n-6, and C18 compared with C20 and C22 PUFAs, appear to increase oxylipins more efficiently in vivo. Blood oxylipin profiles do not necessarily reflect tissue profiles, which has implications for human studies that typically utilize blood as the primary and often only tissue sampled. Future studies of dietary lipids that include oxylipin analysis will therefore provide a greater understanding of their physiological effects.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AdA
- adrenic acid
- ALA
- α-linolenic acid
- COX
- cyclooxygenase
- CYP
- cytochrome P450
- DGLA
- dihomo-γ-linolenic acid
- DiHETrE
- dihydroxy-eicosatrienoic acid
- EDA
- eicosadienoic acid
- GLA
- γ-linolenic acid
- HDoHE
- hydroxy-docosahexaenoic acid
- HEPE
- hydroxy-eicosapentaenoic acid
- LA
- linoleic acid
- LOX
- lipoxygenase
- LT
- leukotriene
- PG
- prostaglandin
- TX
- thromboxane
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant 217412. Additional partial support was provided by the University of Manitoba Graduate Enhancement of Tri-Council Stipends (GETS) program (S.L.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gabbs M., Leng S., Devassy J. G., Monirujjaman M., and Aukema H. M.. 2015. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 6: 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deems R., Buczynski M. W., Bowers-Gentry R., Harkewicz R., and Dennis E. A.. 2007. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 432: 59–82. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi T., Devassy J. G., Gabbs M., Ravandi A., Nagao S., and Aukema H. M.. 2015. Dietary flax oil rich in alpha-linolenic acid reduces renal disease and oxylipin abnormalities, including formation of docosahexaenoic acid derived oxylipins in the CD1-pcy/pcy mouse model of nephronophthisis. Prostaglandins Leukot. Essent. Fatty Acids. 94: 83–89. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri S. P., Love K., Winter T., Gauthier J., Taylor C. G., Blydt-Hansen T., Zahradka P., and Aukema H. M.. 2013. Dietary linoleic acid and alpha-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J. Nutr. 143: 1421–1431. [DOI] [PubMed] [Google Scholar]
- 5.Blasbalg T. L., Hibbeln J. R., Ramsden C. E., Majchrzak S. F., and Rawlings R. R.. 2011. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93: 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyenet S. J., and Carlson S. E.. 2015. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv. Nutr. 6: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aranceta J., and Perez-Rodrigo C.. 2012. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: a systematic review. Br. J. Nutr. 107 (Suppl. 2): S8–S22. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. National Academies Press, Washington, DC. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services and US Department of Agriculture. 2015. Dietary Guidelines for Americans 2015–2020. Accessed May 20, 2017, at http://health.gov/dietaryguidelines/2015/guidelines/.
- 10.Harris W. S., Mozaffarian D., Rimm E., Kris-Etherton P., Rudel L. L., Appel L. J., Engler M. M., Engler M. B., and Sacks F.. 2009. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 119: 902–907. [DOI] [PubMed] [Google Scholar]
- 11.Devassy J. G., Leng S., Gabbs M., Monirujjaman M., and Aukema H. M.. 2016. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 7: 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall R., Ross R. P., Fitzgerald G. F., and Stanton C.. 2010. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 68: 280–289. [DOI] [PubMed] [Google Scholar]
- 13.Calder P. C. 2015. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 1851: 469–484. [DOI] [PubMed] [Google Scholar]
- 14.Ramsden C. E., Ringel A., Majchrzak-Hong S. F., Yang J., Blanchard H., Zamora D., Loewke J. D., Rapoport S. I., Hibbeln J. R., Davis J. M., et al. 2016. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: implications for idiopathic pain syndromes? Mol. Pain. 12: 1744806916636386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taha A. Y., Blanchard H. C., Cheon Y., Ramadan E., Chen M., Chang L., and Rapoport S. I.. Dietary linoleic acid lowering reduces lipopolysaccharide-induced increase in brain arachidonic acid metabolism. Mol. Neurobiol. Epub ahead of print. Jun 23, 2016; doi:10.1007/s12035-016-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rett B. S., and Whelan J.. 2011. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr. Metab. (Lond). 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onat A., Karadeniz Y., Tusun E., Yuksel H., and Kaya A.. 2016. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev. Cardiovasc. Ther. 14: 513–523. [DOI] [PubMed] [Google Scholar]
- 18.Marks K. A., Kitson A. P., Shaw B., Mutch D. M., and Stark K. D.. 2013. Stearoyl-CoA desaturase 1, elongase 6 and their fatty acid products and precursors are altered in ovariectomized rats with 17beta-estradiol and progesterone treatment. Prostaglandins Leukot. Essent. Fatty Acids. 89: 89–96. [DOI] [PubMed] [Google Scholar]
- 19.Baker E. J., Miles E. A., Burdge G. C., Yaqoob P., and Calder P. C.. 2016. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 64: 30–56. [DOI] [PubMed] [Google Scholar]
- 20.Kitson A. P., Marks K. A., Shaw B., Mutch D. M., and Stark K. D.. 2013. Treatment of ovariectomized rats with 17beta-estradiol increases hepatic delta-6 desaturase enzyme expression and docosahexaenoic acid levels in hepatic and plasma phospholipids. Prostaglandins Leukot. Essent. Fatty Acids. 89: 81–88. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan J. C., Sasser J. M., Pollock D. M., and Pollock J. S.. 2005. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 45: 406–411. [DOI] [PubMed] [Google Scholar]
- 22.Cagen L. M., and Baer P. G.. 1987. Effects of gonadectomy and steroid treatment on renal prostaglandin 9-ketoreductase activity in the rat. Life Sci. 40: 95–100. [DOI] [PubMed] [Google Scholar]
- 23.Zúñiga-Muñoz A. M., Guarner Lans V., Soria-Castro E., Diaz-Diaz E., Torrico-Lavayen R., Tena-Betancourt E., and Pérez-Torres I.. 2015. 17beta estradiol modulates perfusion pressure and expression of 5-LOX and CYP450 4A in the isolated kidney of metabolic syndrome female rats. Int. J. Endocrinol. 2015: 149408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassouna A., Obaia E., Marzouk S., Rateb M., and Haidara M.. 2014. The role of sex hormones in induced-systemic inflammation in female albino rats. Acta Physiol. Hung. 101: 112–127. [DOI] [PubMed] [Google Scholar]
- 25.Somjen D., Kohen F., Limor R., Sharon O., Knoll E., Many A., and Stern N.. 2016. Estradiol-17beta increases 12- and 15-lipoxygenase (type2) expression and activity and reactive oxygen species in human umbilical vascular smooth muscle cells. J. Steroid Biochem. Mol. Biol. 163: 28–34. [DOI] [PubMed] [Google Scholar]
- 26.Aukema H. M., Winter T., Ravandi A., Dalvi S., Miller D. W., and Hatch G. M.. 2016. Generation of bioactive oxylipins from exogenously added arachidonic, eicosapentaenoic and docosahexaenoic acid in primary human brain microvessel endothelial cells. Lipids. 51: 591–599. [DOI] [PubMed] [Google Scholar]
- 27.Hall L. M., and Murphy R. C.. 1998. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass Spectrom. 9: 527–532. [DOI] [PubMed] [Google Scholar]
- 28.Sankaran D., Lu J., Bankovic-Calic N., Ogborn M. R., and Aukema H. M.. 2004. Modulation of renal injury in pcy mice by dietary fat containing n-3 fatty acids depends on the level and type of fat. Lipids. 39: 207–214. [DOI] [PubMed] [Google Scholar]
- 29.Henderson W. R. Jr., Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., et al. 2007. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204: 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonventre J. V., Huang Z., Taheri M. R., O’Leary E., Li E., Moskowitz M. A., and Sapirstein A.. 1997. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 390: 622–625. [DOI] [PubMed] [Google Scholar]
- 31.Taha A. Y., Hennebelle M., Yang J., Zamora D., Rapoport S. I., Hammock B. D., and Ramsden C. E.. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot. Essent. Fatty Acids. Epub ahead of print. May 11, 2016; doi:10.1016/j.plefa.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsden C. E., Ringel A., Feldstein A. E., Taha A. Y., MacIntosh B. A., Hibbeln J. R., Majchrzak-Hong S. F., Faurot K. R., Rapoport S. I., Cheon Y., et al. 2012. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot. Essent. Fatty Acids. 87: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adam O., Tesche A., and Wolfram G.. 2008. Impact of linoleic acid intake on arachidonic acid formation and eicosanoid biosynthesis in humans. Prostaglandins Leukot. Essent. Fatty Acids. 79: 177–181. [DOI] [PubMed] [Google Scholar]
- 34.Ramsden C. E., Hibbeln J. R., Majchrzak S. F., and Davis J. M.. 2010. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br. J. Nutr. 104: 1586–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder P. C. 2010. The American Heart Association advisory on n-6 fatty acids: evidence based or biased evidence? Br. J. Nutr. 104: 1575–1576. [DOI] [PubMed] [Google Scholar]
- 36.Calder P. C., and Deckelbaum R. J.. 2011. Harmful, harmless or helpful? The n-6 fatty acid debate goes on. Curr. Opin. Clin. Nutr. Metab. Care. 14: 113–114. [DOI] [PubMed] [Google Scholar]
- 37.Johnson G. H., and Fritsche K.. 2012. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J. Acad. Nutr. Diet. 112: 1029–1041. [DOI] [PubMed] [Google Scholar]
- 38.Zivkovic A. M., Telis N., German J. B., and Hammock B. D.. 2011. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif. Agric. (Berkeley). 65: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dichlberger A., Schlager S., Maaninka K., Schneider W. J., and Kovanen P. T.. 2014. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J. Lipid Res. 55: 2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witkamp R. 2016. Fatty acids, endocannabinoids and inflammation. Eur. J. Pharmacol. 785: 96–107. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J. Y., Kothapalli K. S., and Brenna J. T.. 2016. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care. 19: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdge G. C., Finnegan Y. E., Minihane A. M., Williams C. M., and Wootton S. A.. 2003. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br. J. Nutr. 90: 311–321. [DOI] [PubMed] [Google Scholar]
- 43.Domenichiello A. F., Kitson A. P., Metherel A. H., Chen C. T., Hopperton K. E., Stavro P. M., and Bazinet R. P.. 2017. Whole-body docosahexaenoic acid synthesis-secretion rates in rats are constant across a large range of dietary alpha-linolenic acid intakes. J. Nutr. 147: 37–44. [DOI] [PubMed] [Google Scholar]
- 44.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 285: 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schebb N. H., Ostermann A. I., Yang J., Hammock B. D., Hahn A., and Schuchardt J. P.. 2014. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat. 113–115: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundström S. L., Yang J., Brannan J. D., Haeggström J. Z., Hammock B. D., Nair P., O’Byrne P., Dahlén S. E., and Wheelock C. E.. 2013. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol. Nutr. Food Res. 57: 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi A., Pergola C., Pace S., Radmark O., Werz O., and Sautebin L.. 2014. In vivo sex differences in leukotriene biosynthesis in zymosan-induced peritonitis. Pharmacol. Res. 87: 1–7. [DOI] [PubMed] [Google Scholar]
- 48.Wang M. H., Zand B. A., Nasjletti A., and Laniado-Schwartzman M.. 2002. Renal 20-hydroxyeicosatetraenoic acid synthesis during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282: R383–R389. [DOI] [PubMed] [Google Scholar]
- 49.Holla V. R., Adas F., Imig J. D., Zhao X., Price E. Jr., Olsen N., Kovacs W. J., Magnuson M. A., Keeney D. S., Breyer M. D., et al. 2001. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc. Natl. Acad. Sci. USA. 98: 5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D. N., Rothe M., et al. 2014. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 55: 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikei K. N., Yeung J., Apopa P. L., Ceja J., Vesci J., Holman T. R., and Holinstat M.. 2012. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J. Lipid Res. 53: 2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong L., Zou H., Yuan C., Hong Y. H., Kuklev D. V., and Smith W. L.. 2016. Different fatty acids compete with arachidonic acid for binding to the allosteric or catalytic subunits of cyclooxygenases to regulate prostanoid synthesis. J. Biol. Chem. 291: 4069–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malkowski M. G., Thuresson E. D., Lakkides K. M., Rieke C. J., Micielli R., Smith W. L., and Garavito R. M.. 2001. Structure of eicosapentaenoic and linoleic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J. Biol. Chem. 276: 37547–37555. [DOI] [PubMed] [Google Scholar]
- 54.Laneuville O., Breuer D. K., Xu N., Huang Z. H., Gage D. A., Watson J. T., Lagarde M., DeWitt D. L., and Smith W. L.. 1995. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)-octadecatrienoic acids from alpha-linolenic acid. J. Biol. Chem. 270: 19330–19336. [DOI] [PubMed] [Google Scholar]
- 55.Vangaveti V. N., Jansen H., Kennedy R. L., and Malabu U. H.. 2016. Hydroxyoctadecadienoic acids: oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 785: 70–76. [DOI] [PubMed] [Google Scholar]
- 56.Wang W., Yang J., Yang H., Sanidad K. Z., Hammock B. D., Kim D., and Zhang G.. 2016. Effects of high-fat diet on plasma profiles of eicosanoid metabolites in mice. Prostaglandins Other Lipid Mediat. 127: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willenberg I., Ostermann A. I., and Schebb N. H.. 2015. Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC-MS analysis of oxylipins. Anal. Bioanal. Chem. 407: 2675–2683. [DOI] [PubMed] [Google Scholar]
- 58.Ostermann A. I., Willenberg I., and Schebb N. H.. 2015. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Anal. Bioanal. Chem. 407: 1403–1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.