Abstract
Blood eosinophil counts and serum periostin levels are biomarkers of type 2 inflammation. Although serum levels of HDL and apoA-I have been associated with less severe airflow obstruction in asthma, it is not known whether serum lipids or lipoprotein particles are correlated with type 2 inflammation in asthmatics. Here, we assessed whether serum lipids and lipoproteins correlated with blood eosinophil counts or serum periostin levels in 165 atopic asthmatics and 163 nonasthmatic subjects with and without atopy. Serum lipids and lipoproteins were quantified using standard laboratory assays and NMR spectroscopy. Absolute blood eosinophils were quantified by complete blood counts. Periostin levels were measured using the Elecsys® periostin assay. In atopic asthmatics, blood eosinophils negatively correlated with serum HDL cholesterol and total HDL particles measured by NMR spectroscopy (HDLNMR). Serum periostin levels negatively correlated with total HDLNMR. In contrast, blood eosinophil counts positively correlated with serum triglyceride levels. This study demonstrates for the first time that HDL particles were negatively correlated, whereas serum triglycerides were positively correlated, with blood eosinophils in atopic asthmatics. This supports the concept that serum levels of HDL and triglycerides may be linked to systemic type 2 inflammation in atopic asthma.
Keywords: apolipoproteins, immunology, clinical studies, lung, inflammation, eosinophils, periostin, asthma
A type 2 high asthma endotype has been identified that is characterized by airway and systemic eosinophilic inflammation (1–3). Biomarkers to define type 2 high asthma include blood eosinophil counts and serum periostin levels. Blood eosinophil counts correlate with disease severity in eosinophil-predominant asthma and predict responsiveness to treatment with anti-interleukin (IL)-5 monoclonal antibodies (4–17). Increased serum levels of periostin, which can be induced by IL-13 in airway epithelial cells (18–20), have also been used to identify type 2 high asthmatics for subgroup analysis in clinical trials of anti-IL-13 monoclonal antibody therapy (21–24).
There is an increasing recognition that apolipoprotein pathways may participate in the pathogenesis of lung disease (25). Both apoE and apoA-I mimetic peptides have been shown to suppress type 2 inflammation in murine models of allergen-induced airway disease (26–28). Endothelial lipase, which is a phospholipase that hydrolyzes phospholipids and promotes the catabolism of HDL particles, is expressed in the lung where it may modulate eosinophilic inflammation. Consistent with this, allergen-challenged endothelial lipase knockout mice have high serum HDL levels and decreased eosinophilic lung inflammation (29). Among obese adolescent asthmatics, serum HDL levels have been inversely correlated with monocyte activation and numbers of patrolling monocytes (30). We have also recently shown that serum levels of HDL cholesterol (HDL-C), large HDL particles measured by NMR spectroscopy (HDLNMR particles), and apoA-I are positively associated with forced expiratory volume in 1 s (FEV1) in atopic asthmatics, whereas a negative association existed with LDL cholesterol (LDL-C), triglycerides, and apoB (31). This suggests that a link may exist between airflow obstruction and serum lipids and lipoproteins in atopic asthmatics. Here, we hypothesized that similar associations might exist between serum lipids or lipoproteins and the type 2 inflammatory markers, blood eosinophils, and serum periostin in asthmatic and nonasthmatic subjects.
MATERIALS AND METHODS
Study population
Informed consent was obtained from 163 healthy nonasthmatic subjects and 165 clinically stable atopic asthmatics for participation in institutional review board-approved protocols, 96-H-0100 and 13-H-0059, at the National Heart, Lung, and Blood Institute between 1999 and 2015. All subjects from these protocols who met criteria for the current study and had analyzable serum samples were included in the study. These subjects were current nonsmokers and had undergone chest radiography to exclude other lung diseases. Asthma was diagnosed according to National Heart, Lung, and Blood Institute guidelines (32) and severe asthma was defined using European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines (32, 33). All asthmatics demonstrated either reversible airflow obstruction following inhalation of a short-acting β2-agonist or airway hyperreactivity to methacholine bronchoprovocation testing, as well as a history and physical examination consistent with asthma. All nonasthmatic subjects demonstrated absence of airway hyperreactivity by methacholine bronchoprovocation challenge testing. Reported spirometry values were those obtained prior to administration of a bronchodilator.
Atopy was defined by a positive clinical history and skin test reactivity to at least one allergen or a history of severe allergy or anaphylaxis. A negative clinical history of allergy and negative allergy skin testing defined nonatopic subjects. Allergy skin testing at the National Institutes of Health was performed using a Multi-Test II® applicator (a generous gift from Lincoln Diagnostics, Decatur, IL) and an allergen panel that included cat dander, Dermatophagoides farinae, short ragweed, timothy grass, Aspergillus fumigatus, and cockroach antigen (HollisterStier, Spokane, WA).
Lipid, apolipoprotein, eosinophil, and periostin assays
Blood and serum samples were collected in the nonfasting state. Serum samples were stored at −80°C and thawed on ice prior to analysis. Standard laboratory tests and serum lipid profiles were performed in the Clinical Laboratory Improvement Amendment-certified National Institutes of Health Clinical Research Center Clinical Laboratory. The absolute number of blood eosinophils (cells per microliter) was quantified through complete blood counts with differential using an automated hematology analyzer. Serum apoA-I and apoB levels were measured using a Dimension Vista® Intelligent Lab system (Siemens Healthcare, Erlangen, Germany). Serum was diluted 1:10,000 in PBS prior to quantification of apoE using a commercially available ELISA with a lower limit of detection of 0.02 ng/ml (MABTECH Inc., Cincinnati, OH).
NMR spectroscopy was performed using a Vantera clinical analyzer (LipoScience Inc., Raleigh, NC). Serum periostin levels were measured using the Elecsys® periostin assay (Roche Diagnostics, Penzberg, Germany) (34). We utilized NMR spectroscopy to quantify lipoprotein particle subsets based upon size, as this approach has been proposed to be similar or superior to the standard measurement of HDL-C for the assessment of cardiovascular risk (35, 36). NMR spectroscopy is a method that analyzes distinct signals emitted from each lipoprotein particle class when exposed to electromagnetic pulses. A direct correlation exists between the NMR signal amplitude and the concentration of the lipoprotein particle, which can then be identified by comparison to a reference library of lipoproteins.
Statistics
The null hypothesis was that there were no significant correlations between blood eosinophil counts or serum periostin levels and serum lipids or lipoproteins (HDL-C, total and large HDLNMR particles, LDL-C, triglycerides, apoA-I, apoB, and apoE) in atopic asthmatics. Statistical analyses were performed using SAS Enterprise Guide version 4.3 (SAS Institute Inc., Cary, NC). All statistical tests were two-tailed and performed at the 0.05 α level. Results are presented as means ± SDs and medians (and interquartile range). Nonsymmetric or skewed variables were transformed to natural logarithms prior to further analysis. One-way ANOVA with Bonferroni correction was used to assess raw differences between groups. Correlation analyses were performed using Pearson correlation. Multivariable regression analysis by fitting linear models adjusted for predefined variables [age, sex, race, BMI, and C-reactive protein (CRP) levels] were utilized to assess group differences in serum lipid or lipoprotein levels. While multiplicity adjustment was applied to between group comparisons as stated above, adjustment was not applied to global hypothesis testing. Multivariable regressions with these same variables were also performed to assess relationships between eosinophils or periostin and serum lipid levels. Lastly, sensitivity analyses were performed by excluding race from the multivariable models, as well as to assess the effect of lipid profile modifying medication or inhaled corticosteroid use.
RESULTS
The study cohort consisted of three groups; 165 atopic asthmatics, 79 atopic nonasthmatics, and 84 nonatopic nonasthmatics (Table 1). As compared with nonatopic nonasthmatics, asthmatic subjects were older and had higher BMI, absolute blood eosinophil counts (hereafter referred to as eosinophils), serum total IgE, and serum CRP levels. Asthmatics also had lower FEV1 (percent predicted) and FEV1/forced vital capacity ratio as compared with both nonatopic nonasthmatics and atopic nonasthmatics. Atopic nonasthmatics had similar characteristics as nonatopic nonasthmatics, except that atopic nonasthmatics had higher total IgE and eosinophils. Serum periostin levels (hereafter referred to as periostin) did not differ between the three groups. Asthmatics and nonatopic nonasthmatics had proportionally more women as compared with atopic nonasthmatics.
TABLE 1.
Study group demographics
| NN (n = 84) | AN (n = 79) | AA (n = 165) | |
| Age | 32 ± 13.5 | 34 ± 12.4 | 37.3 ± 14.2a |
| Sex, female/male (%) | 59/25 (70/30) | 39/40 (49/51)b | 106/59 (64/36) |
| Race, white/black/other (%) | 56/17/11 (67/20/13) | 52/14/13 (66/18/16) | 101/44/20 (61/27/12) |
| BMI (kg/m2) | 25.5 ± 5.1 | 26 ± 5.1 | 28.6 ± 7.2c |
| Percent predicted FEV1 (%) | 110 ± 15 | 109 ± 13 | 86 ± 21c |
| FEV1/FVC ratio (%) | 85 ± 5 | 84 ± 6 | 71 ± 13c |
| IgE (IU/ml)e | 21.5 (9, 48)d | 78 (36, 221)d | 232 (96.5, 472.5)d |
| Eosinophils (per μl)e | 100 (63, 144.5)d | 134 (82, 216)d | 232 (138, 370)d |
| CRP (mg/l)e | 0.9 (0.38, 2.22) | 0.8 (0.43, 2.34) | 1.40 (0.6, 4.78)c |
| Serum periostin (ng/ml) | 50.8 ± 12.2 | 54.2 ± 16.6 | 54.7 ± 14.9 |
| Subjects taking LMM | 6 | 3 | 8 |
| Subjects using ICS | 0 | 0 | 76 |
Values shown are number of subjects (for sex and race) or means ± SDs unless otherwise noted. Significant differences between groups (P ≤ 0.05) were assessed by one-way ANOVA with Bonferroni t-tests. NN, nonatopic nonasthmatics; AN, atopic nonasthmatics; AA, atopic asthmatics; FVC, forced vital capacity; LMM, lipid profile-modifying medications.
AA is significantly different from NN.
AN is significantly different from both AA and NN.
AA is significantly different from both AN and NN.
All three groups differ from each other significantly.
Values shown are median (interquartile range).
Unadjusted standard lipid profile results are presented in Table 2. Asthmatics had significantly higher total cholesterol as compared with both nonatopic and atopic nonasthmatics and significantly higher serum LDL-C as compared with atopic nonasthmatics. After adjusting for differences in age, sex, race, BMI, and serum CRP, the only significant difference was a higher mean serum HDL-C in asthmatics as compared with nonatopic nonasthmatics (Table 3). Because there were no significant differences between atopic nonasthmatics and nonatopic nonasthmatics after adjusting for confounding factors, these were combined into a single nonasthmatic group, hereafter referred to as nonasthmatics, for the remaining analyses.
TABLE 2.
Standard clinical lipid profiles of the three study groups
| NN (n = 84) | AN (n = 79) | AA (n = 165) | |
| Total cholesterol (mg/dl) | 175 ± 33 | 174 ± 37 | 189 ± 36a |
| Triglycerides (mg/dl)c | 101 (70, 141) | 93 (71, 146) | 108 (76, 158) |
| LDL-C (mg/dl) | 102 ± 31 | 99 ± 29 | 110 ± 32b |
| apoE (μg/ml)c | 20 (13, 32) | 20 (12, 28) | 23 (15, 33) |
| HDL-C (mg/dl) | 50 ± 16 | 49 ± 17 | 54 ± 18 |
| apoA1 (mg/dl) | 168 ± 30 | 159 ± 31 | 170 ± 35 |
| apoB (mg/dl) | 86 ± 23 | 85 ± 24 | 91 ± 23 |
Values shown are means ± SDs except as noted. Significant differences between groups (P ≤ 0.05) were assessed by one-way ANOVA with Bonferroni t-tests. NN, nonatopic nonasthmatics; AN, atopic nonasthmatics; AA, atopic asthmatics.
AA is significantly different from both AN and NN.
AA is significantly different from AN.
Values shown are median (interquartile range).
TABLE 3.
Adjusted means for the standard clinical lipid profile from multivariable regression analysis
| NN [mean (95% CI)] | n | AN [mean (95% CI)] | n | AA [mean (95% CI)] | n | |
| Total cholesterol (mg/dl) | 178 (171, 186) | 84 | 177 (169, 184) | 79 | 186 (181, 192) | 164 |
| Triglycerides (mg/dl) | 117 (105, 130) | 84 | 109 (98, 121) | 79 | 112 (103, 121) | 164 |
| LDL-C (mg/dl) | 107 (99, 114) | 83 | 103 (95, 110) | 77 | 110 (104, 115) | 163 |
| apoE (μg/ml) | 23 (20, 26) | 78 | 20 (17, 23) | 68 | 24 (21, 26) | 139 |
| HDL-C (mg/dl) | 45 (42, 49) | 84 | 48 (44, 51) | 79 | 52 (49, 54)a | 164 |
| apoA-I (mg/dl) | 161 (154, 168) | 84 | 157 (151, 164) | 79 | 164 (159, 169) | 164 |
| apoB (mg/dl) | 92 (87, 97) | 84 | 88 (83, 92) | 79 | 91 (88, 95) | 164 |
Adjusted (least squares) means for clinical lipid profile parameters for the three groups, from multivariable regression using linear models incorporating age, BMI, race, sex, and CRP as covariates. NN, nonatopic nonasthmatics; AN, atopic nonasthmatics; AA, atopic asthmatics.
AA and AN are no different from NN with regard to all lipid panel parameters except HDL-C, which is significantly higher (P < 0.05) in the AA group compared with NN.
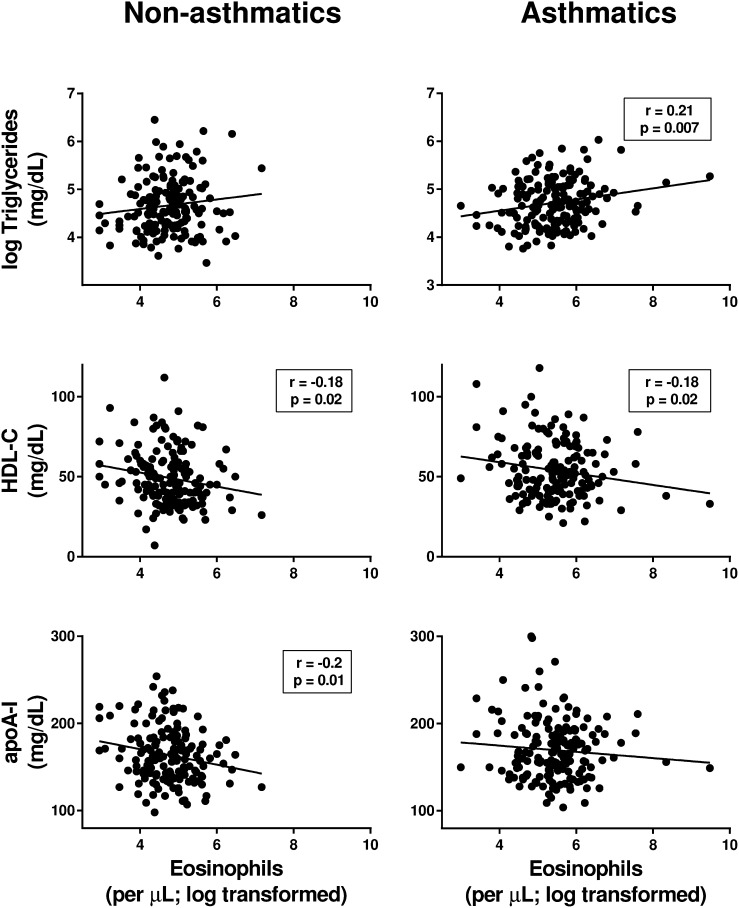
We next examined whether eosinophils or periostin were correlated with standard measurements of serum lipids, apolipoproteins, and lipoprotein particles. In asthmatics, eosinophils were positively correlated with triglycerides and negatively correlated with HDL-C, whereas in nonasthmatics eosinophils were negatively correlated with both HDL-C and apoA-I (Fig. 1). No correlations were found between eosinophils and serum levels of total cholesterol, LDL-C, apoB, or apoE. In addition, there was no correlation between periostin and serum levels of HDL-C, apoA-I, total cholesterol, LDL-C, triglycerides, apoB, or apoE.
Fig. 1.
Blood eosinophils in asthmatics are positively correlated with serum triglyceride levels and negatively correlated with serum HDL-C. Correlations between absolute blood eosinophil counts (cells per microliter, log transformed) with serum levels of triglycerides (log transformed), HDL-C and apo A-I in nonasthmatics and asthmatics are shown. Pearson’s correlation coefficients and associated P values are shown for significant relationships only.
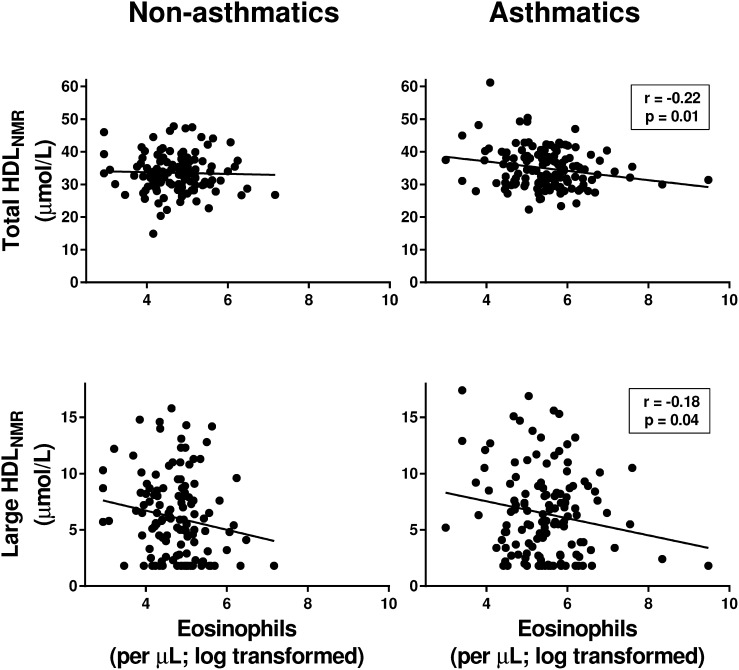
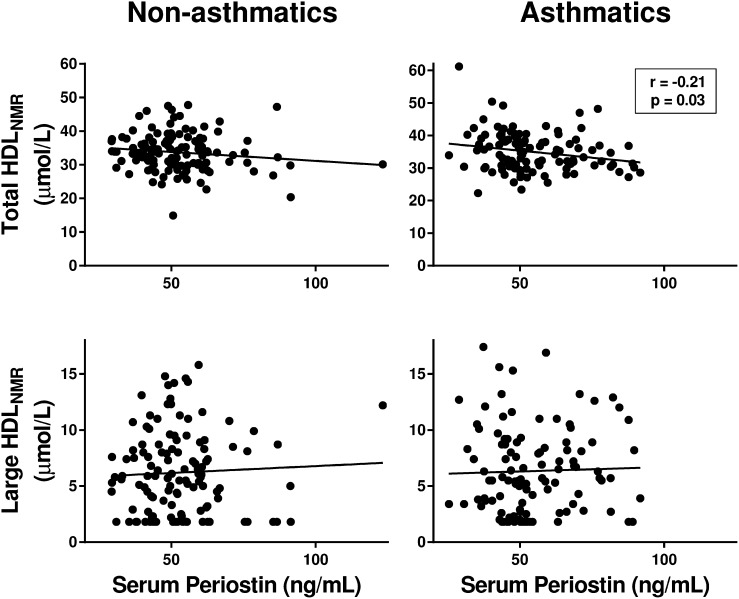
We then used NMR spectroscopy to assess whether the negative correlation between HDL-C and eosinophils was mediated by a subset of HDL particles. In asthmatics, eosinophils were negatively correlated with the concentration of total HDL (total HDLNMR) and large HDL particles (large HDLNMR) (Fig. 2). Periostin was similarly negatively correlated with total HDLNMR in asthmatics, whereas a correlation was not found with large HDLNMR (Fig. 3).
Fig. 2.
Blood eosinophils in asthmatics are negatively correlated with the concentration of total and large HDLNMR particles in serum. Correlations between absolute blood eosinophil counts (cells per microliter, log transformed) with HDLNMR particles in serum, as measured by NMR spectroscopy, in nonasthmatics and asthmatics are shown. Pearson’s correlation coefficients and associated P values are shown for significant relationships only.
Fig. 3.
Serum periostin levels in asthmatics are negatively correlated with the concentration of total HDLNMR particles in serum. Correlations between serum periostin levels and HDLNMR particles in serum, as measured by NMR spectroscopy, in nonasthmatics and asthmatics are shown. Pearson’s correlation coefficients and associated P values are shown for significant relationships only.
To account for the possible effects of age, sex, race, BMI, and serum CRP on the correlation between eosinophils and lipid parameters, we performed a multivariable regression analysis using linear models incorporating these covariates for each group. As shown in Table 4, only in asthmatics were eosinophils positively correlated with triglycerides (P = 0.01) and negatively correlated with HDL-C and total HDLNMR (P = 0.04 and 0.01, respectively). In addition, the negative correlation between large HDLNMR particles and serum eosinophils in asthmatics approached statistical significance (P = 0.06). In contrast, in nonasthmatics, there were no significant correlations between eosinophils and any of the lipid values. As shown in Table 5, similar analyses for periostin showed that the negative correlation with total HDLNMR remained significant in asthmatics (P = 0.001).
TABLE 4.
Multivariable regression analysis results for associations between lipids or lipoproteins and eosinophil counts
| Lipid/Lipoprotein Fractions | Nonasthmatics | Asthmatics | ||||
| Intercept | Estimate (95% CI) | P | Intercept | Estimate (95% CI) | P | |
| Total cholesterol (mg/dl) | 127.66 | 2.35 (−5.00, 9.70) | 0.53 | 166.46 | 0.30 (−5.82, 6.42) | 0.92 |
| Triglycerides (mg/dl) | 4.66 | 0.001(−0.11, 0.11) | 0.99 | 4.00 | 0.10 (0.02, 0.17) | 0.01 |
| LDL-C (mg/dl) | 50.36 | 2.65 (−3.95, 9.25) | 0.43 | 94.39 | 0.90 (−4.87, 6.67) | 0.76 |
| apoE (μg/ml) | 9.58 | −0.03 (−0.17, 0.11) | 0.64 | 9.35 | 0.06 (−0.04, 0.16) | 0.25 |
| HDL-C (mg/dl) | 57.39 | −1.44 (−4.84, 1.97) | 0.41 | 66.03 | −2.98 (−5.76, −0.19) | 0.04 |
| apoA-I (mg/dl) | 202.25 | −3.73 (−9.81, 2.36) | 0.23 | 171.82 | −2.34 (−7.80, 3.11) | 0.4 |
| apoB (mg/dl) | 62.57 | 1.54 (−3.30, 6.38) | 0.53 | 80.83 | 0.85 (−3.03, 4.72) | 0.67 |
| HDL particles (μmol/l) | ||||||
| Total HDLNMR | 35.20 | 0.54 (−0.87, 1.95) | 0.45 | 42.43 | −1.43 (−2.47, −0.38) | 0.01 |
| Large HDLNMR | 6.82 | −0.17 (−0.98, 0.64) | 0.68 | 10.68 | −0.62 (−1.27, 0.03) | 0.0596 |
Intercepts and estimates for NMR lipid parameters from multivariable regression, using linear models incorporating BMI, log CRP, age, sex, and race as covariates, to test for significant associations between absolute eosinophil counts and lipid or lipoprotein parameters. P values for significant associations are shown in bold italicized text.
TABLE 5.
Multivariable regression analysis results for associations between lipids or lipoproteins and serum periostin levels
| Lipid/Lipoprotein Fractions | Nonasthmatics | Asthmatics | ||||
| Intercept | Estimate (95% CI) | P | Intercept | Estimate (95% CI) | P | |
| Total cholesterol (mg/dl) | 140.68 | 0.12 (−0.27, 0.51) | 0.53 | 159.23 | 0.18 (−0.21, 0.57) | 0.36 |
| Triglycerides (mg/dl) | 4.50 | 0.003 (−0.003, 0.01) | 0.27 | 4.76 | −0.001 (−0.01, 0.004) | 0.76 |
| LDL-C (mg/dl) | 65.94 | 0.04 (−0.32, 0.39) | 0.84 | 71.09 | 0.33 (−0.03, 0.69) | 0.07 |
| apoE (μg/ml) | 9.47 | −0.001 (−0.01, 0.01) | 0.88 | 9.56 | 0.001 (−0.01, 0.01) | 0.69 |
| HDL-C (mg/dl) | 51.86 | 0.01 (−0.17, 0.19) | 0.90 | 60.99 | −0.11 (−0.31, 0.09) | 0.28 |
| apoA-I (mg/dl) | 196.14 | −0.12 (−0.45, 0.20) | 0.46 | 194.80 | −0.36 (−0.73, 0.01) | 0.06 |
| apoB (mg/dl) | 76.65 | −0.01 (−0.26, 0.24) | 0.94 | 78.97 | 0.10 (−0.15, 0.35) | 0.42 |
| HDL particles (μmol/l) | ||||||
| Total HDLNMR | 41.52 | −0.06 (−0.12, 0.01) | 0.11 | 43.70 | −0.12 (−0.19, −0.05) | 0.001 |
| Large HDLNMR | 6.36 | 0.003 (−0.04, 0.04) | 0.90 | 9.00 | −0.02 (−0.06, 0.03) | 0.46 |
Intercepts and estimates for NMR lipid parameters from multivariable regression, using linear models incorporating BMI, log CRP, age, sex, and race as covariates, to test for significant associations between serum periostin levels and lipid or lipoprotein parameters. P values for significant associations are shown in bold italicized text.
A sensitivity analysis was performed excluding 17 subjects who might have been taking lipid profile-modifying medications. Eight asthmatic subjects, three atopic nonasthmatics, and six nonatopic nonasthmatics were either taking a lipid-modifying medication or had missing information regarding medication use (Table 1). When the potential effects of age, sex, race, BMI, and CRP were accounted for using multivariable regression, significant relationships between eosinophils and HDL-C (P = 0.007) and total and large HDLNMR particles (P = 0.01), as well as triglycerides (P = 0.03), persisted in asthmatics. No significant relationships were observed between eosinophils and any lipid parameter in nonasthmatics after exclusion of subjects taking lipid profile-modifying medications. Similar sensitivity analyses performed for periostin showed persistence of the negative correlation between periostin and total HDLNMR (P = 0.001) in asthmatics.
As the two groups were not significantly different regarding racial composition (P = 0.24), additional sensitivity analyses with multivariable regression, including all previously specified variables except for race, were performed. After excluding race from the model, eosinophils remained significantly negatively correlated with HDL-C, total HDLNMR, and large HDLNMR (P = 0.03, 0.01, and 0.05, respectively) and positively correlated with triglycerides (P = 0.007), independent of age, sex, BMI, and CRP levels in asthmatics. Similarly, sensitivity analyses showed that periostin remained significantly negatively correlated with total HDLNMR in asthmatics (P = 0.003).
Because inhaled corticosteroids can potentially alter eosinophil counts and possibly HDL-C levels, we performed a post hoc subgroup analysis of asthmatics who were using inhaled corticosteroids (ICSs) and those who were not (n = 76 and 89, respectively). Mean HDL-C level in those asthmatics using ICSs was 55.3 (±17.7) mg/dl and 53.0 (±17.3) mg/dl in those not using ICSs at the time of study (P = 0.4; one-way ANOVA). Subjects who were using ICSs at the time of study had slightly higher mean eosinophil counts than those who were not (270 ± 3 eosinophils/μl vs. 197 ± 2 eosinophils/μl, respectively; P = 0.02). Similar to the overall group results, Pearson correlation between HDL-C and eosinophils showed a negative association between HDL-C and eosinophils in both of these subgroups (r = −0.199 and r = −0.188, respectively), which approached statistical significance (P = 0.08 for both). We did not separately assess the very small subgroup of asthmatics who were taking oral corticosteroids (n = 6) at the time of study. Only three asthmatic subjects were taking medications for the treatment of diabetes mellitus. Therefore, we did not perform any further subgroup analysis with regard to diabetics.
DISCUSSION
Here, we assessed whether correlations exist between serum levels of lipids, apolipoproteins, and lipoprotein particles with biomarkers of type 2 inflammation. We report that serum levels of HDL-C are negatively correlated, whereas serum triglycerides are positively correlated, with blood eosinophil counts in atopic asthmatics, independent of age, sex, race, BMI, and serum CRP levels. Furthermore, the negative association with blood eosinophil counts was mediated by large HDLNMR particles. In addition, serum periostin was inversely associated with the concentration of total HDLNMR particles in asthmatics.
HDL mediates reverse cholesterol transport, which effluxes phospholipids and cholesterol out of cells, to facilitate its atheroprotective effects (37). In addition, HDL has anti-oxidant, anti-thrombotic, and anti-inflammatory functions. In particular, HDL-mediated cholesterol efflux can decrease cellular cholesterol content and thereby reduce receptor localization to lipid raft microdomains, which attenuates cytokine receptor signaling, as well as the ability of antigen-presenting dendritic cells to activate T cell responses (38–41). We hypothesize that these anti-inflammatory properties of HDL may contribute to the negative correlation with blood eosinophil counts in atopic asthmatics.
Similar to the associations of HDL and triglycerides with lower and higher cardiovascular risk, respectively, we now show that HDL particles are negatively associated, whereas triglycerides are positively associated, with type 2 inflammation in asthma (42, 43). Although qualitatively similar correlations were initially observed in nonasthmatics, these relationships became nonsignificant after adjusting for age, sex, race, BMI, and CRP levels. While the correlation coefficients for the significant relationships between eosinophils and lipoproteins and triglycerides are not large, these findings nevertheless suggest that serum lipids and lipoproteins are associated with systemic type 2 inflammation in asthma. This level of correlation could be seen to be consistent with the biology of systemic type 2 inflammation, which is very complex and regulated by numerous factors, just two of which may include HDL and triglycerides (1). That these relationships could be biologically relevant in asthma is suggested by the robustness of the results in asthmatics even after adjusting for multiple potential confounders, including the use of lipid profile modifying medications or inhaled corticosteroids. In addition, these results are consistent with the associations we previously described between FEV1 and serum lipids and lipoproteins in asthmatics (31).
Several prior studies have reported contrasting findings regarding the association between lipids and eosinophils or periostin in general populations. For example, similar to our analysis, the prospective LifeLines Cohort Study of 13,301 subjects from the Netherlands found that higher eosinophil counts were associated with lower serum HDL levels, as well as higher serum levels of triglycerides, total cholesterol, and LDL (44). Similarly, a multiple regression analysis of 822 Korean subjects with type 2 diabetes mellitus found that serum HDL-C levels were negatively correlated with blood eosinophil counts (45). However, a study of 3,282 Taiwanese subjects found no significant correlations between eosinophils and lipid levels using multivariable regression that included age, gender, BMI, and smoking status (46). Plasma periostin levels have also been associated with plasma triglycerides, TNF-α levels, and insulin resistance in 161 Chinese subjects with obesity and type 2 diabetes mellitus (47).
There have been a number of studies and reviews that have examined the efficacy of statins in asthma, as these cholesterol-lowering agents have anti-inflammatory and anti-oxidant effects and are a mainstay of risk reduction in cardiovascular disease (48–50). However, statins have not been demonstrated to be consistently effective for the treatment of asthma, which may reflect a variety of factors that have been reviewed elsewhere (48). We have found that serum cholesterol levels are not associated with blood eosinophil counts, serum periostin levels, or FEV1, which suggests that modifying cholesterol levels alone may not be sufficient to manage asthma control. HDL particles are comprised of at least 85 different proteins, which raises the hypothesis that HDL-associated proteins, rather than its cholesterol content, may be relevant for the associations that we have shown with biomarkers of type 2 inflammation and airflow obstruction (51).
It is important to clarify several points regarding our study. First, although asthmatics had both higher adjusted HDL-C levels as well as higher eosinophil counts, there is a negative correlation between these two variables. This apparent discrepancy is not an inconsistency; simply comparing group means does not allow examination of the relationship between HDL-C and eosinophils at the level of the individual subject. Consequently, we performed correlation analyses precisely to be able to determine whether these variables could be linked at the level of each individual subject. Second, lipids and lipoprotein levels were measured from samples collected in the nonfasting state. Most lipid parameters, other than triglycerides, collected in the nonfasting state have been shown to be similar to fasting levels, and also predictive of cardiovascular outcomes such as myocardial infarction, stroke and death. Nonfasting measurements of serum lipids have now become standard practice except in special circumstances such as severe hypertriglyceridemia (52). Only four subjects from our entire cohort had triglyceride levels that exceeded 400 mg/dl, so we believe that it is unlikely that the nonfasting lipid measurements impacted our findings. Third, apoA-I is the major protein component of HDL, facilitating reverse cholesterol transport (53). Although we previously found a positive association between serum apoA-I levels and FEV1 in atopic asthmatics, in the current study no association was found between serum apoA-I levels and blood eosinophil counts or serum periostin levels (31). This raises the possibility that protein or lipid components of HDL other than apoA-I may be the link between HDL and biomarkers of type 2 inflammation. Fourth, although we did not find a difference in standard lipid values between the nonatopic and atopic nonasthmatics, we cannot exclude the possibility that our sample size was too small to detect a significant difference between these two groups. Lastly, it is important to emphasize that the associations that we report between biomarkers of type 2 inflammation and lipids or lipoproteins in atopic asthmatics do not establish causality. Thus, for example, we cannot infer based on our data that type 2 inflammation results in lower serum HDL levels; or the reverse, that higher serum HDL levels result in lower eosinophil counts.
Prior studies have assessed differences in lipid profiles between asthmatics and normal healthy individuals, as well as the relationships between serum lipids and asthma, with divergent results. Potential explanations for these heterogeneous findings include differences in the assays used, the demographic characteristics and cultural and genetic backgrounds of the populations studied, and definitions of asthma used in these studies (31, 54). Here, we took care to characterize the clinical phenotype of asthmatics versus nonasthmatics, but we acknowledge that other factors, such as the metabolic syndrome, could potentially be modifying the observed relationships in our study. Additionally, this was a cross-sectional study and these relationships may change over time. Thus, we have initiated the collection of longitudinal data to address this concern.
In conclusion, to the best of our knowledge, this is the first report of an association between biomarkers of type 2 inflammation and serum HDL and triglycerides in human atopic asthma. These results extend our previous finding that showed a positive correlation in atopic asthmatics between FEV1 and serum HDL-C, whereas FEV1 and triglycerides were negatively correlated (31). Collectively, these results show that serum levels of HDL and triglycerides are correlated with both systemic type 2 inflammation and airflow obstruction in atopic asthma. Our findings provide new insights regarding potential mechanisms that may modulate type 2 inflammation in allergic asthma. If, indeed, these results are confirmed by additional studies, they will support further investigations to determine how lipids or lipoproteins might modulate disease pathogenesis in allergic asthma, and shed light on potential new targets for therapy.
Footnotes
Abbreviations:
- CRP
- C-reactive protein
- FEV1
- forced expiratory volume in 1 s
- HDL-C
- HDL cholesterol
- HDLNMR
- HDL particles measured by NMR spectroscopy
- ICS
- inhaled corticosteroid
- IL
- interleukin
- LDL-C
- LDL cholesterol
This work was supported by Division of Intramural Research, National Institutes of Health, National Heart, Lung, and Blood Institute Grant 1 ZIA HL006166-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Fahy J. V. 2015. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 15: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier M., Ray A., and Wenzel S. E.. 2015. Evolving concepts of asthma. Am. J. Respir. Crit. Care Med. 192: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff P. G., Modrek B., Choy D. F., Jia G., Abbas A. R., Ellwanger A., Koth L. I., Arron J. R., and Fahy J. V.. 2009. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arron J. R., and Izuhara K.. 2015. Asthma biomarkers: what constitutes a ‘gold standard’? Thorax. 70: 105–107. [DOI] [PubMed] [Google Scholar]
- 5.Szefler S. J., Wenzel S., Brown R., Erzurum S. C., Fahy J. V., Hamilton R. G., Hunt J. F., Kita H., Liu A. H., Panettieri R. A. Jr., et al. . 2012. Asthma outcomes: biomarkers. J. Allergy Clin. Immunol. 129: S9–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn B. R., Robin E. D., Theodore J., and Van Kessel A.. 1975. Total eosinophil counts in the management of bronchial asthma. N. Engl. J. Med. 292: 1152–1155. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J., Chanez P., Lacoste J. Y., Barneon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P., et al. . 1990. Eosinophilic inflammation in asthma. N. Engl. J. Med. 323: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 8.Ulrik C. S., and Frederiksen J.. 1995. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 108: 10–15. [DOI] [PubMed] [Google Scholar]
- 9.Malinovschi A., Fonseca J. A., Jacinto T., Alving K., and Janson C.. 2013. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J. Allergy Clin. Immunol. 132: 821–827.e1-5. [DOI] [PubMed] [Google Scholar]
- 10.Katz L. E., Gleich G. J., Hartley B. F., Yancey S. W., and Ortega H. G.. 2014. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann. Am. Thorac. Soc. 11: 531–536. [DOI] [PubMed] [Google Scholar]
- 11.Zeiger R. S., Schatz M., Li Q., Chen W., Khatry D. B., Gossage D., and Tran T. N.. 2014. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J. Allergy Clin. Immunol. Pract. 2: 741–750. [DOI] [PubMed] [Google Scholar]
- 12.Price D. B., Rigazio A., Campbell J. D., Bleecker E. R., Corrigan C. J., Thomas M., Wenzel S. E., Wilson A. M., Small M. B., Gopalan G., et al. . 2015. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir. Med. 3: 849–858. [DOI] [PubMed] [Google Scholar]
- 13.Bel E. H., Wenzel S. E., Thompson P. J., Prazma C. M., Keene O. N., Yancey S. W., Ortega H. G., and Pavord I. D.; SIRIUS Investigators. 2014. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 371: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 14.Ortega H. G., Liu M. C., Pavord I. D., Brusselle G. G., FitzGerald J. M., Chetta A., Humbert M., Katz L. E., Keene O. N., Yancey S. W., et al. ; MENSA Investigators. 2014. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 15.Pavord I. D., Korn S., Howarth P., Bleecker E. R., Buhl R., Keene O. N., Ortega H., and Chanez P.. 2012. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 380: 651–659. [DOI] [PubMed] [Google Scholar]
- 16.Castro M., Zangrilli J., Wechsler M. E., Bateman E. D., Brusselle G. G., Bardin P., Murphy K., Maspero J. F., O’Brien C., and Korn S.. 2015. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 3: 355–366. [DOI] [PubMed] [Google Scholar]
- 17.Castro M., Wenzel S. E., Bleecker E. R., Pizzichini E., Kuna P., Busse W. W., Gossage D. L., Ward C. K., Wu Y., Wang B., et al. . 2014. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir. Med. 2: 879–890. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff P. G., Boushey H. A., Dolganov G. M., Barker C. S., Yang Y. H., Donnelly S., Ellwanger A., Sidhu S. S., Dao-Pick T. P., Pantoja C., et al. . 2007. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl. Acad. Sci. USA. 104: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidhu S. S., Yuan S., Innes A. L., Kerr S., Woodruff P. G., Hou L., Muller S. J., and Fahy J. V.. 2010. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc. Natl. Acad. Sci. USA. 107: 14170–14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G., Erickson R. W., Choy D. F., Mosesova S., Wu L. C., Solberg O. D., Shikotra A., Carter R., Audusseau S., Hamid Q., et al. ; Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma Study Group. 2012. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J. Allergy Clin. Immunol. 130: 647–654.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corren J., Lemanske R. F., Hanania N. A., Korenblat P. E., Parsey M. V., Arron J. R., Harris J. M., Scheerens H., Wu L. C., Su Z., et al. . 2011. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 22.Hanania N. A., Noonan M., Corren J., Korenblat P., Zheng Y., Fischer S. K., Cheu M., Putnam W. S., Murray E., Scheerens H., et al. . 2015. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 70: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanania N. A., Korenblat P., Chapman K. R., Bateman E. D., Kopecky P., Paggiaro P., Yokoyama A., Olsson J., Gray S., Holweg C. T., et al. . 2016. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 4: 781–796. [DOI] [PubMed] [Google Scholar]
- 24.Brightling C. E., Chanez P., Leigh R., O’Byrne P. M., Korn S., She D., May R. D., Streicher K., Ranade K., and Piper E.. 2015. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 3: 692–701. [DOI] [PubMed] [Google Scholar]
- 25.Yao X., Gordon E. M., Figueroa D. M., Barochia A. V., and Levine S. J.. 2016. Emerging roles of apolipoprotein E and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am. J. Respir. Cell Mol. Biol. 55: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao X., Fredriksson K., Yu Z. X., Xu X., Raghavachari N., Keeran K. J., Zywicke G. J., Kwak M., Amar M. J., Remaley A. T., et al. . 2010. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am. J. Respir. Crit. Care Med. 182: 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X., Dai C., Fredriksson K., Dagur P. K., McCoy J. P., Qu X., Yu Z. X., Keeran K. J., Zywicke G. J., Amar M. J., et al. . 2011. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J. Immunol. 186: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nandedkar S. D., Weihrauch D., Xu H., Shi Y., Feroah T., Hutchins W., Rickaby D. A., Duzgunes N., Hillery C. A., Konduri K. S., et al. . 2011. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J. Lipid Res. 52: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otera H., Ishida T., Nishiuma T., Kobayashi K., Kotani Y., Yasuda T., Kundu R. K., Quertermous T., Hirata K., and Nishimura Y.. 2009. Targeted inactivation of endothelial lipase attenuates lung allergic inflammation through raising plasma HDL level and inhibiting eosinophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 296: L594–L602. [DOI] [PubMed] [Google Scholar]
- 30.Rastogi D., Fraser S., Oh J., Huber A. M., Schulman Y., Bhagtani R. H., Khan Z. S., Tesfa L., Hall C. B., and Macian F.. 2015. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am. J. Respir. Crit. Care Med. 191: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barochia A. V., Kaler M., Cuento R. A., Gordon E. M., Weir N. A., Sampson M., Fontana J. R., MacDonald S., Moss J., Manganiello V., et al. . 2015. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am. J. Respir. Crit. Care Med. 191: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma: Full Report 2007. 2007. U.S. Department of Health and Human Services (NIH) Publication No. 07-4051. [Google Scholar]
- 33.Chung K. F., Wenzel S. E., Brozek J. L., Bush A., Castro M., Sterk P. J., Adcock I. M., Bateman E. D., Bel E. H., Bleecker E. R., et al. . 2014. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 43: 343–373. [Erratum. 2014. Eur. Respir. J. 43: 1216.] [DOI] [PubMed] [Google Scholar]
- 34.Palme S., Christenson R. H., Jortani S. A., Ostlund R. E., Kolm R., Kopal G., and Laubender R. P.. 2017. Multicenter evaluation of analytical characteristics of the Elecsys(R) periostin immunoassay. Clin. Biochem. 50: 139–144. [DOI] [PubMed] [Google Scholar]
- 35.deGoma E. M., and Rader D. J.. 2012. High-density lipoprotein particle number: a better measure to quantify high-density lipoprotein? J. Am. Coll. Cardiol. 60: 517–520. [DOI] [PubMed] [Google Scholar]
- 36.Mackey R. H., McTigue K. M., Chang Y. F., Barinas-Mitchell E., Evans R. W., Tinker L. F., Lewis C. E., Manson J. E., Stefanick M. L., Howard B. V., et al. . 2015. Lipoprotein particles and size, total and high molecular weight adiponectin, and leptin in relation to incident coronary heart disease among severely obese postmenopausal women: the Women’s Health Initiative Observational Study. BBA Clin. 3: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., and Fogelman A. M.. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772. [DOI] [PubMed] [Google Scholar]
- 38.Yvan-Charvet L., Pagler T., Gautier E. L., Avagyan S., Siry R. L., Han S., Welch C. L., Wang N., Randolph G. J., Snoeck H. W., et al. . 2010. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 328: 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansson G. K., and Bjorkholm M.. 2010. Medicine. Tackling two diseases with HDL. Science. 328: 1641–1642. [DOI] [PubMed] [Google Scholar]
- 40.Wang S. H., Yuan S. G., Peng D. Q., and Zhao S. P.. 2012. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 225: 105–114. [DOI] [PubMed] [Google Scholar]
- 41.Tiniakou I., Drakos E., Sinatkas V., Van Eck M., Zannis V. I., Boumpas D., Verginis P., and Kardassis D.. 2015. High-density lipoprotein attenuates Th1 and th17 autoimmune responses by modulating dendritic cell maturation and function. J. Immunol. 194: 4676–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rader D. J., and Hovingh G. K.. HDL and cardiovascular disease. 2014. Lancet. 384: 618–625. [DOI] [PubMed] [Google Scholar]
- 43.Nordestgaard B. G., and Varbo A.. 2014. Triglycerides and cardiovascular disease. Lancet. 384: 626–635. [DOI] [PubMed] [Google Scholar]
- 44.Amini M., Bashirova D., Prins B. P., Corpeleijn E. , LifeLines Cohort Study, Bruinenberg M., Franke L., Harst P. V., Navis G., Wolffenbuttel B. H., et al. . 2016. Eosinophil count is a common factor for complex metabolic and pulmonary traits and diseases: the LifeLines Cohort Study. PLoS One. 11: e0168480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim W. S., Kim H. J., Kang E. S., Ahn C. W., Lim S. K., Lee H. C., and Cha B. S.. 2006. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res. Clin. Pract. 73: 284–291. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z. S., Chien K. L., Yang C. Y., Tsai K. S., and Wang C. H.. 2001. Peripheral differential leukocyte counts in humans vary with hyperlipidemia, smoking, and body mass index. Lipids. 36: 237–245. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y., Qu H., Wang H., Wei H., Wu J., Duan Y., Liu D., and Deng H.. 2016. Plasma periostin levels are increased in Chinese subjects with obesity and type 2 diabetes and are positively correlated with glucose and lipid parameters. Mediators Inflamm. 2016: 6423637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharjee D., Chogtu B., and Magazine R.. 2015. Statins in asthma: potential beneficial effects and limitations. Pulm. Med. 2015: 835204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker D. Y., and Edwards K. L.. 2013. Statins in the treatment of asthma. Am. J. Health Syst. Pharm. 70: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 50.Yuan C., Zhou L., Cheng J., Zhang J., Teng Y., Huang M., Adcock I. M., Barnes P. J., and Yao X.. 2012. Statins as potential therapeutic drug for asthma? Respir. Res. 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah A. S., Tan L., Long J. L., and Davidson W. S.. 2013. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 54: 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nordestgaard B. G., Langsted A., Mora S., Kolovou G., Baum H., Bruckert E., Watts G. F., Sypniewska G., Wiklund O., Boren J., et al. . 2016. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 37: 1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remaley A. T., Norata G. D., and Catapano A. L.. 2014. Novel concepts in HDL pharmacology. Cardiovasc. Res. 103: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fessler M. B. 2015. Revisiting “good” and “bad” cholesterol. The battle over flow through arteries now shifts to flow through airways. Am. J. Respir. Crit. Care Med. 191: 969–970. [DOI] [PMC free article] [PubMed] [Google Scholar]