Abstract
The role of APOE in the risk of Alzheimer’s disease (AD) has largely focused on its effects on AD pathological processes. However, there are increasing data that APOE genotype affects processes in normal brains. Studies of young cognitively normal humans show effects of APOE genotype on brain structure and activity. Studies of normal APOE knock-in mice show effects of APOE genotype on brain structure, neuronal markers, and behavior. APOE interactions with molecules important for lipid efflux and lipid endocytosis underlie effects of APOE genotype on neuroinflammation and lipoprotein composition. These effects provide important targets for new therapies for reduction of the risk of AD before any signs of pathogenesis.
Keywords: apolipoprotein E, Alzheimer’s disease, apolipoproteins, inflammation, lipids/efflux, ATP binding cassette transporter A1
APOE genotype has the most profound genetic risk on late onset Alzheimer’s disease (AD) (1). APOE4 promotes earlier amyloid deposition and clinical symptoms of AD by about 15 years per allele (2). With an allele frequency of 0.14, APOE4 is present in approximately 25% of the US population (3). Thus, there are nearly 80 million people in the US who carry this risk, without any risk-altering treatments. APOE2, in contrast, lowers the AD risk in about 35 million US individuals. The main questions that these observations raise are: how does APOE genotype affect the risk of AD, and what can be done to decrease that risk in individuals? In this review, we will be examining whether the roles of APOE in neuroinflammation or lipid homeostasis before AD pathogenesis may predispose the brain to damage that occurs later in aging with the accumulation of the Aβ peptide.
THE EFFECTS OF APOE GENOTYPE ON INFLAMMATION
Inflammation is a potential early indicator of AD risk or AD onset in humans because genetic factors related to immune functions and inflammation have been identified in genome-wide association studies of AD (4). APOE is one of these AD risk genes related to neuroinflammation, as evidenced by several in vitro and in vivo systems. In vivo studies rely largely on mice with the coding sequence of the human APOE alleles replacing the mouse APOE gene as the best animal model for normal APOE regulation and function (5). The APOE4 knock-in mice are more susceptible to inflammation induced by lipopolysaccharide (6) or by Aβ deposition (7) compared with APOE2 and APOE3 mice. APOE4 mice are also more susceptible to brain damage that has strong inflammatory components, such as traumatic brain injury (8) and experimental autoimmune encephalomyelitis (9). In APOE mouse models, peptides based on the APOE receptor binding domain prevent or alleviate effects of inflammation-related insults, such as lipopolysaccharide-induced inflammation (10), traumatic brain injury (11), intracerebral hemorrhage (12), and focal ischemia (13). Similar effects are seen in vitro. APOE isoforms affect inflammatory processes in microglia and astrocytes, with APOE4 promoting the strongest inflammatory effects (10, 14, 15). An APOE peptide inhibits inflammatory processes in isolated microglia (16) through the APOE receptor, LRP1 (17). APOE similarly induces an anti-inflammatory phenotype in isolated macrophages through the APOE receptors, ApoER2 and VLDLR (18). Interestingly, blocking inflammatory signaling increases APOE expression in microglia (19), suggesting that APOE levels and inflammation are in a negative feedback loop, with APOE inhibiting inflammation and inflammation inhibiting APOE levels. These data indicate that APOE is associated with increased inflammatory responses before and after the onset of AD pathogenesis.
THE EFFECTS OF APOE GENOTYPE ON LIPID HOMEOSTASIS
APOE is one of the primary apolipoproteins in CNS lipid metabolism (20). Thus, an effect of APOE genotype on brain lipid homeostasis may underlie the AD risk associated with APOE. As with inflammation, this possibility is supported by the identification of lipid-related genetic risk factors for AD (21), particularly APOJ (or clusterin) (22, 23). Although APOJ is not as strong of a genetic risk factor as APOE [the polymorphic site in APOJ has an odds ratio of about 0.9 for the minor allele, compared with an odds ratio of about 5 for the APOE-ε4 allele (24)], both APOE and APOJ are components of CNS lipoproteins (25) and are associated with the functions of CNS lipoproteins: lipid efflux and lipid delivery (26).
APOE and APOJ interact with lipid debris in the brain (27) and APOE is necessary for the removal of degenerating membrane after injury (28). APOE lipoproteins also accumulate lipid from a cellular efflux mechanism with the ABCA1 transporter (29). Deletion of the ABCA1 gene decreases levels of APOE (30, 31) and increases the deposition of Aβ (31) in the brain. APOE isoforms differ in their ability to promote cholesterol efflux from cells, with APOE2 having the greatest efficiency and APOE4 the least (32, 33). This efflux is the first step in the generation of APOE-lipid complexes. APOE4 was found to be part of smaller complexes in normal mouse brains (34, 35), and in mouse brain expressing different APOE isoforms from virus (36, 37). In contrast, APOE2 was associated with larger complexes (36). The relevance of these findings to humans was demonstrated through analysis of human cerebrospinal fluid (CSF), with APOE complexes largest in APOE2.3 individuals and smallest in APOE4.4 individuals (38). Consistent with the hypothesis that APOE4 is associated with smaller lipoproteins, APOE4 lipoproteins promoted less cholesterol efflux than APOE3 lipoproteins (39), and APOE4-positive individuals had more lipid-depleted APOE in their CSF than APOE4-negative individuals (40).
Lipid delivery to cells occurs as APOE and APOJ are endocytosed via members of the LDL receptor family (41); endocytosis promotes neurite outgrowth (42), neuronal sprouting (43), and synapse formation (44). APOE and APOJ also promote endocytosis through TREM2 (45, 46), another prominent genetic risk factor for AD (47). These processes involve clearance into neurons as well as glia (48, 49) or across the blood brain barrier (50). The need for clearance of lipids from the brain may increase with age as membrane damage accumulates and neuronal loss occurs.
The effects of the reduced lipidation capacity of APOE4 may result in reduced neuronal protection or repair. Neuronal injury increases brain APOE levels (51), although the increase is not immediate (52). The presence of an APOE4 allele decreases the brain’s neuronal reparative capacity in AD patients (53). We hypothesize that reduced lipidation of CNS lipoproteins may be an important risk factor of AD; biomarker-based APOE lipidation may be useful in measuring levels of neuroprotection in the brain environment (38). The effects of APOE genotype on lipidation may be causally related to some effects of inflammation, due to direct effects on high levels of cholesterol on inflammation (54) or to the connections of regulatory systems controlling brain lipid homeostasis and inflammation (55).
THE EFFECTS OF APOE GENOTYPE ON APOE LEVELS AND POSTTRANSLATIONAL MODIFICATION
Humans with the APOE4 allele have smaller APOE lipoproteins and lower APOE levels in the CSF and plasma, whereas those with the APOE2 allele have larger APOE lipoproteins and higher APOE levels (56, 57). APOE4 knock-in mice also have lower levels of APOE in the brain, CSF, plasma, and interstitial fluid compared with APOE3 or APOE2 mice (15, 58, 59). The lower levels of APOE may be due to increased degradation of APOE4 compared with the other isoforms (59). If APOE4 individuals have both smaller lipoproteins and less APOE, then there could be a twofold impact on lipid clearance and delivery processes that contributes to the increased risk for AD.
There are also important posttranslational modifications to the APOE protein. Most notably, APOE4 lacks cysteine residues for the formation of APOE-APOE homodimers and APOE-APOAII heterodimers (26), whereas APOE3 contains one cysteine and APOE2 contains two cysteines for dimer formation. APOE4 is associated with enhanced cleavage of the C terminus of APOE (60), which exacerbates the effects of Aβ on inflammation and behavioral deficits in mice (61). This cleaved APOE4 is neuron specific and induced by neuronal stress (62), with the APOE4 fragments inducing neuronal dysfunction (63). Finally, the APOE protein is modified by O-glycosylation (64), and to a greater extent in the CNS than in the periphery (26). We have identified biochemical differences in modified versions of brain APOE: unmodified APOE is solubilized only in the presence of detergent and modified APOE is solubilized in saline (65). The ratio of these different forms is altered by APOE genotypes in mouse and human brains (65). The APOE isoform effects on APOE levels and on dimer formation support loss-of-function explanations for the effects of APOE4, with APOE4 less able to clear debris and deliver lipids than APOE2 or APOE3. In contrast, effects of APOE cleavage fragments on neurotoxicity (66) or the inhibitory effects of APOE4 toward neuronal sprouting (67) support a gain-of-function explanation. Understanding of APOE functions requires a better understanding of the different forms of APOE present in the CNS, particularly because treatment approaches could involve the increase or the decrease of APOE4 levels.
THE EFFECTS OF APOE GENOTYPE ON NORMAL CNS FUNCTIONS
APOE genotype affects a number of CNS phenotypes in young individuals, as demonstrated both in mice and in humans (65). APOE4 knock-in mice have several differences compared with APOE3 mice. In measures of behavior, APOE4 is associated with deficits in spatial learning and memory (68–71). In measures of neuronal complexity, APOE4 is associated with reduced dendritic arborization (72, 73), neuronal activity (74), the balance of excitatory and inhibitory neurons (75), neurotransmitter release (76–78), and dendritic spine density (68, 72, 79). In measures of immunohistochemistry, APOE4 is associated with alterations in levels of VGlut1 (34, 80) and in levels of specific APOE receptors (81). Finally, in biochemical measures, APOE4 is associated with alterations in APOE solubilization (65) and presynaptic metabolic abnormalities (80). Thus, in normal mice, APOE4 is associated with many different aspects of brain function, effects important for later brain impairments.
In measures of normal human behavior, APOE4 is associated with reduced verbal memory (82), as well as visual recall and memory retention (83). In measures of human brain activity using functional magnetic resonance imaging, APOE4 is associated with increased brain activity in the default mode network, and the hippocampus during an encoding task (84). Indeed, medial temporal lobe (MTL) activation is altered by APOE genotype during diverse behavioral tasks (85–87) and APOE4 carriers have reduced grid-cell-like representations in the entorhinal cortex and increased hippocampal activation (88). APOE genotype in the absence of AD is also associated with differences in brain structure. APOE4 is associated with differences in the MTL at birth (89, 90) [the effects of APOE4 on MTL structure in older individuals is mixed (84, 91–93)]. There are differences in brain connectivity based on APOE and APOJ genotypes determined by diffusion tensor imaging (94). Differences in brain structure in APOE4 individuals are also supported by the observation that dendritic spine density in the hippocampus is lower in aged APOE4 individuals with no evidence of Aβ deposition (79). Some of these differences are consistent with increased brain activity or connectivity in young individuals with APOE4. The antagonistic pleiotropy hypothesis posits that APOE4 has a positive effect on brain activity and behavior at young ages, but is detrimental at older ages (95). In general, the human studies and mouse studies together have supported the hypothesis that APOE genotype impacts normal brain structure and function independent of AD pathology.
APOE-DIRECTED PREVENTATIVE TREATMENTS
Understanding of the basic biology of APOE helps to identify mechanism-based therapies that could rescue APOE4 phenotypes. In normal brains, these phenotypes could predispose to Aβ deposition with aging, which could be prevented by early prophylactic approaches. For example, as mentioned above, APOE mimetic peptides could serve as a therapeutic approach for APOE4 individuals for AD, as well as other diseases with neuro-inflammatory components (96). The introduction of active APOE peptides could alleviate conditions caused by lower APOE levels in APOE4 individuals (59).
Another potential AD preventative treatment is the class of nonsteroidal anti-inflammatory drugs (NSAIDs). Epidemiological studies have repeatedly shown that early NSAID use is associated with reduced AD risk in humans (97–101), but NSAIDs have been unsuccessful at treating AD in clinical trials (102), or preventing AD in short-term prevention trials of the elderly (103). Interestingly, the preventative effect of NSAIDs may be most powerful in those with the APOE4 risk genotype (97, 104–106). These findings suggest that NSAIDs are protective against AD, but only before accumulation of the neuropathological changes associated with AD (103). We have tested this hypothesis by treating APOE4 mice with the NSAID, ibuprofen. Ibuprofen rescues the effects of APOE4 genotype on reduced dendritic spine density and on the altered distribution of APOE in brain fractions (65). These effects of ibuprofen support the epidemiological data that NSAIDs may reduce AD risk factors in normal individuals.
Yet another approach is to counteract the effects of APOE4 genotypes on the deficient APOE levels and reduced APOE4 lipidation. APOE and related molecules are regulated as part of the LXR/RXR transcriptional system, making that an attractive target for drug discovery (107). LXR activation promotes brain lipid efflux through induction of genes, such as APOE and ABCA1, and, in mouse models, leads to a decrease in lipids in synaptosomes (108). As mentioned above, reducing ABCA1 in genetic knock-out models decreases APOE and increases Aβ in mouse brain (30, 31). LXR agonists increase APOE and ABCA1, reducing Aβ levels (59, 109), improving behavior (109, 110), and increasing synaptic plasticity (111). An RXR agonist, bexarotene, reduces Aβ accumulation in a mouse model (112), dependent on the presence of both APOE and ABCA1 (113). Bexarotene also rescues the impaired APOE lipidation and reversed behavioral deficits in APOE4 mice (34). Finally, induction of ABCA1 activity could be a useful AD therapeutic approach: an agonist for ABCA1 reversed the effects of APOE4 on reduced lipoprotein lipidation, synaptic markers, and behavioral deficits (35). Specifically increasing the function of ABCA1 is a particularly interesting approach to altering APOE lipid metabolism, because it relies only on promoting lipid efflux through ABCA1, and not induction of the other genes of the LXR transcription system (107).
CONCLUSIONS
The unparalleled effect of APOE on AD risk in older individuals and its varied effects on the function of younger brains emphasize the need to study AD prevention strategies related to APOE. Studies on APOE in inflammation and lipid homeostasis are providing mechanisms for how brain alterations associated with APOE4 might be rescued (Fig. 1). The many people who have inherited this strong predisposition to AD have no treatments to help them avoid AD and, with increased access to genome sequencing, more of them are recognizing that they are at frighteningly high risk. This population of APOE4-positive individuals provides a logical target for in-depth studies of promising AD prevention approaches that are not necessarily related to the neuropathological accumulation of Aβ (114). While prevention approaches to AD are difficult to evaluate, given the need to measure long-term effects, they provide hope if the approaches of treating individuals after the onset of AD pathogenesis are unsuccessful.
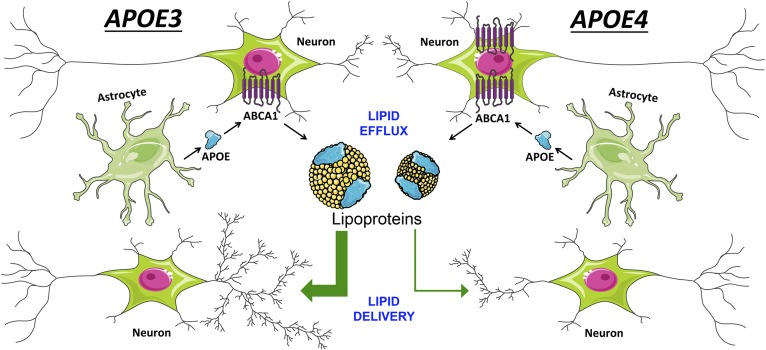
Fig. 1.
CNS APOE functions. CNS APOE (in blue) is secreted from glia. It is lipidated through interactions with ABCA1 on neurons and glia, forming high-density CNS lipoproteins (in yellow). These lipoproteins can deliver lipids to neurons to promote neuronal complexity. Compared with APOE3, APOE4 is less able to promote cholesterol efflux, forming smaller CNS lipoproteins, and less able to deliver lipids to neurons for neuroprotective functions. APOE lipoproteins also inhibit glial inflammation, with the smaller and fewer APOE4 lipoproteins less capable of this function. Chronic glial inflammation in the APOE4 brain contributes to neuronal dysfunction and impaired APOE regulation, but these APOE4 phenotypes can be mitigated through treatments with anti-inflammatory agents (NSAID).
Footnotes
Abbreviations:
- AD
- Alzheimer’s disease
- CSF
- cerebrospinal fluid
- MTL
- medial temporal lobe
- NSAID
- nonsteroidal anti-inflammatory drug
This work was supported by a gift in honor of Rolland “Pap” McCracken for the prevention and treatment of Alzheimer’s disease.
REFERENCES
- 1.Raber J., Huang Y., and Ashford J. W.. 2004. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging. 25: 641–650. [DOI] [PubMed] [Google Scholar]
- 2.Jansen W. J., Ossenkoppele R., Knol D. L., Tijms B. M., Scheltens P., Verhey F. R., Visser P. J.; Amyloid Biomarker Study Group, Aalten P., Aarsland D., et al. 2015. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 313: 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C. C., Kanekiyo T., Xu H., and Bu G.. 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9: 106–118. [Erratum. 2013. Nat. Rev. Neurol. 9: 184.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik M., Parikh I., Vasquez J. B., Smith C., Tai L., Bu G., LaDu M. J., Fardo D. W., Rebeck G. W., and Estus S.. 2015. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol. Neurodegener. 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan P. M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R. L., Quarfordt S. H., and Maeda N.. 1997. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272: 17972–17980. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y., Nwabuisi-Heath E., Dumanis S. B., Tai L. M., Yu C., Rebeck G. W., and LaDu M. J.. 2012. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 60: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez G. A., Tai L. M., LaDu M. J., and Rebeck G. W.. 2014. Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition. J. Neuroinflammation. 11: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannix R. C., Zhang J., Park J., Zhang X., Bilal K., Walker K., Tanzi R. E., Tesco G., and Whalen M. J.. 2011. Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 31: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu J. L., Zhao C. B., Vollmer T., Coons S., Lin H. J., Marsh S., Treiman D. M., and Shi J.. 2009. APOE 4 polymorphism results in early cognitive deficits in an EAE model. Biochem. Biophys. Res. Commun. 384: 466–470. [DOI] [PubMed] [Google Scholar]
- 10.Lynch J. R., Tang W., Wang H., Vitek M. P., Bennett E. R., Sullivan P. M., Warner D. S., and Laskowitz D. T.. 2003. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 278: 48529–48533. [DOI] [PubMed] [Google Scholar]
- 11.Lynch J. R., Wang H., Mace B., Leinenweber S., Warner D. S., Bennett E. R., Vitek M. P., McKenna S., and Laskowitz D. T.. 2005. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp. Neurol. 192: 109–116. [DOI] [PubMed] [Google Scholar]
- 12.James M. L., Sullivan P. M., Lascola C. D., Vitek M. P., and Laskowitz D. T.. 2009. Pharmacogenomic effects of apolipoprotein E on intracerebral hemorrhage. Stroke. 40: 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Anderson L. G., Lascola C. D., James M. L., Venkatraman T. N., Bennett E. R., Acheson S. K., Vitek M. P., and Laskowitz D. T.. 2013. Apolipoprotein E mimetic peptides improve outcome after focal ischemia. Exp. Neurol. 241: 67–74. [DOI] [PubMed] [Google Scholar]
- 14.Guo L., LaDu M. J., and Van Eldik L. J.. 2004. A dual role for apolipoprotein E in neuroinflammation: anti- and pro-inflammatory activity. J. Mol. Neurosci. 23: 205–212. [DOI] [PubMed] [Google Scholar]
- 15.Vitek M. P., Brown C. M., and Colton C. A.. 2009. APOE genotype-specific differences in the innate immune response. Neurobiol. Aging. 30: 1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocivavsek A., Burns M. P., and Rebeck G. W.. 2009. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 57: 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocivavsek A., Mikhailenko I., Strickland D. K., and Rebeck G. W.. 2009. Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation. J. Neuroimmunol. 214: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baitsch D., Bock H. H., Engel T., Telgmann R., Muller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., et al. 2011. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocivavsek A., and Rebeck G. W.. 2009. Inhibition of c-Jun N-terminal kinase increases apoE expression in vitro and in vivo. Biochem. Biophys. Res. Commun. 387: 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitas R. E., Boyles J. K., Lee S. H., Hui D., and Weisgraber K. H.. 1987. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 262: 14352–14360. [PubMed] [Google Scholar]
- 21.Karch C. M., and Goate A. M.. 2015. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 77: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M. L., Pahwa J. S., Moskvina V., Dowzell K., Williams A., et al. 2009. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 41: 1088–1093. [Erratum. 2009. Nat. Genet. 41: 1156. Erratum. 2013. Nat. Genet. 45: 712.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert J. C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M. J., Tavernier B., et al. 2009. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 41: 1094–1099. [DOI] [PubMed] [Google Scholar]
- 24.Jun G., Naj A. C., Beecham G. W., Wang L. S., Buros J., Gallins P. J., Buxbaum J. D., Ertekin-Taner N., Fallin M. D., Friedland R., et al. 2010. Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 67: 1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghini I., Barja F., Pometta D., and James R. W.. 1995. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta. 1255: 192–200. [DOI] [PubMed] [Google Scholar]
- 26.Rebeck G. W., Alonzo N. C., Berezovska O., Harr S. D., Knowles R. B., Growdon J. H., Hyman B. T., and Mendez A. J.. 1998. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Exp. Neurol. 149: 175–182. [DOI] [PubMed] [Google Scholar]
- 27.White F., Nicoll J. A., and Horsburgh K.. 2001. Alterations in ApoE and ApoJ in relation to degeneration and regeneration in a mouse model of entorhinal cortex lesion. Exp. Neurol. 169: 307–318. [DOI] [PubMed] [Google Scholar]
- 28.Fagan A. M., Murphy B. A., Patel S. N., Kilbridge J. F., Mobley W. C., Bu G., and Holtzman D. M.. 1998. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (−/−) mice but impaired clearance of axonal degeneration products following injury. Exp. Neurol. 151: 314–325. [DOI] [PubMed] [Google Scholar]
- 29.Koldamova R., Fitz N. F., and Lefterov I.. 2014. ATP-binding cassette transporter A1: from metabolism to neurodegeneration. Neurobiol. Dis. 72 Pt. A: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch-Reinshagen V., Zhou S., Burgess B. L., Bernier L., McIsaac S. A., Chan J. Y., Tansley G. H., Cohn J. S., Hayden M. R., and Wellington C. L.. 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279: 41197–41207. [DOI] [PubMed] [Google Scholar]
- 31.Wahrle S. E., Jiang H., Parsadanian M., Hartman R. E., Bales K. R., Paul S. M., and Holtzman D. M.. 2005. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J. Biol. Chem. 280: 43236–43242. [DOI] [PubMed] [Google Scholar]
- 32.Michikawa M., Fan Q. W., Isobe I., and Yanagisawa K.. 2000. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 74: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 33.Minagawa H., Gong J. S., Jung C. G., Watanabe A., Lund-Katz S., Phillips M. C., Saito H., and Michikawa M.. 2009. Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J. Neurosci. Res. 87: 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehm-Cagan A., and Michaelson D. M.. 2014. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J. Neurosci. 34: 7293–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehm-Cagan A., Bar R., Liraz O., Bielicki J. K., Johansson J. O., and Michaelson D. M.. 2016. ABCA1 agonist reverses the apoE4-driven cognitive and brain pathologies. J. Alzheimers Dis. 54: 1219–1233. [DOI] [PubMed] [Google Scholar]
- 36.Hu J., Liu C. C., Chen X. F., Zhang Y. W., Xu H., and Bu G.. 2015. Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice. Mol. Neurodegener. 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodart J. C., Marr R. A., Koistinaho M., Gregersen B. M., Malkani S., Verma I. M., and Paul S. M.. 2005. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 102: 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinsinger N. M., Gachechiladze M. A., and Rebeck G. W.. 2016. Apolipoprotein E genotype affects size of apoE complexes in cerebrospinal fluid. J. Neuropathol. Exp. Neurol. 75: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yassine H. N., Feng Q., Chiang J., Petrosspour L. M., Fonteh A. N., Chui H. C., and Harrington M. G.. 2016. ABCA1-mediated cholesterol efflux capacity to cerebrospinal fluid is reduced in patients with mild cognitive impairment and Alzheimer’s disease. J. Am. Heart Assoc. 5: e002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson A. J., Bayer-Carter J. L., Green P. S., Montine T. J., Wilkinson C. W., Baker L. D., Watson G. S., Bonner L. M., Callaghan M., Leverenz J. B., et al. 2013. Effect of apolipoprotein E genotype and diet on apolipoprotein E lipidation and amyloid peptides: randomized clinical trial. JAMA Neurol. 70: 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris-White M. E., and Frautschy S. A.. 2005. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer’s and cognition. Curr. Drug Targets CNS Neurol. Disord. 4: 469–480. [DOI] [PubMed] [Google Scholar]
- 42.Nathan B. P., Bellosta S., Sanan D. A., Weisgraber K. H., Mahley R. W., and Pitas R. E.. 1994. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 264: 850–852. [DOI] [PubMed] [Google Scholar]
- 43.Teter B., Xu P. T., Gilbert J. R., Roses A. D., Galasko D., and Cole G. M.. 1999. Human apolipoprotein E isoform-specific differences in neuronal sprouting in organotypic hippocampal culture. J. Neurochem. 73: 2613–2616. [DOI] [PubMed] [Google Scholar]
- 44.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., and Pfrieger F. W.. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 294: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 45.Yeh F. L., Wang Y., Tom I., Gonzalez L. C., and Sheng M.. 2016. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 91: 328–340. [DOI] [PubMed] [Google Scholar]
- 46.Atagi Y., Liu C. C., Painter M. M., Chen X. F., Verbeeck C., Zheng H., Li X., Rademakers R., Kang S. S., Xu H., et al. 2015. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem. 290: 26043–26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P. V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A. I., Lah J. J., et al. 2013. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulder S. D., Nielsen H. M., Blankenstein M. A., Eikelenboom P., and Veerhuis R.. 2014. Apolipoproteins E and J interfere with amyloid-beta uptake by primary human astrocytes and microglia in vitro. Glia. 62: 493–503. [DOI] [PubMed] [Google Scholar]
- 49.Cole G. M., Beech W., Frautschy S. A., Sigel J., Glasgow C., and Ard M. D.. 1999. Lipoprotein effects on Abeta accumulation and degradation by microglia in vitro. J. Neurosci. Res. 57: 504–520. [PubMed] [Google Scholar]
- 50.Bell R. D., Sagare A. P., Friedman A. E., Bedi G. S., Holtzman D. M., Deane R., and Zlokovic B. V.. 2007. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J. Cereb. Blood Flow Metab. 27: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ignatius M. J., Gebicke-Harter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., and Shooter E. M.. 1986. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc. Natl. Acad. Sci. USA. 83: 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washington P. M., and Burns M. P.. The effect of the APOE4 gene on accumulation of Aβ40 after brain injury cannot be reversed by increasing apoE4 protein. J. Neuropathol. Exp. Neurol. Epub ahead of print. June 12, 2016; doi:10.1093/jnen/nlw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arendt T., Schindler C., Bruckner M. K., Eschrich K., Bigl V., Zedlick D., and Marcova L.. 1997. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein epsilon 4 allele. J. Neurosci. 17: 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ringheim G. E., and Szczepanik A. M.. 2006. Brain inflammation, cholesterol, and glutamate as interconnected participants in the pathology of Alzheimer’s disease. Curr. Pharm. Des. 12: 719–738. [DOI] [PubMed] [Google Scholar]
- 55.Courtney R., and Landreth G. E.. 2016. LXR regulation of brain cholesterol: from development to disease. Trends Endocrinol. Metab. 27: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., et al. 2011. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3: 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruchaga C., Kauwe J. S., Nowotny P., Bales K., Pickering E. H., Mayo K., Bertelsen S., Hinrichs A. , Alzheimer’s Disease Neuroimaging Initiative, Fagan A. M., et al. 2012. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum. Mol. Genet. 21: 4558–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan P. M., Han B., Liu F., Mace B. E., Ervin J. F., Wu S., Koger D., Paul S., and Bales K. R.. 2011. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol. Aging. 32: 791–801. [DOI] [PubMed] [Google Scholar]
- 59.Riddell D. R., Zhou H., Atchison K., Warwick H. K., Atkinson P. J., Jefferson J., Xu L., Aschmies S., Kirksey Y., Hu Y., et al. 2008. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J. Neurosci. 28: 11445–11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris F. M., Brecht W. J., Xu Q., Tesseur I., Kekonius L., Wyss-Coray T., Fish J. D., Masliah E., Hopkins P. C., Scearce-Levie K., et al. 2003. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc. Natl. Acad. Sci. USA. 100: 10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bien-Ly N., Andrews-Zwilling Y., Xu Q., Bernardo A., Wang C., and Huang Y.. 2011. C-terminal-truncated apolipoprotein (apo) E4 inefficiently clears amyloid-beta (Abeta) and acts in concert with Abeta to elicit neuronal and behavioral deficits in mice. Proc. Natl. Acad. Sci. USA. 108: 4236–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brecht W. J., Harris F. M., Chang S., Tesseur I., Yu G. Q., Xu Q., Dee Fish J., Wyss-Coray T., Buttini M., Mucke L., et al. 2004. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 24: 2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Y., Liu X. Q., Wyss-Coray T., Brecht W. J., Sanan D. A., and Mahley R. W.. 2001. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc. Natl. Acad. Sci. USA. 98: 8838–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wernette-Hammond M. E., Lauer S. J., Corsini A., Walker D., Taylor J. M., and Rall S. C. Jr. 1989. Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J. Biol. Chem. 264: 9094–9101. [PubMed] [Google Scholar]
- 65.DiBattista A. M., Dumanis S. B., Newman J., and Rebeck G. W.. 2016. Identification and modification of amyloid-independent phenotypes of APOE4 mice. Exp. Neurol. 280: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tolar M., Keller J. N., Chan S., Mattson M. P., Marques M. A., and Crutcher K. A.. 1999. Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. J. Neurosci. 19: 7100–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teter B., Xu P. T., Gilbert J. R., Roses A. D., Galasko D., and Cole G. M.. 2002. Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. J. Neurosci. Res. 68: 331–336. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez G. A., Burns M. P., Weeber E. J., and Rebeck G. W.. 2013. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn. Mem. 20: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bour A., Grootendorst J., Vogel E., Kelche C., Dodart J. C., Bales K., Moreau P. H., Sullivan P. M., and Mathis C.. 2008. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 193: 174–182. [DOI] [PubMed] [Google Scholar]
- 70.Grootendorst J., Bour A., Vogel E., Kelche C., Sullivan P. M., Dodart J. C., Bales K., and Mathis C.. 2005. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav. Brain Res. 159: 1–14. [DOI] [PubMed] [Google Scholar]
- 71.Knoferle J., Yoon S. Y., Walker D., Leung L., Gillespie A. K., Tong L. M., Bien-Ly N., and Huang Y.. 2014. Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. J. Neurosci. 34: 14069–14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumanis S. B., Tesoriero J. A., Babus L. W., Nguyen M. T., Trotter J. H., Ladu M. J., Weeber E. J., Turner R. S., Xu B., Rebeck G. W., et al. 2009. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J. Neurosci. 29: 15317–15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C., Wilson W. A., Moore S. D., Mace B. E., Maeda N., Schmechel D. E., and Sullivan P. M.. 2005. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol. Dis. 18: 390–398. [DOI] [PubMed] [Google Scholar]
- 74.Gillespie A. K., Jones E. A., Lin Y. H., Karlsson M. P., Kay K., Yoon S. Y., Tong L. M., Nova P., Carr J. S., Frank L. M., et al. 2016. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron. 90: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leung L., Andrews-Zwilling Y., Yoon S. Y., Jain S., Ring K., Dai J., Wang M. M., Tong L., Walker D., and Huang Y.. 2012. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS One. 7: e53569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein R. C., Acheson S. K., Mace B. E., Sullivan P. M., and Moore S. D.. 2014. Altered neurotransmission in the lateral amygdala in aged human apoE4 targeted replacement mice. Neurobiol. Aging. 35: 2046–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein R. C., Mace B. E., Moore S. D., and Sullivan P. M.. 2010. Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience. 171: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolejší E., Liraz O., Rudajev V., Zimcik P., Dolezal V., and Michaelson D. M.. 2016. Apolipoprotein E4 reduces evoked hippocampal acetylcholine release in adult mice. J. Neurochem. 136: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji Y., Gong Y., Gan W., Beach T., Holtzman D. M., and Wisniewski T.. 2003. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 122: 305–315. [DOI] [PubMed] [Google Scholar]
- 80.Dumanis S. B., DiBattista A. M., Miessau M., Moussa C. E., and Rebeck G. W.. 2013. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J. Neurochem. 124: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilat-Frenkel M., Boehm-Cagan A., Liraz O., Xian X., Herz J., and Michaelson D. M.. 2014. Involvement of the Apoer2 and Lrp1 receptors in mediating the pathological effects of ApoE4 in vivo. Curr. Alzheimer Res. 11: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caselli R. J., Reiman E. M., Osborne D., Hentz J. G., Baxter L. C., Hernandez J. L., and Alexander G. G.. 2004. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 62: 1990–1995. [DOI] [PubMed] [Google Scholar]
- 83.Acevedo S. F., Piper B. J., Craytor M. J., Benice T. S., and Raber J.. 2010. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr. Res. 67: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filippini N., Rao A., Wetten S., Gibson R. A., Borrie M., Guzman D., Kertesz A., Loy-English I., Williams J., Nichols T., et al. 2009. Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer’s disease. Neuroimage. 44: 724–728. [DOI] [PubMed] [Google Scholar]
- 85.Rusted J. M., Evans S. L., King S. L., Dowell N., Tabet N., and Tofts P. S.. 2013. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage. 65: 364–373. [DOI] [PubMed] [Google Scholar]
- 86.Green A. E., Gray J. R., Deyoung C. G., Mhyre T. R., Padilla R., Dibattista A. M., and William Rebeck G.. 2014. A combined effect of two Alzheimer’s risk genes on medial temporal activity during executive attention in young adults. Neuropsychologia. 56: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borghesani P. R., Johnson L. C., Shelton A. L., Peskind E. R., Aylward E. H., Schellenberg G. D., and Cherrier M. M.. 2008. Altered medial temporal lobe responses during visuospatial encoding in healthy APOE*4 carriers. Neurobiol. Aging. 29: 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kunz L., Schroder T. N., Lee H., Montag C., Lachmann B., Sariyska R., Reuter M., Stirnberg R., Stocker T., Messing-Floeter P. C., et al. 2015. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science. 350: 430–433. [DOI] [PubMed] [Google Scholar]
- 89.Dean D. C. III, Jerskey B. A., Chen K., Protas H., Thiyyagura P., Roontiva A., O’Muircheartaigh J., Dirks H., Waskiewicz N., Lehman K., et al. 2014. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knickmeyer R. C., Wang J., Zhu H., Geng X., Woolson S., Hamer R. M., Konneker T., Lin W., Styner M., and Gilmore J. H.. 2014. Common variants in psychiatric risk genes predict brain structure at birth. Cereb. Cortex. 24: 1230–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Battista A. M., Heinsinger N. M., and Rebeck G. W.. 2016. Alzheimer’s disease genetic risk factor APOE-ε4 also affects normal brain function. Curr. Alzheimer Res. 13: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matura S., Prvulovic D., Jurcoane A., Hartmann D., Miller J., Scheibe M., O’Dwyer L., Oertel-Knochel V., Knochel C., Reinke B., et al. 2014. Differential effects of the ApoE4 genotype on brain structure and function. Neuroimage. 89: 81–91. [DOI] [PubMed] [Google Scholar]
- 93.O’Dwyer L., Lamberton F., Matura S., Tanner C., Scheibe M., Miller J., Rujescu D., Prvulovic D., and Hampel H.. 2012. Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS One. 7: e48895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevens B. W., DiBattista A. M., William Rebeck G., and Green A. E.. 2014. A gene-brain-cognition pathway for the effect of an Alzheimers risk gene on working memory in young adults. Neuropsychologia. 61: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han S. D., and Bondi M. W.. 2008. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 4: 251–254. [DOI] [PubMed] [Google Scholar]
- 96.Laskowitz D. T., and Vitek M. P.. 2007. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 8: 959–969. [DOI] [PubMed] [Google Scholar]
- 97.Cornelius C., Fastbom J., Winblad B., and Viitanen M.. 2004. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology. 23: 135–143. [DOI] [PubMed] [Google Scholar]
- 98.Lindsay J., Laurin D., Verreault R., Hebert R., Helliwell B., Hill G. B., and McDowell I.. 2002. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 156: 445–453. [DOI] [PubMed] [Google Scholar]
- 99.Stewart W. F., Kawas C., Corrada M., and Metter E. J.. 1997. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 48: 626–632. [DOI] [PubMed] [Google Scholar]
- 100.Zandi P. P., Anthony J. C., Hayden K. M., Mehta K., Mayer L., and Breitner J. C.; Cache County Study Investigators. 2002. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 59: 880–886. [DOI] [PubMed] [Google Scholar]
- 101. in’t Veld, B. A., A. Ruitenberg, A. Hofman, B. H. Stricker, and M. M. Breteler. 2001. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol. Aging. 22: 407–412. [DOI] [PubMed] [Google Scholar]
- 102.Pasqualetti P., Bonomini C., Dal Forno G., Paulon L., Sinforiani E., Marra C., Zanetti O., and Rossini P. M.. 2009. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin. Exp. Res. 21: 102–110. [DOI] [PubMed] [Google Scholar]
- 103.Breitner J. C., Baker L. D., Montine T. J., Meinert C. L., Lyketsos C. G., Ashe K. H., Brandt J., Craft S., Evans D. E., Green R. C., et al. 2011. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 7: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayden K. M., Zandi P. P., Khachaturian A. S., Szekely C. A., Fotuhi M., Norton M. C., Tschanz J. T., Pieper C. F., Corcoran C., Lyketsos C. G., et al. 2007. Does NSAID use modify cognitive trajectories in the elderly? The Cache County Study. Neurology. 69: 275–282. [DOI] [PubMed] [Google Scholar]
- 105.Szekely C. A., Breitner J. C., Fitzpatrick A. L., Rea T. D., Psaty B. M., Kuller L. H., and Zandi P. P.. 2008. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 70: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yip A. G., Green R. C., Huyck M., Cupples L. A., Farrer L. A., and Group M. S.. 2005. Nonsteroidal anti-inflammatory drug use and Alzheimer’s disease risk: the MIRAGE study. BMC Geriatr. 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong C., and Tontonoz P.. 2014. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13: 433–444. [DOI] [PubMed] [Google Scholar]
- 108.Eckert G. P., Vardanian L., Rebeck G. W., and Burns M. P.. 2007. Regulation of central nervous system cholesterol homeostasis by the liver X receptor agonist TO-901317. Neurosci. Lett. 423: 47–52. [DOI] [PubMed] [Google Scholar]
- 109.Donkin J. J., Stukas S., Hirsch-Reinshagen V., Namjoshi D., Wilkinson A., May S., Chan J., Fan J., Collins J., and Wellington C. L.. 2010. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 285: 34144–34154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fitz N. F., Castranio E. L., Carter A. Y., Kodali R., Lefterov I., and Koldamova R.. 2014. Improvement of memory deficits and amyloid-β clearance in aged APP23 mice treated with a combination of anti-amyloid-β antibody and LXR agonist. J. Alzheimers Dis. 41: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandoval-Hernández A. G., Buitrago L., Moreno H., Cardona-Gomez G. P., and Arboleda G.. 2015. Role of liver X receptor in AD pathophysiology. PLoS One. 10: e0145467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., Casali B. T., Restivo J. L., Goebel W. D., James M. J., et al. 2012. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 335: 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corona A. W., Kodoma N., Casali B. T., and Landreth G. E.. 2016. ABCA1 is necessary for bexarotene-mediated clearance of soluble amyloid beta from the hippocampus of APP/PS1 mice. J. Neuroimmune Pharmacol. 11: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sperling R., Mormino E., and Johnson K.. 2014. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 84: 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]