Supplemental Digital Content is Available in the Text.
Key Words: therapeutic drug monitoring, myelosuppression, leukocytopenia, hepatotoxicity, thiopurines
Abstract
Background:
Thiopurines are the prerequisite for immunomodulation in inflammatory bowel disease (IBD) therapy. When administered in high (oncological) dose, thiopurine metabolites act as purine antagonists, causing DNA-strand breakage and myelotoxicity. In lower IBD dosages, the mode of action is primarily restricted to anti-inflammatory effects. Then, myelosuppression and hepatotoxicity are the most common adverse events of thiopurines. The aim of this study was to assess the effect of thiopurine metabolites on hematologic and hepatic parameters and to determine which patient characteristics are related to generation of thiopurine metabolites.
Methods:
The authors scrutinized the therapeutic drug monitoring database of the VU University medical center and subsequently merged this database with the Clinical Laboratory database of our hospital covering the same time period (2010–2015).
Results:
The authors included 940 laboratory findings of 424 unique patients in this study. Concentrations of 6-thioguanine nucleotides (6-TGN) correlated negatively with red blood cell count, white blood cell count, and neutrophil count in both azathioprine (AZA) and mercaptopurine users. There was a positive correlation with mean corpuscular volume. In patients using 6-thioguanine, 6-TGN concentrations correlated positively with white blood cell count. Furthermore, there was an inverse correlation between patient's age and 6-TGN concentrations in patients using AZA or 6-thioguanine, and we observed an inverse correlation between body mass index and 6-TGN concentrations in patients using AZA or mercaptopurine. No relations were observed with liver test abnormalities.
Conclusions:
Thiopurine derivative therapy influenced bone marrow production and the size of red blood cells. Age and body mass index were important pharmacokinetic factors in the generation of 6-TGN.
INTRODUCTION
As per inflammatory bowel disease (IBD) guidelines,1,2 thiopurine derivatives are regularly used drugs to maintain remission in patients with IBD [ie, Crohn disease (CD) and ulcerative colitis (UC)]. Thiopurine derivatives in IBD refer to 3 different chemical compounds: azathioprine (AZA), mercaptopurine (MP), and 6-thioguanine (TG). The metabolism of AZA and MP is fairly similar and complex, whereas the metabolism of TG is more straightforward leading to the principally pharmacologically active metabolites, 6-thioguanine nucleotides (6-TGN).3 AZA and MP, also known as conventional thiopurines, are in part converted into 6-methylmercaptopurine (6-MeMP) because of enzymatic activity of thiopurine-S-methyltransferase (TPMT) (Fig. 1).4,5 In IBD, the anti-inflammatory function of thiopurines is merely ascribed to the formation of 6-TGN, which cause apoptosis of activated T-lymphocytes when administered in low dosage, by inhibiting the small GTPase Rac1.6–8 When administered in high doses, especially in patients with hematological-oncology diseases, or in patients with low or absent TPMT function, 6-TGN concentrations are grossly elevated and may induce cytotoxicity by inhibiting DNA, RNA, and protein synthesis.3,9 Furthermore, in these patients, high concentrations of 6-MeMP inhibit de novo purine synthesis, thus contributing to (oncologic) cytotoxicity as well. On the other hand, 6-MeMP is involved in the development of several (mostly hepatotoxic) adverse events.10 During thiopurine therapy, in up to 20% of the cases, hepatotoxicity, defined as an elevation of one or more liver tests, occurs.11–13 Additionally, about 4% of patients may develop leukocytopenia.14,15
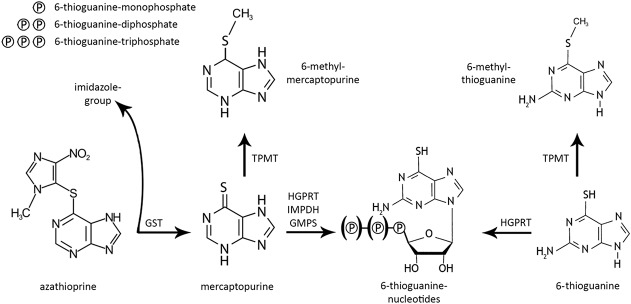
FIGURE 1.
Simplified overview of thiopurine metabolism. Azathioprine is converted into mercaptopurine by glutathione-S-transferase (GST) with the separation of an imidazole-group. Mercaptopurine is converted into the pharmacologically active 6-thioguanine nucleotides following conversion by hypoxanthine-guanine phosphoribosyltransferase (HGPRT), inosine-5'-monophosphate dehydrogenase (IMPDH), and guanosine monophosphate synthetase (GMPS). Mercaptopurine could also be converted into 6-methylmercaptopurine by the activity of the TPMT enzyme. 6-Thioguanine is more directly converted into 6-thioguaninenucleotides by the activity of HGPRT. Comparable to mercaptopurine, 6-thioguanine could also be converted into 6-methyl-thioguanine by the activity of TPMT. Adapted from van Asseldonk et al.3 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
In this study, we aimed to assess the effect of thiopurine metabolites on hematologic indices and liver tests. Moreover, we wanted to determine which patient characteristics might influence the generation of specific thiopurine metabolites.
MATERIALS AND METHODS
Over a time period of 5 years (January 1, 2011–December 31, 2015), the therapeutic drug monitoring database of the department of Clinical Pharmacology of the VU University Medical Center (VUmc) was scrutinized for thiopurine metabolite measurements.16 The Clinical Pharmacology laboratory of VUmc is one of the few laboratory facilities to determine metabolites of thiopurines of patients in the Netherlands. We merged this therapeutic drug monitoring database and the Clinical Laboratory database of our hospital covering the same time period. All patients with laboratory measurements within a time frame of 3 days before or after thiopurine metabolite determination were included in this observational database study. Patient characteristics were extracted from patient charts.
Data Extraction
Thiopurine metabolites were determined in red blood cells (RBCs) using a slightly adapted method by Dervieux et al.17,18 Quality of the measurements was warranted by internal and external verifications. In each 15 samples, one control sample is measured and taken into a trend analysis. Furthermore, our laboratory is connected to the Dutch Foundation for Quality Assessment in Medical Laboratories (SKML) for external quality control. The lower limit of detection was 30 pmol/8 × 108 RBC for 6-TGN and 15 pmol/8 × 108 RBC for 6-MMP, whereas the lower limit of quantification was 70 and 100 pmol/8 × 108 RBC, respectively. To make our study comparable with international literature, in which the method by Lennard et al19 is most commonly used, concentrations of 6-TGN were subdivided by a factor 2.6, as described previously.18,20 6-MeMP outcomes are similar for both methods. Additionally, concentrations of 6-TGN in the RBC were converted into leukocyte concentrations of 6-TGN (L-6TGN) by multiplication with 21 (for AZA/MP) or 3.5 (for TG), as previously described.3,21
Apart from the metabolite concentrations (6-MeMP, reference value <5700 pmol/8 × 108 RBC and 6-TGN, reference interval 235–450 pmol/8 × 108 RBC),11 we systematically assessed the following characteristics, when available: age, sex, weight, height, body mass index (BMI), diagnosis [CD; UC; IBD unclassified or other, such as microscopic colitis, celiac disease, or autoimmune pancreatitis], thiopurine derivatives AZA, MP, TG, and dosage of thiopurine therapy.
From the laboratory database, we extracted the following hematologic data: hemoglobin concentration (Hb, reference interval: male = 8.5–11.0 × 109/L, female = 7.5–10.0 × 109/L); white blood cell (WBC) count (reference interval 4.0–10.0 × 109/L); mean corpuscular volume (MCV, reference interval 80–100 fL); absolute neutrophil count (ANC, reference interval 1.5–8.0 × 109/L); and platelet count (PC, reference interval 150–350 × 109/L). Furthermore, we assessed the values of alanine aminotransferase (ALT, reference interval: male = 10–45 U/L, female = 7–35 U/L), aspartate aminotransferase (AST, reference interval: male = 14–20 U/L, female = 10–36 U/L), alkaline phosphatase (AP, reference interval 25–100 U/L), and gamma-glutamyl transferase (GGT, reference interval 8–65 U/L).
As suggested in the common terminology criteria for adverse events (CTCAE), myelosuppression was defined as either WBC below 3.0 × 109/L or PC under 75 × 109/L. Hepatotoxicity was defined as at least one of AST, ALT, AP, or GGT over twice the upper reference limit of normal.22
Data Presentation and Statistics
Descriptive data were presented as numbers with percentages and tabulated. Continuous data were presented as median with range or mean with SD, according to the distribution. Categorical data were compared using the Pearson χ2 test. Correlations were computed using the Spearman rank correlation coefficient. Statistical analyses were performed using SPSS statistics (version 22.0; IBM, New York, NY).
Ethics Approval
This study was approved by the Medical Ethics Review Committee (METc) of the VU University Medical Center with file-number 2016-319.
RESULTS
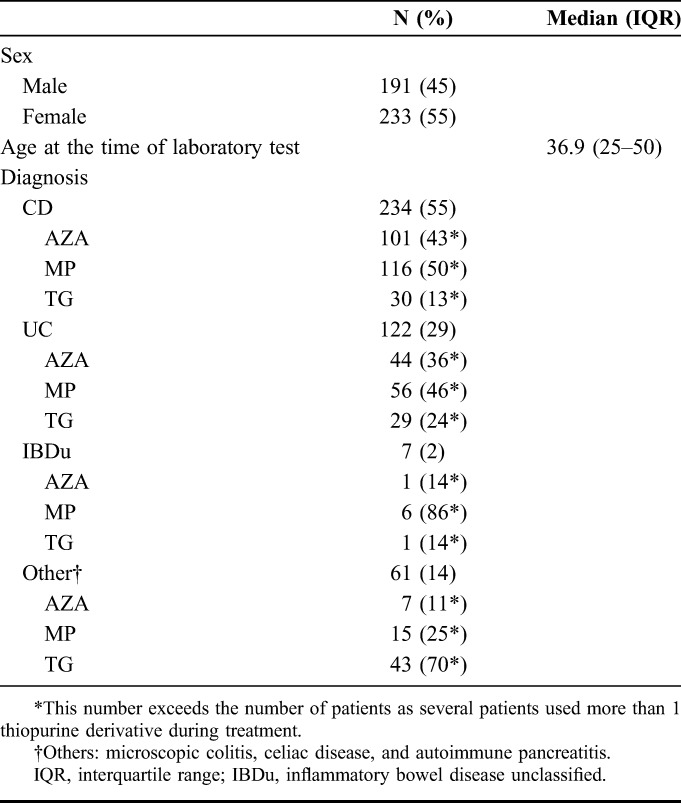
In total, we studied 940 laboratory results of 424 unique patients in which thiopurine metabolite measurements were performed. In 796 measurements, this was combined with the assessment of either hematological indices (n = 37) or liver tests (n = 10), or both (n = 749). The remaining 144 measurements were solely metabolite measurements. All included patients had at least one measurement combined with hematological indices or liver tests. Patient characteristics are given in Table 1. CD was diagnosed in 234 patients, and there were 122 patients with UC, 7 with IBD unclassified, and 61 patients had a diagnosis other than IBD [ie, microscopic colitis (n = 25), coeliac disease (n = 33), or autoimmune pancreatitis (n = 3)].
TABLE 1.
Clinical and Demographic Characteristics of Patients Treated With Thiopurines
Azathioprine
In patients using AZA (n = 270), median 6-TGN concentration was 179 pmol/8 × 108 RBC (range 12–767) and median 6-MeMP concentration was 1318 pmol/8 × 108 RBC (range 15–22,500). There was a negative correlation between (6-TGN) and RBC, WBC count and neutrophil count, and (6-TGN) correlated positively with MCV (r = −0.16, −0.28, −0.19, and 0.27, respectively). 6-MeMP concentrations were neither correlated with hematologic parameters nor to liver enzymes (Fig. 2).
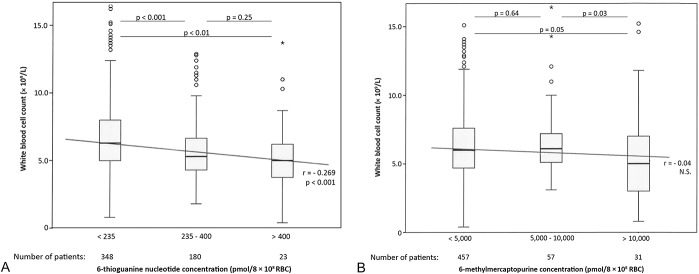
FIGURE 2.
A, Correlation between 6-thioguanine nucleotide concentrations and WBC count in patients using either azathioprine or mercaptopurine (n = 551). B, Correlation between 6-methylmercaptopurine concentrations and WBC count in patients using either azathioprine or mercaptopurine (n = 545). In these figures, the correlation between WBC count and, respectively, 6-thioguaninenucleotide and 6-methylmercaptopurine concentrations is depicted among patients using azathioprine or mercaptopurine. The box-and-whisker plots show inter-group variations; the diagonal line represents the Spearman correlation coefficient. o = Outlier, * = far outlier (1.5 × IQR)
When we subdivide the patients using AZA into groups based on their diagnosis, correlations with 6-TGN were either similar to those in the total group or did not reach statistical significance. There were no altered correlations compared with the total group (see Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/TDM/A187).
The median dose of AZA was 125 mg/d (range 25–200). In patients with allopurinol cotherapy, median dosage of AZA was 50 mg/d. Dosing was correlated with 6-MeMP concentrations (r = 0.51, P < 0.001) but not to 6-TGN concentrations (P = 0.61). When this group was subdivided into allopurinol cousers (n = 19) and patients without allopurinol cotherapy (n = 251), dosing correlated with 6-MeMP and 6-TGN concentrations only in the group without allopurinol (r = 0.42, P < 0.001 and r = 0.16, P = 0.02, respectively).
Mercaptopurine
In the MP group (n = 404), median 6-TGN concentration was 190 pmol/8 × 108 RBC (range 17–789) and median 6-MeMP concentration was 798 pmol/8 × 108 RBC (range 15–33,000), and correlations were seen in the same hematologic parameters as in the AZA group (Fig. 2). 6-MeMP concentrations were only positively correlated with ALT and had a negative correlation with AP.
In the MP group, as comparable to the AZA group, results in the different disease groups did not differ from correlations with 6-TGN in the total group (see Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/TDM/A187).
The median dose of MP was 75 mg/d (range 25–150). In patients with allopurinol cotherapy, median dosage of MP was 25 mg/d. Dosing was positively correlated with 6-MeMP concentrations (r = 0.62, P < 0.001) but negatively to 6-TGN concentrations (r = -0.11, P = 0.048). When we subdivide this group into allopurinol users (n = 146) and patients without allopurinol cotherapy (n = 258), dosing correlated with 6-MeMP in both groups (r = 0.42, P < 0.001 and r = 0.28, P < 0.001, respectively) and to 6-TGN concentrations only in the group with allopurinol (r = 0.23, P < 0.01).
6-Thioguanine
In the TG group (n = 266), where the median 6-TGN concentration was 356 pmol/8 × 108 RBC (range 12–2364), (6-TGN) correlated positively with WBC count and PC, and no correlation was seen with other hematologic parameters (Fig. 3). The correlation with WBC count was not reproduced in different disease subgroups. Dosing in the TG group (median 20 mg/d, range 10–40) was slightly positively correlated with 6-TGN concentration in RBC (r = 0.19, P < 0.01). These results are summarized in Supplemental Digital Content 1 (see Supplementary Table 3, http://links.lww.com/TDM/A187).
FIGURE 3.
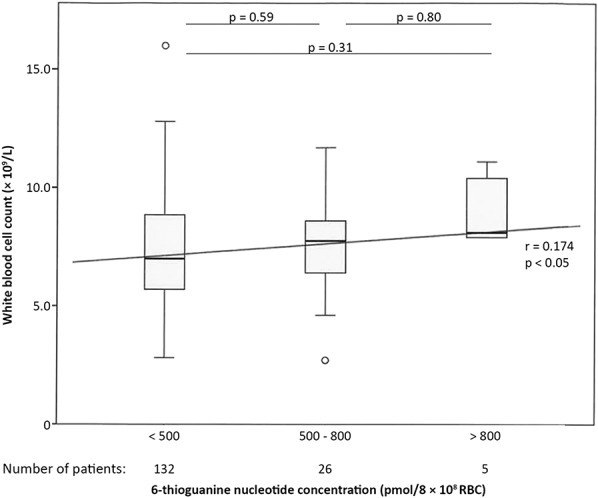
Correlation between 6-thioguanine nucleotide concentrations and WBC count in patients using 6-thioguanine (n = 163). In this figure, the correlation between the concentration of 6-thioguanine nucleotides and WBC count is depicted among patients using 6-thioguanine. The box-and-whisker plots show inter-group variations; the diagonal line represents the Spearman correlation coefficient.
Comparing Patient Characteristics With 6-TGN Concentrations
Patient's age was inversely correlated with 6-TGN concentrations in AZA and TG users, and there was a trend in MP users (r = −0.09, P = 0.08) (Table 2). In all drug groups, mean (6-TGN) was lower in patients older than 50 years, compared with patients between 18 and 50 years old, yet in none of the drug groups, this difference reached statistical significance, except in the group of TG-using patients with a mean (6-TGN) of 445 ± 269 pmol/8 × 108 RBC in patients under the age of 50 compared with a mean (6-TGN) of 315 ± 151 pmol/8 × 108 RBC in patients older than 50 years (P < 001). In our cohort, dosing was lower in patients older than 50 years using AZA compared with younger patients receiving this drug (1.3 versus 2.2 mg/kg, P < 0.001). In patients receiving either MP or TG, there was no difference in dosing.
TABLE 2.
Correlation Between 6-TGN Concentrations and Different Patient Characteristics Subdivided for Medicament Group
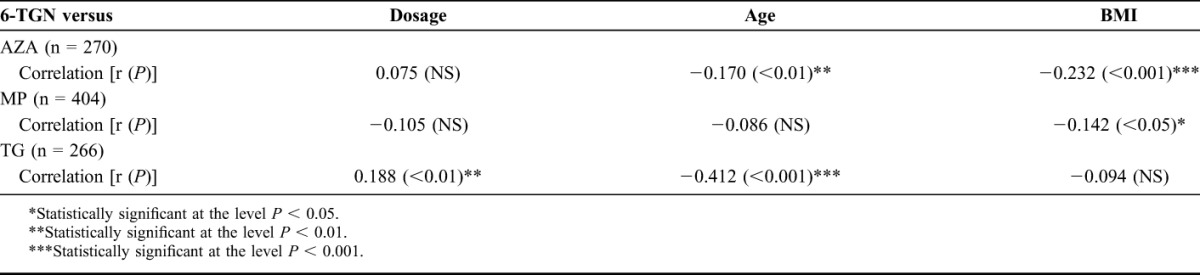
Patients' BMI was inversely correlated in patients using AZA and MP, but no correlation was seen in TG-using patients. Furthermore, there was an inverse correlation between body weight–adjusted dose (mg/kg) and body weight (kg) in all 3 thiopurine-derivative groups (AZA: r = −0.43; MP: r = −0.33; TG: r = −0.35, all P < 0.001). 6-MeMP concentrations were inversely correlated with patient's age (r = −0.19, P < 0.001) and BMI (r = −0.21, P < 0.001).
Of the measurements of all patients using AZA, 6-TGN concentrations were under the lower reference level (<235 pmol/8 × 108 RBC) in 192 measurements (71%) and above the upper reference level (>450 pmol/8 × 108 RBC) in 5 measurements (2%). In the 404 measurements of patients with MP therapy, low 6-TGN concentrations were observed 244 times (60%) and high 6-TGN concentrations 26 times (6%).
Leukocytopenia and Hepatotoxicity
Laboratory data regarding WBC or PC were available in 738 measurements (79%). In 21 measurements with myelosuppression based on laboratory values (3 in AZA, 16 in MP, and 2 in TG therapy), concentrations of 6-TGN (median 318 versus 184 pmol/8 × 108, P = 0.003) and concentrations of 6-MMP (median 4020 versus 1025 pmol/8 × 108, P = 0.012) were significantly higher in patients with myelosuppression compared with patients without myelosuppression in the group of conventional thiopurine users. Additionally, in this group, incidence of myelosuppression was higher in the group with 6-TGN concentrations outside the therapeutic range (>450 pmol/8 × 108 RBC) compared with patients with 6-TGN concentrations within the therapeutic range (16% versus 2%, P = 0.001). In the group of TG users, there were 2 patients with laboratory signs of myelosuppression (6-TGN concentration 170 and 537 pmol/8 × 108). Further statistical tests were not performed.
Median concentrations of 6-TGN in the leukocyte were higher in the patient group with myelotoxicity compared with patients with WBC >3.0 (3950 versus 3230 pmol/8 × 108); however, this difference did not reach statistical significance (P = 0.17). Taken together, there is a slight negative correlation between WBC and 6-TGN concentrations in leukocytes (r = −0.08, P = 0.03). Finally, we observed an increase in MCV when leukocytic 6-TGN concentrations (regardless of which thiopurine derivative was used) were higher (r = 0.178, P < 0.001).
Liver test values were available in 759 measurements (81%), and biochemical signs of hepatotoxicity were observed in 61 measurements (8%; 20 in AZA, 29 in MP, and 12 in TG therapy). In the 49 measurements with laboratory signs of hepatotoxicity using conventional thiopurine derivatives, no differences were found in median 6-MeMP concentrations (AZA: P = 0.78, MP: P = 0.92) or 6-TGN concentrations (conventional thiopurines: P = 0.09, TG: P = 0.28). In all patients with biochemical hepatotoxicity, PC was comparable to patients without signs of hepatotoxicity (median 313 versus 292 × 109/L, P = 0.11).
6-MeMP concentrations were above the upper limit of normal (>5700 pmol/8 × 108 RBC) in 91 out of 674 (14%) measurements during AZA or MP therapy. The incidence of hepatotoxicity was higher in patients with high 6-MeMP concentrations (16% versus 8%, P = 0.04) and the incidence of myelotoxicity (9% versus 2%, P = 0.002).
DISCUSSION
In this study, 940 laboratory parameters of 424 unique patients treated with thiopurines were systematically assessed. We demonstrated that, in the groups of conventional thiopurines, concentrations of 6-TGN were significantly correlated with a decrease in Hb, WBC, and ANC. Furthermore, the higher the concentration of 6-TGN in RBC, the higher the volume of these RBC, visualized as a positive correlation with MCV in these groups. In the patients treated with TG, we were not able to reproduce these findings, which implicates that the effect of TG on bone marrow function is less.
The association of hematologic indices and thiopurine metabolites has been investigated before, especially focusing on MCV. In a recent review, it has been suggested that a change in MCV was useful in guiding intracellular metabolite levels.23 This was confirmed by a post hoc analysis of the SONIC trial, in which a change in MCV above 7 fL was associated with a higher proportion of steroid-free remission.24 In these series, we confirmed this positive correlation between higher 6-TGN concentrations and higher MCV, in both erythrocytes and leukocytes.
Furthermore, the effect of 6-TGN concentration on other hematologic indices has been described in several small studies (including 32 to 168 patients), showing an inverse correlation with WBC (especially lymphocyte count), Hb, ANC, and PC.25–28 Most of these findings were reproduced in our larger cohort. Interestingly, as shown in Figures 2A, 3, the correlation of 6-TGN with WBC count was inverse in our group with AZA and MP, but we observed a slight positive correlation in the group with 6-thioguanine users. Although this relationship has to be interpreted with caution because of the small number of patients in this group compared with the AZA and MP group, this underlines the clinical observation that leukocytopenia is less common in TG users.
Among the patients with signs of hepatotoxicity, no differences were found in 6-MeMP or 6-TGN concentrations compared with patients without hepatotoxicity. However, when the 6-MeMP group is subdivided in high (>5700 pmol/8 × 108 RBC) and regular (≤5700 pmol/8 × 108 RBC) concentrations, there is a higher incidence of hepatotoxicity and myelotoxicity in patients with high 6-MeMP concentrations, which is in line with previously published data.9,16,29
Additionally, we observed an inverse correlation between 6-TGN concentrations and BMI, in line with earlier reports.30,31 Whilst this correlation has been reported before, the mechanism remains unclear. However, when we explored this correlation further, we found an inverse correlation between body weight–adjusted dose (mg/kg) and body weight in all 3 thiopurine groups, suggesting that this difference is probably because of lower dosing rather than a pharmacodynamics mechanism in these patients.
Statistical significance was not reached correlating patient's age with 6-TGN concentrations. However, when patients in the age group 18–50 years old were compared with patients older than 50 years, we demonstrated that 6-TGN concentrations were lower in older patients, which is in line with earlier reports.32 This age-related effect might be explained by a “start low-go slow” treatment regimen, because of the supposed higher risk of infections and malignancies in the elderly. Although 6-MeMP concentrations were inversely correlated with both BMI and patient's age, this suggests a pharmacokinetic change in drug metabolism in patients with either higher age or higher BMI.
One of the limitations of this study is the fact that, because of the retrospective, intercept-cohort design of this study, laboratory measurements were not performed structurally (per protocol). Because metabolite measurement was not performed as a routine, the reason for measuring metabolites might therefore account for slight selection bias in this cohort. Unfortunately, information regarding (potentially myelotoxic, immunosuppressive, or hepatotoxic) comedication or TPMT status was not present in our data set, which may account for a slight bias of the results. Furthermore, because of the retrospective nature of this study, in some cases, essential information about dosing or body weight was missing, leading to exclusion of these cases for analysis. Finally, to make our results comparable with international literature, we computed our metabolite data derived using a slightly adapted Dervieux method into estimated Lennard values. This method is used globally, and results have been validated previously.18 Nevertheless, as this is a calculated result rather than a measured result, this might account for biasing results. However, because correlations and comparisons were based on original Dervieux concentrations, we believe this did not impair the equations in our cohort.
CONCLUSION
In conclusion, we demonstrated that 6-thioguanine nucleotides were inversely correlated with WBC count (more specifically neutrophil count), RBC, patient's age, and patient's BMI in AZA or MP users and 6-TGN concentrations were positively correlated with MCV. Liver test abnormalities were, apart from a slightly elevated ALT, not correlated with 6-MeMP concentrations in the total group; however, the incidence of hepatotoxicity was 2-fold higher in the group with 6-MeMP concentrations above 5700 pmol/8 × 108 RBC compared with patients with regular 6-MeMP concentrations. In the group of 6-thioguanine users, 6-TGN concentrations were slightly positively correlated with WBC count and PC. Overall, thiopurine therapy has a major influence on hematological parameters.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
REFERENCES
- 1.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 3.Van Asseldonk DP, de Boer NK, Peters GJ, et al. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981–997. [DOI] [PubMed] [Google Scholar]
- 4.Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149–1157. [DOI] [PubMed] [Google Scholar]
- 5.Zaza G, Cheok M, Krynetskaia N, et al. Thiopurine pathway. Pharmacogenet Genomics. 2010;20:573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seinen ML, van Nieuw Amerongen GP, de Boer NK, et al. Rac1 as a potential pharmacodynamic biomarker for thiopurine therapy in inflammatory bowel disease. Ther Drug Monit. 2016;38:621–627. [DOI] [PubMed] [Google Scholar]
- 8.Seinen ML, van Nieuw Amerongen GP, de Boer NK, et al. Rac attack: modulation of the small GTPase Rac in inflammatory bowel disease and thiopurine therapy. Mol Diagn Ther. 2016;20:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer NK, van Bodegraven AA, Jharap B, et al. Drug insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686–694. [DOI] [PubMed] [Google Scholar]
- 10.Quemeneur L, Gerland LM, Flacher M, et al. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170:4986–4995. [DOI] [PubMed] [Google Scholar]
- 11.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. [DOI] [PubMed] [Google Scholar]
- 12.Schwab M, Schaffeler E, Marx C, et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics. 2002;12:429–436. [DOI] [PubMed] [Google Scholar]
- 13.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649. [DOI] [PubMed] [Google Scholar]
- 15.Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer B, Kreijne JE, van Moorsel SA, et al. 6-methylmercaptopurine induced leukocytopenia during thiopurine therapy in IBD patients. J Gastroenterol Hepatol. 2017;32:1183–1190. [DOI] [PubMed] [Google Scholar]
- 17.Dervieux T, Meyer G, Barham R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51:2074–2084. [DOI] [PubMed] [Google Scholar]
- 18.de Graaf P, Vos RM, de Boer NK, et al. Limited stability of thiopurine metabolites in blood samples: relevant in research and clinical practise. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1437–1442. [DOI] [PubMed] [Google Scholar]
- 19.Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583:83–90. [DOI] [PubMed] [Google Scholar]
- 20.Shipkova M, Armstrong VW, Wieland E, et al. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin Chem. 2003;49:260–268. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster DL, Patel N, Lennard L, et al. Leucocyte versus erythrocyte thioguanine nucleotide concentrations in children taking thiopurines for acute lymphoblastic leukaemia. Cancer Chemother Pharmacol. 2002;50:33–36. [DOI] [PubMed] [Google Scholar]
- 22.Program CTE. Common Terminology Criteria for Adverse Events (CTCAE). CTEP [Internet]. 2006. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed May 2, 2017. [Google Scholar]
- 23.Dujardin RW, Meijer B, de Boer NK, et al. Usefulness of mean corpuscular volume as a surrogate marker for monitoring thiopurine treatment in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016;28:991–996. [DOI] [PubMed] [Google Scholar]
- 24.Bouguen G, Sninsky C, Tang KL, et al. Change in erythrocyte mean corpuscular volume during combination therapy with azathioprine and infliximab is associated with mucosal healing: a post hoc analysis from SONIC. Inflamm Bowel Dis. 2015;21:606–614. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TM, Le Gall C, Lachaux A, et al. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int J Clin Pharmacol Ther. 2010;48:275–281. [DOI] [PubMed] [Google Scholar]
- 26.Kopylov U, Battat R, Benmassaoud A, et al. Hematologic indices as surrogate markers for monitoring thiopurine therapy in IBD. Dig Dis Sci. 2015;60:478–484. [DOI] [PubMed] [Google Scholar]
- 27.D'Halluin PN, Tribut O, Branger B, et al. RBC 6-TGN and hematological parameters in patients with Crohn's disease treated by azathioprine. Gastroenterol Clin Biol. 2005;29:1264–1269. [DOI] [PubMed] [Google Scholar]
- 28.Heerasing NM, Ng JF, Dowling D. Does lymphopenia or macrocytosis reflect 6-thioguanine levels in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine? Intern Med J. 2016;46:465–469. [DOI] [PubMed] [Google Scholar]
- 29.Chaparro M, Ordas I, Cabre E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–1410. [DOI] [PubMed] [Google Scholar]
- 30.Poon SS, Asher R, Jackson R, et al. Body mass index and smoking affect thioguanine nucleotide levels in inflammatory bowel disease. J Crohns Colitis. 2015;9:640–646. [DOI] [PubMed] [Google Scholar]
- 31.Holtmann MH, Krummenauer F, Claas C, et al. Significant differences between Crohn's disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: results from a European multicenter study in 1,176 patients. Dig Dis Sci. 2010;55:1066–1078. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.