Supplemental Digital Content is available in the text.
Key Words: hemodynamics, magnetic resonance imaging, heart Defects, congenital, respiration, vasodilation
Abstract
Purpose:
Progressive impairment of hemodynamics in patients with Fontan circulation is common, multifactorial, and associated with decreased quality of life and increased morbidity. We sought to assess hemodynamic differences between patients with preserved (preserved Fontans) and those with impaired circulation (impaired Fontans) after pulmonary vasodilation using oxygen and under forced breathing conditions.
Materials and Methods:
Real-time phase-contrast cardiovascular magnetic resonance was performed using non–ECG triggered echo-planar imaging (temporal resolution=24 to 28 ms) in the ascending aorta (AAo) and superior vena cava (SVC)/inferior vena cava (IVC) on room air, after 100% oxygen inhalation (4 L/min; 10 min) and on forced breathing in 29 Fontan patients (17.2±7.3 y) and in 32 controls on room air (13.4±3.7 y). The simultaneously recorded patients’ respiratory cycle was divided into 4 segments (expiration, end-expiration, inspiration, and end-inspiration) to generate respiratory-dependent stroke volumes (SVs). The imaging data were matched with physiological data and analyzed with home-made software.
Results:
The mean SVi (AAo) was 46.1±11.1 mL/m2 in preserved Fontans versus 30.4±6.2 mL/m2 in impaired Fontans (P=0.002) and 51.1±6.9 mL/m2 in controls (P=0.107). The cutoff value for differentiation of Fontan groups was SVi (AAo, end-expiratory) of 32.1 mL/m2. After hyperoxygenation, the mean SVi (AAo) increased to 48.7±12.7 mL/m2 in preserved Fontans (P=0.045) but remained unchanged in impaired Fontans (31.1±5.8 mL/m2, P=0.665). Simultaneously, heart rates decreased from 75.2±15.9 to 70.8±16.4 bpm (preserved; P=0.000) but remained unchanged in impaired circulation (baseline: 84.1±9.8 bpm, P=0.612). Compared with physiological respiration, forced breathing increased the maximum respiratory-related cardiac index difference (ΔCImax) in preserved Fontans (SVC: 2.5-fold, P=0.000; and IVC: 1.8-fold, P=0.000) and to a lower extent in impaired Fontans (both veins, 1.5-fold; P(SVC)=0.011, P(IVC)=0.013). There was no impact on mean blood flow.
Conclusions:
Oxygen affected the pulmonary vascular system by vasodilation and increased SVi in preserved Fontans but had no effect on impaired Fontans. Forced breathing increased ΔCImax but did not change the mean blood flow by sole activation of the ventilatory pump. End-expiratory aortic SVi represents a valuable measure for classifying the severity of Fontan hemodynamics impairment.
Blood circulation in healthy individuals is based on complex relationships between at least 3 different physiological pumping systems: the heart, the peripheral muscle pump, and the ventilatory pump.1 However, patients with functionally univentricular hearts and palliative Fontan-type operations have to compensate for the lack of a subpulmonary ventricle that would normally serve as a power source to drive blood through the lungs.2 Hence, the associated passive systemic venous return through the pulmonary system in these patients is substantially influenced by the respiratory pump system providing blood flow mainly by intrathoracic pressure changes. Although remarkable progress in surgical operation procedures has been achieved in recent years, improving the quality of life and reducing the mortality rate, late failure of the Fontan circulation due to chamber dysfunction, hypoplastic pulmonary vessels, elevated pulmonary vascular resistance (PVR), and increased central venous pressure with protein-losing enteropathy still represents a major problem.3,4 Until now, a reliable noninvasive assessment of Fontan circulation quality for early identification of patients at risk remains a challenge in clinical routine work. To investigate the effectiveness of the ventilatory pump, several researchers have studied the vasodilatory effect of oxygen on the pulmonary vasculature.5–8 Although reported results are contradictory, there is some evidence that in healthy volunteers hyperoxemia increases the pulmonary flow and decreases the heart rate, whereas the cardiac output is only marginally altered.
The aim of our study was to find out whether provoked changes of pulmonary blood flow influencing the ventricular preload are capable of differentiating between patients with adequate systemic blood supply after Fontan completion (preserved Fontans) and those candidates with restricted Fontan circulation (impaired Fontans) who develop signs of failing hemodynamics. To achieve this goal, we postulate that respiratory-related stroke volumes that are accessible by noninvasive real-time phase-contrast cardiovascular magnetic resonance (PC-CMR) represent a suitable measure to (1) test the vasodilatory effect of oxygen provocation and (2) estimate the impact of forced breathing on the pulmonary blood flow in Fontan patients.
MATERIALS AND METHODS
Study Design
From April 2013 to September 2014, we prospectively enrolled 29 patients after total cavopulmonary connection (Fontan type, 28 with extracardiac conduit, 1 with lateral tunnel) and 32 healthy controls. The study was approved by the local ethics institutional review committee (registration number: 55/2013). Written informed consent was obtained from the participants or legal guardians. The study complies with the Declaration of Helsinki.
Exclusion criteria comprised typical contraindications for performing CMR, such as metal-containing mechanical or electronic implants and claustrophobia. After explaining the examination procedure, quantitative real-time PC-CMR measurements were taken in the ascending aorta (AAo; 2 cm distally to the aortic valve), superior vena cava (SVC; before the entry into the right pulmonary artery; Fig. 1), and inferior vena cava (IVC; above the hepatic veins and below the fenestration if present).
FIGURE 1.
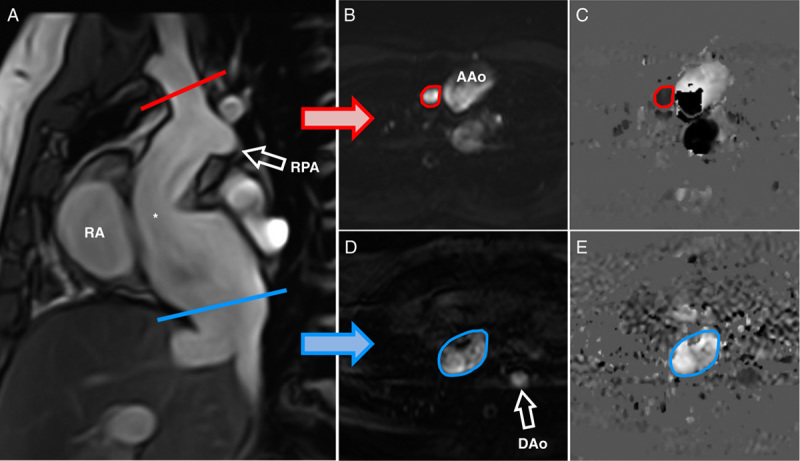
Parasagittal cine-steady-state free precession image in a 31 year-old woman delineating the extracardial Fontan tunnel (A, asterisk) for planning of quantitative real-time PC-CMR in the upper (B, C) and lower caval vein (D, E). End-systolic magnitude images (B, D) and corresponding phase-contrast velocity maps (C, E). DAo indicates descending aorta; RA, right atrium; RPA, right pulmonary artery.
Blood flow was measured (a) under room-air conditions and normal physiological respiration, (b) under room air and forced respiration, and (c) after oxygen administration (100%, 4 L/min; 10 min) using nasal cannulas. Immediately before recording measurements a spirometric equipment including a flow sensor and a nose clip to simultaneously register respiration-related pulmonary volume changes was introduced (BlueCherry Spirostik, Geratherm-Respiratory GmbH, Bad Kissingen, Germany). Healthy subjects were examined under room-air conditions and normal physiological respiration.
Classification of Fontan Patients
The study was intended to differentiate Fontan patients with preserved hemodynamics from those with impaired hemodynamics. The Fontan circulation was classified by defining a 12-parameter risk score according to a modified version of the reference9: fenestration due to elevated PVR, protein-losing enteropathy, high central venous pressure (>15 mm Hg), oxygen saturation (<90%), substantial collateral flow (collateral vessels clearly visible in contrast-enhanced angiography), severely reduced exercise capacity (peak oxygen uptake <50% of normal), substantial atrioventricular valve insufficiency, ejection fraction (<50%), dilatation of the tunnel, diaphragmal paresis, cardiac index (≤2.5 L/min/m2), and morphologically right systemic ventricle. It is to be noted that the presence of fenestration in our institution is given only in case of high PVR to provide sufficient stroke volumes (SVs) and was therefore defined as a risk factor. A risk score ≤3 represents patients with preserved hemodynamics, whereas a score >3 classifies patients with impaired hemodynamics.
Quantitative Real-time PC-CMR
Quantitative flow measurements were performed with a multitransmit 3 T magnetic resonance imaging system (Achieva; Philips Healthcare, Best, The Netherlands). The maximum gradient performance was 80 mT/m and slew rate was 200 T/m/s, and signal detection was performed with a 32-element phased-array coil. After routine examination to assess cardiac function and morphology, additional steady-state free-precession acquisitions (repetition time [TR]/echo time [TE]/flip angle=2.7 ms/1.35 ms/40 degrees) were obtained along the vessel’s course to guarantee accurate through-plane PC-CMR planning.
For real-time PC-CMR a free-breathing, non–electrocardiographically (ECG) triggered flow-sensitive echo-planar imaging (EPI) sequence was used in accordance with the literature.10 To achieve a temporal resolution of 24 to 28 ms, sensitivity encoding (SENSE, reduction factor=4) and half-Fourier (factor=0.6) technique was applied. With this acceleration techniques the echo-train length (EPI factor=17) was shortened to reduce image degrading due to phase errors. Spatial resolution was 2.68×2.68×6 mm3 using a matrix size of 112×112 and a TR/TEeff/flip angle of 12 to 14 ms/3.3 ms/40 degrees. The bandwidth in EPI frequency direction was 3215 Hz. Flow measurements were recorded with a velocity-encoding value of 200 cm/s for AAo and 70 cm/s for caval veins. Each data set included 500 flow-sensitive images acquired during a 12 to 14 s interval (angle dependent) to obtain respiratory-related flow information from 3 to 6 breathing cycles. For offline assignment ECG and respiratory data were simultaneously recorded by the scanner’s wireless physiology unit.
Flow quantification was performed using software developed in our institution. In summary, non–ECG triggered flow-sensitive image data were matched with simultaneously registered physiological data allowing exact assignment of R-waves and breathing curves. The patient’s respiration curve was split into 4 intervals—inspiration, end-inspiration, expiration, and end-expiration—whereby all flow data belonging to 1 single time frame contributed to the formation of virtual SVs (=respiratory-dependent SVs; Figs. 2, 3). It is to be noted that flow information is typically gathered from multiple heart beat segments (see Text document, Supplemental Digital Content 1, http://links.lww.com/JTI/A79), which describes the evaluation software in detail). Calculation of mean SVs was achieved by exploiting all complete cardiac cycles available during the entire acquisition interval, including the effect of breathing and thus representing a surrogate parameter to conventional quantitative flow measurements.
FIGURE 2.
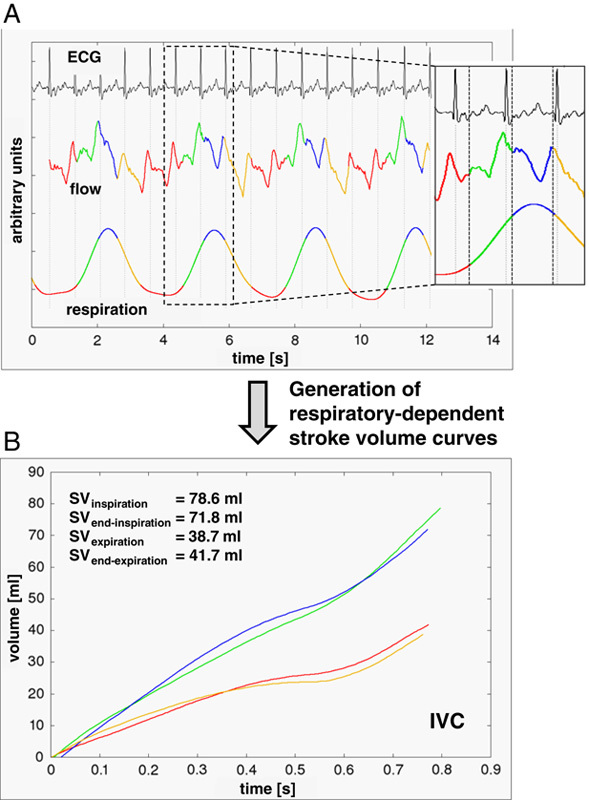
A, Subdivision of the patient’s breathing curve into 4 respiratory phases (green=inspiration; blue=end-inspiration; yellow=expiration; red=end-expiration). As demonstrated in the small picture detail, heart intervals belong typically to different respiratory phases. B, Generation of respiratory-dependent stroke volume (SV) curves by accordingly assigning flow data to the appropriate respiratory phases. Data taken from the inferior vena cava (IVC) of a 25-year-old woman with preserved hemodynamics under normal physiological breathing (room-air condition).
FIGURE 3.
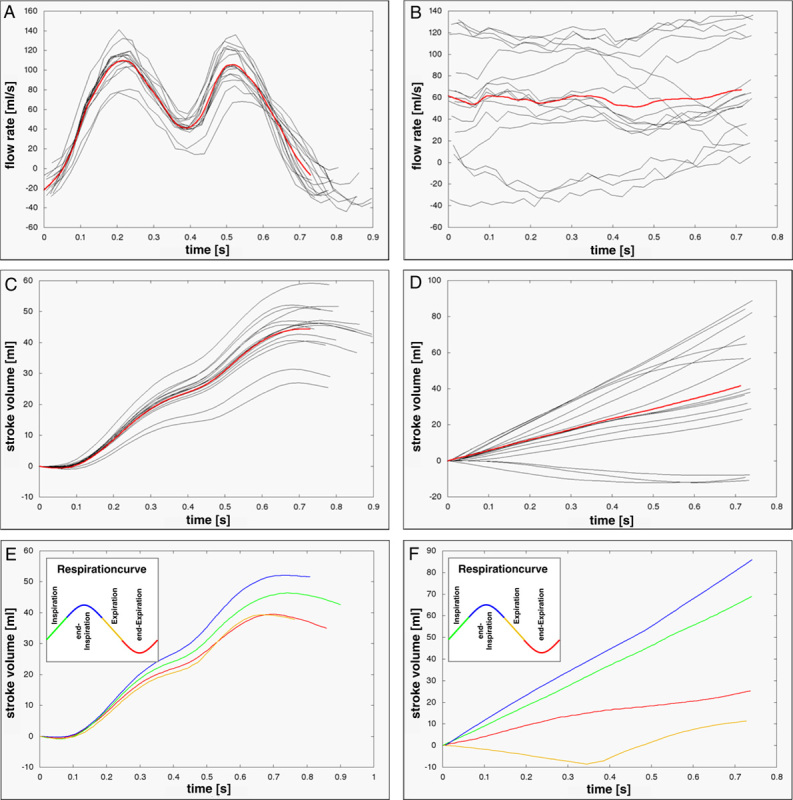
Flow rates (A, B) and stroke volumes (C, D) obtained in the IVC from a volunteer (left, in a 15 year-old woman, room air) and a Fontan patient (right, 28 year-old man) by real-time PC-CMR demonstrating the considerable physiological variation between different heart cycles. Red lines represent average across all heartbeats. E and F, Respiratory-dependent stroke volumes reveal highest flow toward the lungs during end-inspiration (blue) and inspiration (green). Compared with controls an occasionally weak retrograde flow was detected during end-expiration (red) and expiration (yellow) in Fontans.
Statistical Analysis
Statistical analysis was performed using SPSS software (Version 21.0.0.0, IBM Deutschland GmbH). The Shapiro-Wilk test was used to test data on normal distribution and the Levene statistics for variance homogeneity. To compare flow rates and stroke volumes normalized to body surface area (SVi) of preserved versus impaired Fontans, an unpaired Student t test was used for normally distributed data; otherwise the Mann-Whitney U test was used. Comparing flow rates and SV data obtained under room air versus oxygen and normal versus forced breathing, respectively, a paired Student t test was applied for normally distributed data; otherwise the Wilcoxon test was used. Differences in respiratory-related flow rates were analyzed by applying the analysis of variance statistics. The Bonferroni test was applied to identify statistically different groups. Accordingly, non-normally distributed data were tested by the Friedmann test and differences between groups by the Wilcoxon test. Statistical significance was determined at P-values <0.05.
RESULTS
Baseline characteristics of the 2 patient groups are summarized in Table 1. The main diagnoses leading to total cavopulmonary connection were hypoplastic left heart syndrome (N=9), hypoplastic right heart syndrome (N=7), double-inlet left ventricle (N=7), and double-outlet right ventricle (N=3). Other diagnoses included large ventricular septal defect, tricuspid valve atresia, transposition of great arteries, and coarctation (N=3). Twenty-one patients were in New York Heart Association (NYHA) functional class I, 4 patients in class II, and 4 in class III. The average age of healthy subjects was 13.4±3.7 y.
TABLE 1.
Demographics
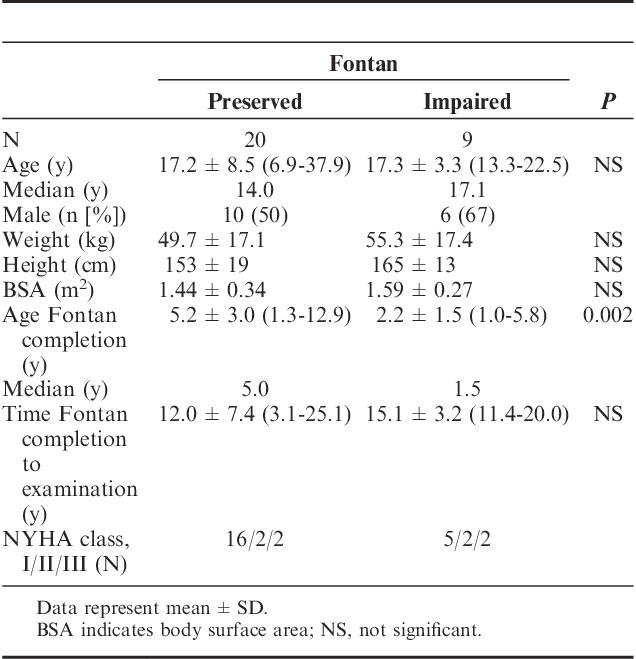
In the subproject “room-air versus oxygen application” 5 data sets (preserved Fontans: N=3) were excluded from the study because of impossibility to generate valid pairs of data from all considered scenarios for statistical analysis. Accordingly, in the second subproject “normal versus forced breathing” 3 data sets (preserved Fontans: N=3) were excluded from the study.
Differentiation of Fontan Hemodynamics Using Stroke Volumes
Room-air Conditions and Normal Physiological Breathing
Compared with healthy controls, the mean aortic SVi and SVi (IVC) in patients with preserved hemodynamics did not differ significantly (P=0.107, respectively 0.070) under room-air conditions and normal physiological breathing, whereas the mean SVi (SVC) was significantly lower in preserved patients (Table 2, 19.6±4.6 to 14.1±6.4 mL/m2; P=0.001). In patients with impaired Fontan circulation the mean SVi in all 3 vessels was statistically lower in comparison with that of controls.
TABLE 2.
Impact of Oxygen Inhalation on Fontan Hemodynamics
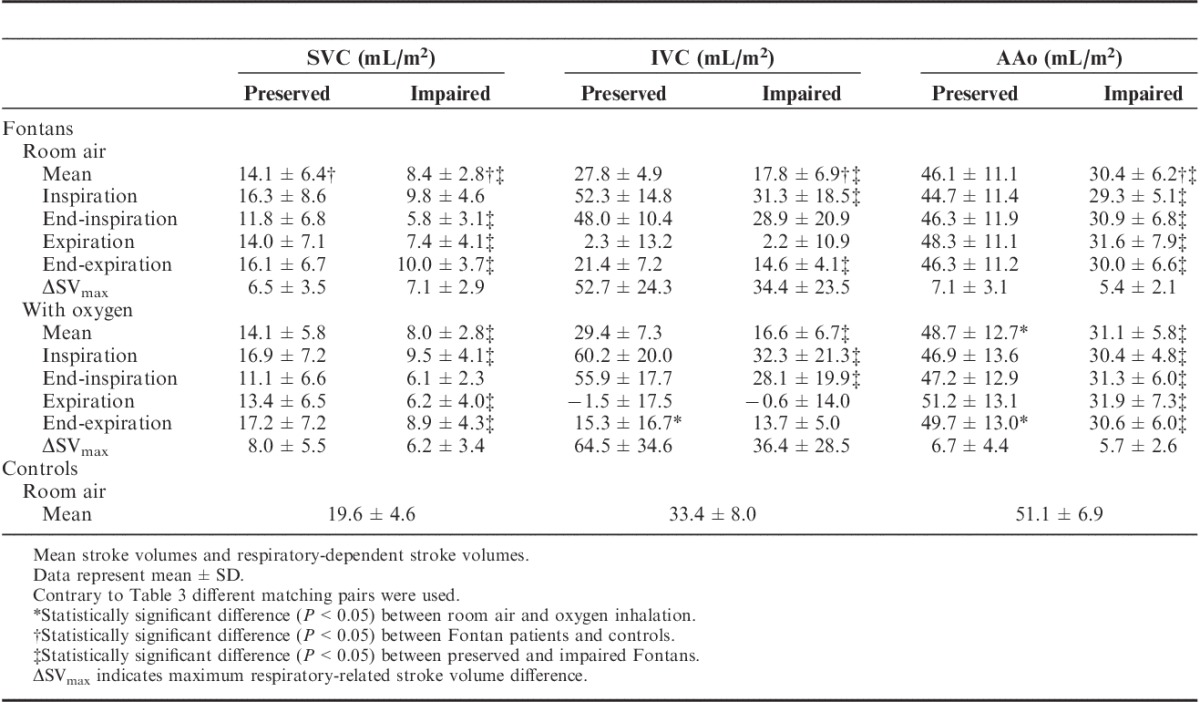
On comparing the 2 patient groups (preserved vs. impaired Fontans) it was found that the mean SVi and respiratory-dependent SVi were in most cases significantly different from each other, showing that generally blood flow was lower in impaired Fontans (Tables 2, 3).
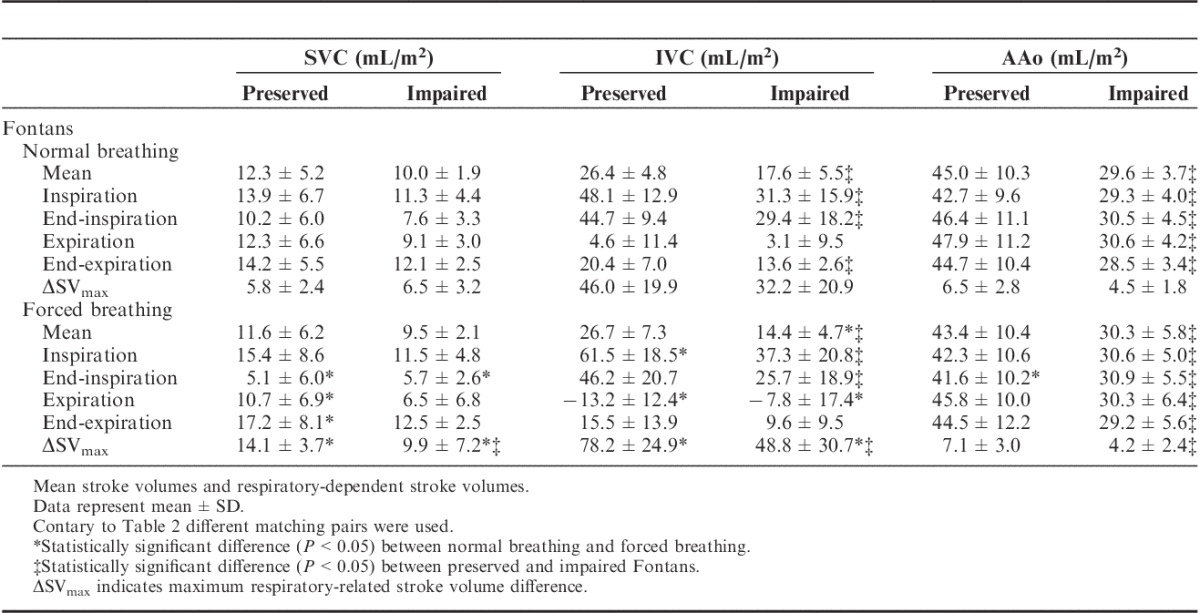
TABLE 3.
Impact of Forced Breathing on Fontan Hemodynamics - stroke volumes
The diagnostic accuracy of SVi was assessed by calculating receiver-operating curves (ROC) and its corresponding area under the curve (AUC). Figure 4 demonstrates the dispersion and overlapping of aortic end-expiratory SVis. The ROC analysis revealed an excellent diagnostic accuracy with an AUC of 0.920 resulting in a positive likelihood ratio (LR+) of 14.5 with a sensitivity of 86% and a specificity of 94%.11 This corresponds to a cutoff value of 32.1 mL/m2 for differentiation of the 2 Fontan groups. Comparable values were found for mean aortic SVi (AUC=0.916; LR+=7.3; sensitivity=86%; specificity=88%; cutoff value=34.3 mL/m2). All other measures showed minor diagnostic accuracy.
FIGURE 4.
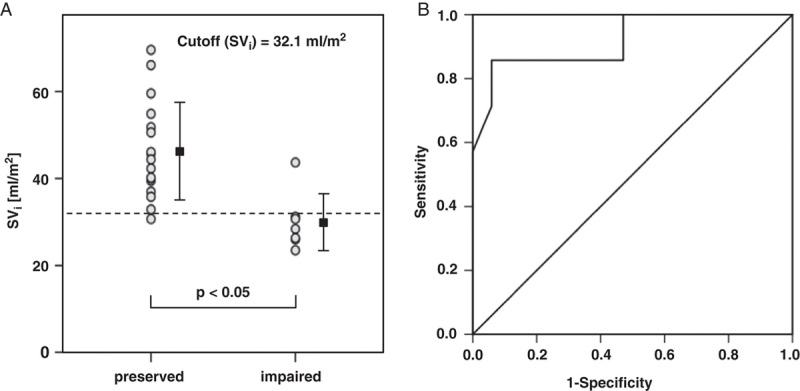
Dot diagram inclusive mean±SD (A) and ROC plot (B) to test the diagnostic accuracy for distinguishing preserved Fontans and impaired Fontans. Data taken from aortic end-expiratory SVi under room-air condition. The AUC was 0.920 and thus represents excellent diagnostic accuracy.
Impact of Oxygen Provocation
The vasodilatory impact of oxygen on pulmonary vessels was identified by a significant increase in mean SVi after oxygen inhalation in the AAo from 46.1±11.1 to 48.7±12.7 mL/m2 (Table 2, P=0.045) in preserved Fontans. The increasing effect was observed in all respiration gates but was most pronounced in end-expiration (46.3±11.2 to 49.7±13.0 mL/m2; P=0.006). The mean SVi remained unchanged in both caval veins (P(IVC)=0.197; P(SVC)=0.962). In this context, heart rate decreased significantly from 75.2±15.9 bpm (baseline) to 70.8±16.4 bpm (P=0.000) after oxygen treatment.
In contrast, in patients with impaired Fontan circulation SVi remained unchanged in all examined vessels. Generally, the mean heart rate was higher (84.1±9.8 bpm; baseline) in this group and was not affected by oxygen (P=0.612).
Impact of Breathing Maneuvers
Instead of testing, the quality of Fontan circulation by oxygen provocation the participants were also asked to breathe in and breathe out deeply during data acquisition. The intensity of this forced respiratory maneuver was controlled by spirometry. On average (all vessels), tidal volumes were 3.2-fold higher during forced respiration compared with that during normal physiological breathing.
Forced breathing had no influence on mean SVi (SVC, IVC, Ao) in preserved Fontans (Table 3). Otherwise, SVi (IVC) increased considerably during inspiration from 48.1±12.9 to 61.5±18.5 mL/m2 (P=0.003) and was significantly reduced during expiration (4.6±11.4 to −13.2±12.4 mL/m2; P=0.000). Addressing SVi (SVC) a substantial increase was found during end-expiration and a decrease during end-inspiration (P=0.015, respectively 0.000).
Comparable with preserved Fontans, forced breathing affects the respiratory-dependent SVi changes in impaired Fontans in a similar manner but with reduced intensity, as indicated by lower maximum respiratory-related stroke volume differences (ΔSVmax) (Table 3).
Blood Flow Rates
Room-air Conditions and Normal Physiological Breathing
Contrary to SVs, a somewhat minor relevance regarding differentiation of Fontan groups was found when absolute blood flow rates were used. This was confirmed by ROC plots, in which AUC values were lower (∼0.800, data not shown) for mean aortic flow rates and end-expiratory aortic flow rates, thus demonstrating a weaker diagnostic accuracy.
At room air and normal physiological breathing the mean aortic flow rate was significantly higher in preserved Fontans compared with that in impaired Fontans (Table 4; P=0.013). The greater aortic flow was present in all respiration intervals except during inspiration. Likewise, the mean IVC flow rate was pronounced in the preserved group in comparison with that in the impaired group (P=0.036), whereas the mean SVC flow rate did not significantly differ between the 2 patient groups (P=1.000).
TABLE 4.
Impact of Forced Breathing on Fontan Hemodynamics - Blood Flow Rates
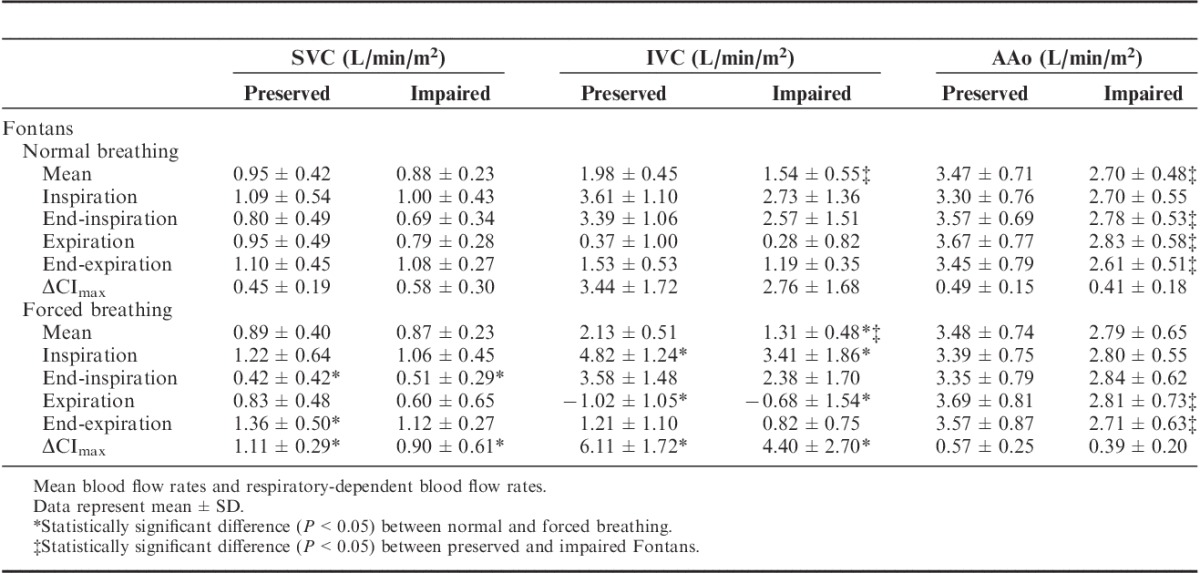
Impact of Oxygen Provocation
In contrast to SVs, aortic blood flow rates (note: flow rate represents the product of SV and heart rate) were not significantly altered after oxygen inhalation in preserved Fontans because of the compensatory effect of reduced heart rates in combination with increasing SVs in this group. As already indicated above, flow rates remained unchanged in impaired Fontans as well because neither heart rates nor SVs were modified after applying oxygen in these patients.
In general, oxygen provocation did not substantially modify the respiration-dependent blood flow rate amplitudes in any of the 3 vessels compared with normal physiological breathing under room-air conditions.
Impact of Breathing Maneuvers
On the basis of the forced breathing procedure, the maximum respiratory-related cardiac index difference (ΔCImax) was almost doubled compared with normal physiological breathing in both caval veins and in both patient groups (P<0.05; Table 4). Otherwise, the mean blood flow rates in all examined vessels remained unchanged except a reduced mean IVC flow rate in patients with impaired Fontan hemodynamics (1.54±0.55 vs. 1.31±0.48 L/min/m2, P=0.017).
Forced breathing significantly increased IVC blood flow during inspiration but induced considerably damped IVC flow during expiration in both patient groups (P<0.05). Likewise, a significant reduction in SVC flow was observed in the end-inspiration interval in preserved (P=0.000) and impaired (P=0.028) patients, whereas a significant increase was found during end-expiration (only preserved Fontans, P=0.010; see Figs. 2A to 2C, Supplemental Digital Content, http://links.lww.com/JTI/A80, http://links.lww.com/JTI/A81, http://links.lww.com/JTI/A82, which demonstrate the respiratory-related blood flow changes from mean).
DISCUSSION
To our knowledge this is the first attempt to assess whether respiratory-related SVi applying real-time PC-CMR is associated with diagnostic markers to classify the quality of Fontan hemodynamics. The main finding in this study is that SVi is generally suitable for differentiating preserved from restricted Fontan hemodynamics. Regarding the mean SVi values in AAo and IVC, respectively, considerably lower SVi was detected in impaired Fontans compared with that in preserved Fontans in all scenarios (room air, oxygen provocation, forced breathing). The high diagnostic value, particularly of aortic end-expiratory SVi, was underlined by ROC statistics, where AUC was >0.9 with LR+ >10. This corresponds to a high conclusive increase in the likelihood of disease—that is, impaired Fontan circulation. Accordingly, the calculated cutoff value of 32.1 mL/m2 implies that below this threshold it is highly likely that patients possess an impaired Fontan circulation. Therefore, simple noninvasive real-time flow measurements may be capable of predicting a complicated clinical course and thus could positively influence drug therapy and clinical decision making.
Several diagnostic parameters such as increased PVR, high ventricular end-diastolic pressure, impaired exercise capacity, protein-losing enteropathy, low arterial oxygen saturation, and plastic bronchitis are associated with failure of Fontan circulation12–14 but none is solely suitable for classification. Just recently, liver stiffness measurements applying noninvasive transient elastography were proposed as a promising tool to identify patients with unfavorable Fontan hemodynamics.15
Referring to the mean aortic SVi data, we found only a minor reduction in preserved Fontans compared with healthy controls (P=0.107) under room-air condition and normal physiological breathing at rest. In contrast, patients with an impaired circulation had a significantly lower mean aortic SVi (P=0.000).
Several studies were carried out to investigate exercise intolerance among Fontan patients and the accompanying hemodynamic causes to gain a better understanding of altered hemodynamic relations as compared with a normal physiological circulation.16–18 In this connection, the authors found a reduced SVi, although the observed difference between patients and controls at rest as well as during exercise was somewhat lower compared with our results in impaired Fontan circulation.16,17 This is possibly related to the subdivision into the 2 different severity levels of Fontan hemodynamics in our study.
An additional main finding is that oxygen provocation increases aortic SVi in preserved Fontans but not in impaired Fontan circulation. This effect could be explained by the vasodilatory effect of oxygen on the pulmonary vascular system in the former group by reducing PVR. Accordingly, blood flow toward the heart is facilitated and ventricular preload is improved.7 This increased blood supply results in secondary lower heart rates and therefore in unchanged flow rates before and after oxygen application. Several researchers also found a decrease in heart rate under oxygen but in healthy volunteers.6,8,19 This physiological effect could help in early identification of patients who start to develop impaired hemodynamics and those who may benefit from timely antipulmonary hypertensive drug therapies like cGMP-specific PDE5 inhibitors (sildenafil)20 or endothelin receptor antagonists (bosentan). Recently, in a randomized, placebo-controlled, double-blind study, improvements in exercise capacity, exercise time, and functional class (NYHA) after bosentan treatment were demonstrated in 69 Fontan patients.21 Although results are promising the authors emphasize that considering high costs and potential adverse effects of bosentan the appropriate onset of treatment is still unknown. Therefore, timely differentiation of responders on pulmonary vasodilators from nonresponders is of great importance.
In contrast, no adaption of SVi and heart rates was observed in impaired Fontans. One explanation could be that maximal dilation of the pulmonary vasculature has already occurred or that diseased vessels were unable to react on oxygen inhalation by vasodilation, resulting in the inability to augment ventricular preload. In the latter case, applying more potent pulmonary vasodilators like nitric oxide22 or iloprost may help to identify patients with treatable PVR. Otherwise it is also conceivable that diastolic dysfunction is responsible for the inability to adapt SVs despite the increased preload.23
Generally, heart rates tended to be higher in impaired Fontans to compensate for the typically lower SVs. The inability of several Fontan patients to adapt cardiac filling and output to physical demands compared with healthy individuals and the chronotropic incompetence was frequently described by others when assessing exercise intolerance.14,17,24 The authors found a reduced forced vital capacity possibly reflecting a smaller lung size combined with chronically diseased pulmonary vessels, which explains the insufficient cardiac output during exercise due to inability to increase the ventricular preload across the pulmonary circulation.12,14 It was assumed that a physiological decrease in PVR, which is needed under exercise conditions to enhance blood flow and thus SV, may be limited in failing Fontans.
Several studies demonstrated the respiratory impact on blood flow in thoracic vessels of healthy volunteers and Fontan patients.9,18,25 The negative intrathoracic pressure that is induced during inspiration results in higher IVC flow by blood pool activation (located in the venous system of the lower body) and by augmentation of hepatic flow during this respiratory phase.18,25 By contrast, blood flow is considerably reduced during expiration. In comparison with healthy controls the missing subpulmonary pumping chamber is presumably responsible for the higher respiration dependency and therefore for pronounced IVC amplitudes in Fontan patients. As shown in Supplemental Digital Content 2 (see Figs., Supplemental Digital Content 2, http://links.lww.com/JTI/A80, http://links.lww.com/JTI/A81, http://links.lww.com/JTI/A82, which demonstrate the respiratory-related blood flow changes from mean), the strongest IVC blood flow in preserved Fontans occurs during inspiration (+90% compared with mean flow rate) but is diminished during expiration (−94%).
In contrast, SVC blood flow was influenced by the patients’ breathing to a less extent (+16% vs. −20%) and was somewhat shifted to the end-expiration phase. This observation contrasts with the results of Hjortdal et al,18 in which the respiratory dependency in SVC could not be detected by dividing the respiration curve only into 2 parts.
In contrast to healthy volunteers, the major respiration-dependent aortic blood flow occurs earlier in Fontan patients during expiration (+6%; see Fig., Supplemental Digital Content 2A, http://links.lww.com/JTI/A80, which demonstrates the respiratory-related blood flow changes from mean).9,26 This is explainable by the absence of a subpulmonary ventricle in such patients leading to a premature arrival of inspiratory venous bulk flow in the lungs and therefore subsequently in the AAo.
When performing a short period of forced breathing (12 to 14 s), the mean SVs remained unchanged in preserved Fontans. In contrast, a substantial decrease in venous return from the IVC was observed in impaired Fontans. In another study examining the contribution of the muscle and ventilator pump during exercise in clinically stable Fontan patients (NYHA class I) and who correspond approximately to our patients with preserved hemodynamics, a significant change in SV by activating the ventilator pump was not observed either.16 Otherwise, when introducing an expiratory load condition on submaximal exercise, the authors found a substantial SVi reduction, assuming that Fontan patients have only an inadequate hemodynamic reserve to react on an elevated metabolic demand. Our results demonstrate that this behavior is already present in patients with impaired hemodynamics wherein a considerable decline of venous return was detected during simple forced breathing conditions (without exercise). As aortic flow remains unchanged it can be assumed that collateral flow, which is defined as the difference between aortic flow and the total caval flow (SVC and IVC), is augmented (0.28 to 0.61 L/min/m2; P=0.104) to compensate for the lower IVC flow in this group. For comparison only, collateral flow was not elevated in preserved Fontans (0.54 to 0.46 L/min/m2; P=0.432). Some hints may be given by a complementary study in which a significant drop of collateral flow was observed if inhaled nitric oxide was applied to lower PVR in Fontan patients.22 Although the exact reasons for this behavior are unknown, there is some evidence that collateral flow may play a balancing role in Fontan hemodynamics.27
In this regard, it should be emphasized that, contrary to the common assumption, forced breathing did not augment cardiac output by increasing the ventricular preload in Fontan patients. No changes in mean blood flow (except IVC flow in impaired Fontans) were seen by exclusively activating the respiratory pump, although ΔCImax was drastically enhanced. On the basis of the presented results (Tables 3, 4) we hypothesize that the increased venous return caused by forced inspiration was fully compensated by the same amount of damped blood flow during forced expiration.
Several limitations should be acknowledged in this context. Currently it remains unclear as to what extent the abdominal-placed air-cushion belt represents the breathing curve correctly in case of subjects with predominately chest breathing. Theoretically, it is conceivable that in such cases the respiratory-related SVis are allocated imprecisely. To avoid such inaccuracies, verification of the scanners’ physiological data was achieved by simultaneously recording spirometric data. In the near future, logging of respiratory information with MR-compatible contact-free monitoring systems should be possible.28
As is generally known, failure of Fontan circulation is linked with reduced SVi. In this study no testing was done to identify the impact of ventricular stiffness on changes in Fontan hemodynamics.
In conclusion, oxygen augments the ventricular preload and thus increases SVi, whereas secondary lowers heart rates in patients with preserved Fontan circulation. The cardiovascular system of patients with impaired hemodynamics has only limited adaptive capabilities. This supports the hypothesis that preserved Fontans are able to decrease PVR by vasodilation, which could be an indication for using pulmonary vasodilators in therapy. Moreover, this study implies that forced breathing considerably increases the maximum respiratory-related cardiac index difference (ΔCImax); however, it has no impact on mean blood flow with the exception of reduced mean IVC blood flow in impaired Fontans by sole activation of the respiratory pump. Finally, the present work suggests that SVi (AAo, end-expiratory) is most suitable to distinguish the quality of Fontan circulation using a cutoff (SVi) value of 32 mL/m2. Therefore, this value is of clinical relevance for timely identification of hemodynamic impairment and early therapy of Fontan patients.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.thoracicimaging.com.
Footnotes
Hermann Körperich and Katja Müller contributed equally to this work.
Supported in part by the Fördergemeinschaft Deutsche Kinderherzzentren e.V.; Project identification No:W-BDO-019/2013. The authors declare no conflict of interest.
REFERENCES
- 1.Rowland TW. The circulatory response to exercise: role of the peripheral pump. Int J Sports Med. 2001;22:558–565. [DOI] [PubMed] [Google Scholar]
- 2.Cordina RL, O’Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. [DOI] [PubMed] [Google Scholar]
- 3.De Vadder K, Van De Bruaene A, Gewillig M, et al. Predicting outcome after Fontan palliation: a single-centre experience, using simple clinical variables. Acta Cardiol. 2014;69:7–14. [DOI] [PubMed] [Google Scholar]
- 4.de Leval MR. The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med. 2005;2:202–208. [DOI] [PubMed] [Google Scholar]
- 5.Bak Z, Sjoberg F, Rousseau A, et al. Human cardiovascular dose-response to supplemental oxygen. Acta Physiol (Oxf). 2007;191:15–24. [DOI] [PubMed] [Google Scholar]
- 6.Ley S, Puderbach M, Risse F, et al. Impact of oxygen inhalation on the pulmonary circulation: assessment by magnetic resonance (MR)-perfusion and MR-flow measurements. Invest Radiol. 2007;42:283–290. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Tesselaar E, Borges JB, et al. Hyperoxia affects the regional pulmonary ventilation/perfusion ratio: an electrical impedance tomography study. Acta Anaesthesiol Scand. 2014;58:716–725. [DOI] [PubMed] [Google Scholar]
- 8.Daly WJ, Bondurant S. Effects of oxygen breathing on the heart rate, blood pressure, and cardiac index of normal men--resting, with reactive hyperemia, and after atropine. J Clin Invest. 1962;41:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korperich H, Barth P, Gieseke J, et al. Impact of respiration on stroke volumes in paediatric controls and in patients after Fontan procedure assessed by MR real-time phase-velocity mapping. Eur Heart J Cardiovasc Imaging. 2015;16:198–209. [DOI] [PubMed] [Google Scholar]
- 10.Korperich H, Gieseke J, Barth P, et al. Flow volume and shunt quantification in pediatric congenital heart disease by real-time magnetic resonance velocity mapping: a validation study. Circulation. 2004;109:1987–1993. [DOI] [PubMed] [Google Scholar]
- 11.Carter JV, Pan J, Rai SN, et al. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159:1638–1645. [DOI] [PubMed] [Google Scholar]
- 12.Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart Fail Clin. 2014;10:105–116. [DOI] [PubMed] [Google Scholar]
- 13.Ohuchi H, Yasuda K, Miyazaki A, et al. Comparison of prognostic variables in children and adults with Fontan circulation. Int J Cardiol. 2014;173:277–283. [DOI] [PubMed] [Google Scholar]
- 14.Opotowsky AR, Landzberg MJ, Earing MG, et al. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol. 2014;307:H110–H117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu FM, Opotowsky AR, Raza R, et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit Heart Dis. 2014;9:438–447. [DOI] [PubMed] [Google Scholar]
- 16.Shafer KM, Garcia JA, Babb TG, et al. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation). J Am Coll Cardiol. 2012;60:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert A, Jensen AS, Mikkelsen UR, et al. Hemodynamic causes of exercise intolerance in Fontan patients. Int J Cardiol. 2014;175:478–483. [DOI] [PubMed] [Google Scholar]
- 18.Hjortdal V, Emmertsen K, Stenbog E, et al. Effects of exercise and respiration on blood flow in total cavopulmonary connection: a real-time magnetic resonance flow study. Circulation. 2003;108:1227–1231. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau A, Bak Z, Janerot-Sjoberg B, et al. Acute hyperoxaemia-induced effects on regional blood flow, oxygen consumption and central circulation in man. Acta Physiol Scand. 2005;183:231–240. [DOI] [PubMed] [Google Scholar]
- 20.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014;7:265–273. [DOI] [PubMed] [Google Scholar]
- 21.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–2030. [DOI] [PubMed] [Google Scholar]
- 22.Latus H, Gerstner B, Kerst G, et al. Effect of inhaled nitric oxide on blood flow dynamics in patients after the Fontan procedure using cardiovascular magnetic resonance flow measurements. Pediatr Cardiol. 2016;37:504–511. [DOI] [PubMed] [Google Scholar]
- 23.La Gerche A, Gewillig M. What limits cardiac performance during exercise in normal subjects and in healthy Fontan patients? Int J Paediatr. 2010;2010:791291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takken T, Hulzebos HJ, Blank AC, et al. Exercise prescription for patients with a Fontan circulation: current evidence and future directions. Neth Heart J. 2007;15:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsia TY, Khambadkone S, Redington AN, et al. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;102:III148–III153. [DOI] [PubMed] [Google Scholar]
- 26.Claessen G, Claus P, Delcroix M, et al. Interaction between respiration and right versus left ventricular volumes at rest and during exercise: a real-time cardiac magnetic resonance study. Am J Physiol Heart Circ Physiol. 2014;306:H816–H824. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead KK, Harris MA, Glatz AC, et al. Status of systemic to pulmonary arterial collateral flow after the fontan procedure. Am J Cardiol. 2015;115:1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maclaren J, Aksoy M, Bammer R. Contact-free physiological monitoring using a markerless optical system. Magn Reson Med. 2015;74:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.thoracicimaging.com.


