Abstract
The crystalline lens plays an important role in the refractive vision of vertebrates by facilitating variable fine focusing of light onto the retina. Loss of lens transparency, or cataract, is a frequently acquired cause of visual impairment in adults and may also present during childhood. Genetic studies have identified mutations in over 30 causative genes for congenital or other early-onset forms of cataract as well as several gene variants associated with age-related cataract. However, the pathogenic mechanisms resulting from genetic determinants of cataract are only just beginning to be understood. Here, we briefly summarize current concepts pointing to differences in the molecular mechanisms underlying congenital and age-related forms of cataract.
Keywords: Cataract, Lens, Genetic, Crystallin, UPR
1. Introduction
Cataract can be defined broadly as any opacity of the crystalline lens. This has been shown to happen whenever the refractive index of the lens varies significantly over distances approximating the wavelength of the transmitted light (Benedek, 1971; Delaye and Tardieu, 1983). This variation in the refractive index can occur as a result of a variety of changes, including alterations of lens cell structure, lens proteins or a combination of both (Hejtmancik et al., 2001). Alterations to the geometric order of the lens and its membranes can amplify these effects and this can increase light scattering. Congenital cataracts are often associated with breakdown of the lens micro-architecture. Vacuoles can form and cause large fluctuations in optical density with concomitant light scattering. In contrast, age related cataracts are often characterized by light scattering and opacity resulting from the buildup of high molecular weight protein aggregates (HMW), generally 1000 Å or more in size. This can also disrupt the short-range ordered packing of lens crystallins, which is critical for maintaining the crystallins in a homogeneous phase, with disastrous consequences for lens transparency as they compose over 90% of soluble lens proteins. However, while formation of large protein complexes might be a common end point in many cataracts, it is important to remember that reduced and disrupted refractive properties in the lens do not result from protein aggregation and precipitation in all cases. Mutations in BFSP2 were first identified in myopic patients in whom opacification of the lens sutures was only found upon closer examination (Zhang et al., 2004). In addition, age related cataract might not be a simple function of protein precipitation, as shown by the existence of multilamellar bodies in age related cataract (Costello et al., 2012). Finally, the two mechanisms of crystallin aggregation and micro-architectural disruption are not exclusive, as the proper lens cell environment is important for the maintenance of lens crystallin structure and existence in a homogeneous phase.
Cataracts can be defined by age at onset, although the boundaries between different types of cataract are approximate. A cataract is termed congenital or infantile if it is observed within the first year of life. If onset occurs within the first decade of life cataract is termed juvenile and a cataract with an onset later but before the age of 45 years is called presenile, with senile or age-related cataracts generally occurring after 50 or perhaps 60 years of age. The situation is complicated because subtle cataracts, which can easily be asymptomatic, might not be brought to clinical attention for years after their onset. In addition, a cataract’s age of onset does not imply a particular etiology. About 8.3–25% of congenital cataracts are hereditary (Francois, 1982; Haargaard et al., 2005; Merin, 1991) with the remainder generally caused by an intrauterine infection (e.g. rubella) or other prenatal insult. Secondary cataracts such as those occurring as part of a systemic disease life (e.g., retinitis pigmentosa) may be delayed as late as the second or third decade of life. However, the age of onset of a cataract serves as a useful metric with which to group cataracts, and it would seem logical that, while cataracts within each group will certainly have a variety of mutations in different genes affecting specific cellular processes they might share some similarities in their general pathogenic mechanisms, as detailed in the following paragraph.
We and others have suggested that when mutations in crystallins or other lens proteins are sufficient in and of themselves to cause protein aggregation rapidly and directly, they usually result in congenital cataract. In contrast, if they are benign enough merely to increase susceptibility to environmental insults, including hyperglycemic and dietary (Weikel et al., 2014), ultraviolet light (Taylor et al., 1988), or oxidative (Brennan et al., 2012; Truscott, 2005) damage, they would tend to contribute to age related cataract (Hejtmancik and Smaoui, 2003; Shiels and Hejtmancik, 2007) by exacerbating the accumulation of damage seen to long lived lens proteins with aging (Truscott and Friedrich, 2016). Consistent with these proposed mechanisms, hereditary congenital cataracts are most often transmitted in a highly penetrant Mendelian fashion, and cataracts with a later origin, including progressive and age-related cataracts, are often multifactorial, with contributions from multiple genes providing from 35% to as much as 58% of the risk (McCarty and Taylor, 2001) as well as environmental insults. Although this makes them significantly less amenable to genetic and biochemical study than congenital cataracts, inroads are beginning to be made into their genetic etiologies and in some cases their molecular mechanisms.
2. Congenital cataract
2.1. Inherited congenital cataract mutations
Isolated or primary congenital cataracts currently have been mapped to at least 44 genetic loci (Table 1), exhibiting a wide variety of lens opacity morphologies including, nuclear, lamellar, sutural, polar and total (Merin, 1991). While the causative genes have not been identified at 11 of these loci the genes that have been discovered tend to fall into a number of functional groups that identify critical biological processes in the eye lens. Of the cataract families for whom the mutant gene is known, about 45% show mutations in lens crystallins, about 12% have mutations in various growth or transcription factors; 16% show mutations in connexins, about 5% each show mutations in intermediate filament proteins, membrane proteins, or the protein degradation apparatus, and about 8% show mutations in a variety of other functionally divergent genes including those for lipid metabolism (Shiels et al., 2010). Inheritance of the same mutation in different families or in different individuals within the same family can result in radically different cataract phenotypes (phenotypic heterogeneity), which suggests additional genetic or environmental factors might modify expression of the mutant protein that is the primary cause of the cataracts. Conversely, mutations in different genes active in apparently unrelated biological processes can cause cataracts having similar or identical morphologies (genotypic heterogeneity), suggesting that the cataract observed clinically might be a final common pathway for a varied spectrum of initial insults.
Table 1.
Loci and genes for cataract (CTRCT).
| Cataract phenotype | Locus | Inheritance | Associated phenotypes | Gene | Phenotype MIM no. |
Gene/locus MIM no. |
|---|---|---|---|---|---|---|
| CTRCT1; multiple types | 1q21.1 | AD/AR | ±microcornea | GJA8 | 116200 | 600897 |
| CTRCT2; multiple types | 2q33.3 | AD | ±microcornea | CRYGC | 604307 | 123680 |
| CTRCT3; multiple types | 22q11.23 | AD | ±microcornea | CRYBB2 | 601547 | 123620 |
| CTRCT4; multiple types | 2q33.3 | AD | ±microcornea | CRYGD | 115700 | 123690 |
| CTRCT5; multiple types | 16q21 | AD/AR | HSF4 | 116800 | 602438 | |
| CTRCT6; multiple types | 1p36.13 | AD/AR | Age-related cortical | EPHA2 | 116600 | 176946 |
| CTRCT7 | 17q24 | AD | ? | 115660 | ? | |
| CTRCT8; multiple types | 1pter-p36.13 | AD | ? | 115665 | ? | |
| CTRCT9; multiple types | 21q22.3 | AD/AR | ±microcornea | CRYAA | 604219 | 123580 |
| CTRCT10; multiple types | 17q11.2 | AD | CRYBA1 | 600881 | 123610 | |
| CTRCT11; multiple types | 10q24.32 | AD | Anterior segment mesenchymal dysgenesis, microphthalmia, neurodevelopmental abnormalities | PITX3 | 610623 | 602669 |
| CTRCT12; multiple types | 3q22.1 | AD | Myopia? | BFSP2 | 611597 | 603212 |
| CTRCT13 | 6p24 | AR | Adult i (blood group) phenotype | GCNT2 | 110800 | 600429 |
| CTRCT14; multiple types | 13q12.1 | AD | GJA3 | 601885 | 121015 | |
| CTRCT15; multiple types | 12q13.3 | AD | MIP | 615274 | 154050 | |
| CTRCT16; multiple types | 11q22.3 | AD/AR | Myopathy, cardiomyopathy | CRYAB | 613763 | 123590 |
| CTRCT17; multiple types | 22q12.1 | AD/AR | CRYBB1 | 611544 | 6009291 | |
| CTRCT18 | 3p21.31 | AR | FYCO1 | 610019 | 607182 | |
| CTRCT19 | 19q13.41 | AR | LIM2 | 615277 | 154045 | |
| CTRCT20; multiple types | 3q27.3 | AD | CRYGS | 116100 | 123730 | |
| CTRCT21; multiple types | 16q22-q23 | AD | ±microcornea | MAF | 610202 | 177075 |
| CTRCT22; multiple types | 22q11.23 | AD/AR | CRYBB3 | 609741 | 123630 | |
| CTRCT23 | 22q12.1 | AD | CRYBA4 | 610425 | 123631 | |
| CTRCT24; anterior polar | 17p13 | AD | ? | 601202 | ? | |
| CTRCT25 | 15q21-q22 | AD | ? | 605728 | ? | |
| CTRCT26; multiple types | 9q13-q22 | AR | ? | 605749 | ? | |
| CTRCT27; nuclear progressive | 2p12 | AD | ? | 607304 | ? | |
| CTRCT28 | 6p12-q12 | Complex | Age-related cortical, susceptibility to | ? | 609026 | ? |
| CTRCT29; coralliform | 2pter-p24 | AD | ? | 115800 | ? | |
| CTRCT30; pulverulent | 10p13 | AD | VIM | 116300 | 193060 | |
| CTRCT31; multiple types | 20q11.21 | AD | CHMP4B | 605387 | 610897 | |
| CTRCT32; multiple types | 14q22-q23 | AD | ? | %115650 | ? | |
| CTRCT33; cortical | 20p12.1 | AR | BFSP1 | 611391 | 603307 | |
| CTRCT34; multiple types | 1p34.3-p32.2 | AR | ±microcornea | ? | 612968 | ? |
| CTRCT35; congenital nuclear | 19q13 | AR | ? | 609376 | ? | |
| CTRCT36 | 9q22.33 | AR | TDRD7 | 613887 | 611258 | |
| CTRCT37; cerulean | 12q24.2-q24.3 | AD | ? | 614422 | ? | |
| CTRCT38 | 7q34 | AR | Senger’s syndrome | AGK | 614691 | 610345 |
| CTRCT39; multiple types | 2q34 | AD | CRYGB | 615188 | 123670 | |
| CTRCT40 | Xp22.13 | X-linked | Nance-Horan (cataract dental) syndrome | NHS | 302200 | 300457 |
| CTRCT41 | 4p16.1 | AD | Wolfram syndrome, (DIDMOAD) | WFS1 | 116400 | 606201 |
| CTRCT42 | 2q34 | AD | CRYBA2 | 115900 | 600836 | |
| CTRCT43 | 17q12 | AD | UNC45B | 616279 | 611220 | |
| CTRCT44 | 21q22.3 | AR | LSS | 616509 | 600909 |
In addition to occurring as an isolated defect, congenital cataracts may be associated with other anterior chamber developmental anomalies such as microphthalmia, aniridia or microcornea. Lens opacities may also be part of multisystem genetic disorders such as chromosome abnormalities, DNA repair deficiencies, Lowe syndrome, or Nance Horan Syndrome. Cataracts resulting from mutations in CRYAB are often associated with myofibrillar myopathy and cardiomyopathy (Selcen and Engel, 2003). In some cases the distinction between syndromic and isolated cataract becomes somewhat arbitrary, such as in anterior segment mesenchymal dysgenesis resulting from mutations in the PITX3 gene (Semina et al., 1998). Here, inherited cataracts may be isolated in some family members and associated with additional findings in others. Some of the more common syndromic cataract mutations that also occur as isolated cataracts are shown in Table 1, while a more complete list is provided in the Molecular and Metabolic Basis of Inherited Diseases (Hejtmancik et al., 2001) and in Cat-Map (Shiels et al., 2010; Zhang et al., 2004).
2.2. Congenital cataract mechanisms
As stated above, congenital cataracts tend to result from mutations with severe functional consequences for the mutant protein structure and function. For transcription factors such as PITX3 and MAF this might mean absence of a transcriptional activator at a critical point in lens development resulting in failure of appropriate lens structures and protein components (Burdon et al., 2003; Ferda et al., 2000; Jamieson et al., 2002; Semina et al., 1998, 2001). For membrane and channel proteins this might mean absent or dramatically inappropriate ion or solute transport (Pal et al., 2000; Shiels et al., 1998). For βγ-crystallins, this usually implies gross disruption of the protein fold, resulting in rapid denaturation of the protein, often accompanied by precipitation from the soluble phase of the lens cytoplasm. A number of these mutations have been studied in detail, providing some insight into the pathogenic mechanisms of congenital cataract, and their common aspects. While there are too many of these to explore them all here, it is instructive to examine a few that have been studied in detail.
One crystallin that has been studied extensively is γS-crystallin, which combines some properties of both the β- and γ-crystallins (Hejtmancik and Piatigorsky, 2000). Mutations in γS-crystallin have been implicated in both congenital cataract and progressive juvenile cataracts (Sun et al., 2005; Vanita et al., 2009). One congenital cataract that has been well studied is that resulting from the c.124G > A (p.Val42Met) mutation, which causes a congenital nuclear cataract in which the central nuclear opacity is denser than that in the periphery (Vanita et al., 2009), as might be expected from the expression patterns of the γ-crystallins. This mutation has been shown to distort the compact packing of the molecule, opening up the tertiary structure and exposing hydrophobic residues normally buried in the internal protein to the surface (Bharat et al., 2014; Vendra et al., 2012). Similar increases of surface hydrophobicity with corresponding decreases in solubility have been shown for other γ-crystallin mutations associated with cataract (Pande et al., 2005, 2010). This then causes both self-aggregation of the crystallin under mild (approximately physiological) conditions as well as an increased sensitivity to both chemical and thermal denaturation. Overall, the mutant precipitates and scatters light more readily than the wild type γS-crystallin.
While the denatured γS-crystallin might normally be expected to be bound up by α-crystallin, which acts as a chaperone, binding of destabilized proteins is also dependent on the dynamic population of folding intermediates, so that it is possible that many or most of the mutant crystallins implicated in congenital cataracts escape binding by α-crystallin (Sathish et al., 2004). Alternatively, the mass of denatured protein and the rapidity with which denaturation occurs might overwhelm the capability of α-crystallin to buffer the lens from partially denatured or damaged proteins. Not only would this lead to the presence of large particles capable of scattering light in the lens, but would also expose the lens to potentially toxic denatured proteins that might then disturb the homeostasis of the lens cells.
That the lens cells themselves might be damaged in congenital cataracts is supported by the disarray in lens microarchitecture seen in many cataracts that have been studied in model systems. One example of this is seen in cataracts caused by a 5-base insertion (c.119_123dup, c.238insGCGGC, p.C42Afs*63) in the CRYGC gene (Ma et al., 2011; Ren et al., 2000; Scott et al., 1994). Unlike the p.Val42Met CRYGS mutation, this one caused a variable phenotype ranging from total to lamellar to nuclear pulverulent. The basis of this cataract is expression of an unstable hybrid protein comprising the first 41 amino acids of γC-crystallin followed by 62 novel amino acids. While this protein is found in both the soluble and insoluble fractions of lens cells, its transgenic expression in the mouse lens leads to degeneration of the lens fiber cells with concomitant destruction of the lens micro-architecture. Initially, the lenses appear normal, but there is vacuolization of the equatorial epithelial and superficial cortical fiber cells by about 21 days after birth, followed by degeneration of the fiber cells with large vacuoles filled with proteinaceous debris. These findings, and especially the lens histology, are most consistent with a direct toxic effect of the mutant protein on the lens cells.
2.3. The unfolded protein response in congenital cataracts
One possible mechanism through which the mutant protein might exert this effect is induction of the unfolded protein response (UPR) and eventually apoptosis (Ikesugi et al., 2006). The UPR consists of an evolutionarily conserved group of adaptive intracellular signaling pathways that serve to reduce stress on the endoplasmic reticulum (ER stress) that occurs when large amounts of denatured, misfolded, or unfolded proteins accumulate within the ER lumen. The UPR is induced by heat shock 70 kDa protein 5, HSPA5 (also called BiP or GRP78) that binds to at least three major ER-resident sensors, IRE1, ATF6, and EIF2AK3, maintaining them in inactive states. When large amounts of unfolded proteins accumulate HSPA5 dissociates from these three sensors, activating them and initiating the UPR. Initially, the UPR attempts to reduce ER stress by decreasing protein synthesis through induction of eukaryotic translation initiation factor 2-alpha kinase 3 (EIF2AK3), which phosphorylates the alpha subunit of eukaryotic translation-initiation factor 2, inactivating it and thus inhibiting initiation of translation. It also upregulates levels of endoplasmic reticulum associated degradation proteins (ERAD), and increasing chaperone levels (Sovolyova et al., 2014). If stress on the ER is severe, the UPR might fail to achieve homeostasis and then it induces apoptosis (Lai et al., 2007; Rasheva and Domingos, 2009), through both the intrinsic and mitochondrial-mediated pathways (Gupta et al., 2010; Szegezdi et al., 2008) and also EIF2AK3 regulation of gene expression. This regulation includes decreased synthesis of microRNAs (Gupta et al., 2012), activation of specific gene transcription by ATF6, including induction of XBP1 and IRE1, which cleaves XBP1 activating it and inducing transcription of a broad array of mediators including P58 (Gorman et al., 2012) and DNA-damage-inducible transcript 3 (DDIT3). DDIT3 belongs to the CCAAT/ enhancer-binding protein (C/EBP) family of transcription factor and inhibits transcription of a number of genes when activated by ER stress, thus promoting apoptosis. Although fiber cells within the lens core lack nuclei and other organelles such as endoplasmic reticulum or Golgi, the lens epithelium and cortical fiber cells still retain their organelles and could participate in the UPR.
While previously, support for the UPR having a major role in cataract was mixed, there is increasing evidence for a potential role, especially in lens epithelial cells, which have a higher level of metabolic activity. One of the first examples of the UPR in a cataract model was its demonstration in lens epithelial cells from galactosemic rats, as well as cultured transformed lens epithelial cells deprived of glucose (Mulhern et al., 2006). Interestingly, the vacuolization seen in the galactosemic cataracts appears similar to that seen in the early stages of the p.C42Afs*63 mutant CRYGC transgenic mouse cataracts mentioned above (Ma et al., 2011). The UPR also has been shown to contribute to a selenite induced cataract model in rats (Palsamy et al., 2014). The UPR was activated in another model in which expression of two abnormal collagens was used to induce cataracts in transgenic mice, although only one of the abnormal collagens could be shown to cause cell death (Firtina et al., 2009). Perhaps more directly apropos of inherited congenital cataract, variable activation of the UPR has been reported in lenses from mice expressing mutant forms of GJA8/Cx50 (p.Ser50Pro, p.Gly22Arg) that arose spontaneously, or transgenic mutant forms of CRYAA (p.Arg49Cys), and CRYBA1 (c.215 + 1G > A splice-site) shown to underlie human congenital cataracts (Alapure et al., 2012; Andley and Goldman, 2015; Ma et al., 2016b). Such induction of the UPR with subsequent apoptosis of at least lens epithelial cells appears to be an excellent candidate for having a role in those congenital cataracts resulting from severe mutations in lens crystallins or proteins vital to lens cell homeostasis.
3. Age related cataract
3.1. Age-related cataract variants and loci
Age-related cataracts (ARC) also have a genetic component, although the sequence variations contributing to ARC tend to increase the risk of disease against a background of environmental insults to which all individuals are exposed presumably by making individuals having the variation more vulnerable to a variety environmental insults accumulated over many years (McCarty and Taylor, 2001; Shiels and Hejtmancik, 2010). Although often occurring in a mixed pattern when advanced, age-related cataracts may be divided into three sub-types based on their location within the lens: nuclear, cortical, and posterior subcapsular (Merin, 1991). Each of these forms of cataract has its own multifactorial epidemiology involving contributions from multiple environmental and genetic risk factors. In contrast to inherited congenital cataracts, relatively few genes or loci have been unambiguously associated with the risk for age-related cataract, perhaps because their complex inheritance pattern and late age of onset makes them more difficult to study.
Sequence changes in or near a number of genes have been associated with ARC. The first of these was the “Osaka” variant (Ala198Val) of the gene for galactokinase-1 (GALK1) (Okano et al., 2001). GALK1 catalyzes phosphorylation of galactose, the first step in metabolism of galactose to glucose. The Osaka variant has been detected at an increased frequency, about 7.8% in a Japanese cohort with age-related cataract while being present in 4.1% of the general population. In heterozygotes this protein variation results in reduced stability of the variant enzyme and mild galactokinase deficiency equivalent to about 20% of normal levels, presumably sufficient to result in cataract as an individual ages. Among others, SNPs in and near the EPH receptor A2 (EPHA2) have been associated with ARC, especially cortical (Shiels et al., 2008; Sundaresan et al., 2012), as have sequence variations in the αA-crystallin gene (CRYAA) (Bhagyalaxmi et al., 2009, 2010; Liao et al., 2014).
Association of the null allele of the GSTM1 locus with ARC, has proved to be inconsistent, as has that for GSTT1 (Liao et al., 2015; Sun et al., 2010). Alterations in the monocarboxylate (creatine) transporter SLC16A12 are also implicated in juvenile cortical and nuclear cataract and have been associated with age-related cataract (Abplanalp et al., 2013). In addition, inconsistent or unconfirmed association of the DNA repair genes WRN, XPD and XRCC1, as well as HSF4 and the kinesin light chain 1 gene KCL1 have been reported with age-related cataract (Jiang et al., 2013a, 2013b; Padma et al., 2011; Su et al., 2013).
In addition, there are mutations resulting in progressive or presenile Mendelian cataracts (Shiels et al., 2010). While the overall spectrum of these mutations is similar to those of congenital cataracts, with the respective frequencies of missense (63% vs. 65%), splice-site (8% vs. 12%), and insertion/deletion resulting in a frameshift (15% vs. 12%) of congenital and progressive cataracts are relatively similar, congenital cataracts might be somewhat enriched for nonsense (12% vs. 6%), and progressive cataracts for insertion/ deletions with no frameshift (18% vs. 3%), consistent with the concept of less severe mutations resulting in later onset cataracts that develop over the course of a prolonged period of time. Also of interest is the high frequency of BFSP2 mutations causing progressive cataracts (44%, with most being insertions or deletions not resulting in a shift in the reading frame) relative to those in other genes.
3.2. Mechanisms of progressive or age-related cataracts
Among mutations implicated in progressive cataract is the p.Gly18Val mutation in CRYGS (Ma et al., 2009; Sun et al., 2005). In contrast to the p.Val42Met mutant γS-crystallin protein, which results in congenital cataract, the p.Gly18Val mutant has a normal protein fold and overall structure under normal physiological conditions. Only under chemical or thermal stress does the protein show distortion of the protein fold. The p.Val42Met mutant γS-crystallin protein is destabilized at lower temperatures and in lower concentrations of chemical denaturants. In an exactly analogous fashion, the protein functions normally in the lens, but under the continued impact of time and environmental insults denatures over a period of years or decades. These two superficially similar mutations in γS-crystallin appear to result in congenital or age related cataract based on their differential effects on the structure and stability of the protein. A similar example is seen in the p.Phe71Leu mutation in CRYAA associated with age related cataract (Bhagyalaxmi et al., 2009). This mutation results in a protein that does not differ significantly from wild-type αA-crystallin under benign conditions with regard to secondary and tertiary structure, hydrophobicity and the apparent molecular mass of the oligomer, however in the mutant, each of these properties is more easily disturbed by heat treatment. In addition, wild-type αA-crystallin showed increased chaperone like activity following heat treatment, but the p.Phe71Leu mutant protein lost a significant amount of its chaperone activity after heat treatment. These are just two examples of relatively mild mutations that result in age-related or progressive cataract. Additional insight is provided by association of SNP rs7278468 in the CRYAA promoter region, which contributes to age-related cataracts by increasing binding of the inhibitory transcription factor KLF10, resulting in decreased α-crystallin expression (Ma et al., 2016a). One might hypothesize that similarly, mutations that severely disrupt homeostatic functions, for example in ion channels, or glycemic control, might cause congenital cataract, but those that simply stress the system might result in ARC.
3.3. Role of α-crystallin
While there are probably a number of pathways through which age related cataracts occur, and components of the UPR have been reported to be increased in age related cataract (Yang et al., 2015), most age related cataracts appear to occur through a prolonged process in which crystallins are slowly denatured, either by environmental insults, intrinsically lower stability of the crystallins themselves, or degradation of lens cell homeostasis (Hejtmancik and Kantorow, 2004). α-Crystallins, with their chaperone like activity and high concentration in the lens, appear to play a central role in delaying this process (Haslbeck et al., 2015). It has long been appreciated that α-crystallin, acting as a molecular chaperone, would bind partially denatured proteins and maintain them in a soluble state in vitro, providing a type of ‘buffer’ against the untoward effects of denatured and precipitated protein (Horwitz, 2003). Using dynamic light scattering, it has been possible to confirm this in the lens cell cytoplasm, and to measure the fraction of free α-crystallin in human lenses (Datiles et al., 2008). The fraction of unbound a-crystallin decreases about 6 fold in clear lenses as individuals age. Moreover, the fraction of unbound α-crystallin in cataractous lenses decreases approximately 10 fold as the AREDS nuclear opacity grade increases from 0 to 2 and greater, even when this is controlled for age. This process continues until there is little or no free α-crystallin remaining in lenses with nuclear cataracts having AREDS scores greater than 2. Overall, for age related cataract, these findings support the classical view that lens opacities result from accumulation of protein aggregates large enough to scatter light about 1000 Å, (Benedek, 1971), even in cases in which the lens cell architecture remains largely intact. It is possible that after saturation of the α-crystallin buffer, the UPR might be activated by unbound denatured protein in ARC. This would be compatible with a slow increase in opacity over years followed by an accelerated phase over weeks to months seen clinically in some individuals.
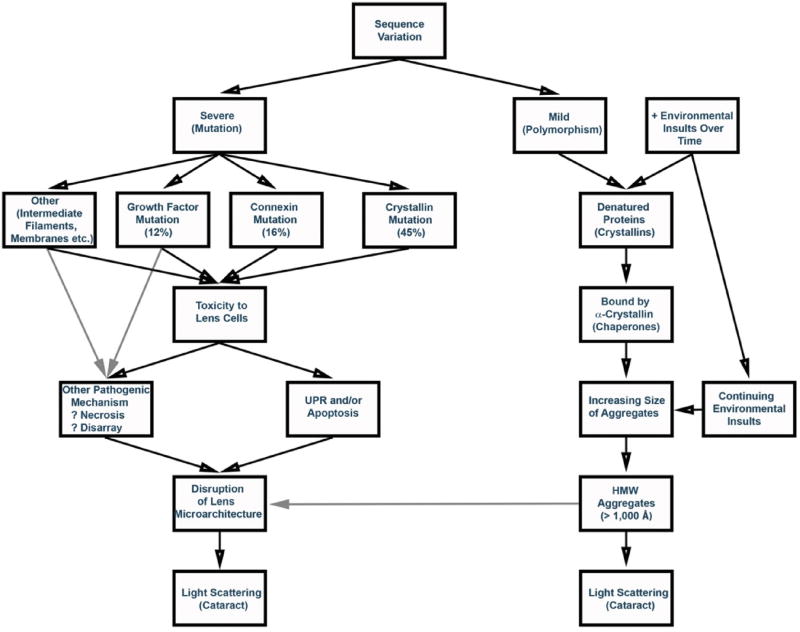
Thus, while cataract can be considered a final common pathway for a number of pathological processes, the molecular mechanisms of many inherited cataracts are beginning to be elucidated and appear to fall into two general groups (Fig. 1). Although there is probably some overlap in the processes, at least many congenital and age-related cataracts appear to occur by distinct mechanisms. Those congenital cataracts whose mechanisms have been delineated appear to occur through mutations causing severe insults to crystallin stability or dramatic loss of lens cell homeostasis, often with resulting activation of the UPR and accompanying apoptosis. In contrast, progressive or age related cataracts often appear to follow a relatively gradual process consisting of denaturation of increasing amounts of βγ-crystallins that are then bound by α-crystallin until this ‘buffer’ is completely consumed. At that point the aggregates grow in size until they become large enough to scatter light, with resulting opacity. There will certainly be exceptions to this general process, e.g., amyloid-like fibrils appear to be involved in some cataracts (Meehan et al., 2004; Sandilands et al., 2002; Xi et al., 2015), although these mechanisms are not mutually exclusive and can overlap (Xi et al., 2015). However, based on the present state of knowledge regarding inherited cataracts, this appears to be a reasonable working hypothesis at the present time.
Fig. 1.
Possible genetic pathways leading to cataracts. Severe mutations would be more likely to cause highly penetrant Mendelian congenital cataracts, while mild changes would be likely to increase susceptibility to environmental insults and lead to multifactorial age-related cataracts. Black arrows show demonstrated pathways and gray arrows show likely pathways.
3.4. Anti-cataract therapeutics and future directions
Despite advances in its surgical treatment, age-related cataract remains a leading cause of low vision and blindness worldwide (Pascolini and Mariotti, 2012). With continued aging of global populations the socioeconomic burden of cataract is projected to increase and this has prompted the search for non-surgical means to reverse, delay or prevent cataract formation (Moreau and King, 2012; Toh et al., 2007). However, recent progress in therapy for both congenital and age-related cataracts provides some hope. Lin et al. claim to have developed a modification of conventional surgery for congenital cataracts that enables lens regeneration from endogenous stem cells thereby avoiding the need to implant an artificial intraocular lens (Lin et al., 2016). While this would presumably not be applicable to genetic cataracts, which would regenerate with the causative mutation, these might possibly be amenable to a combined approach incorporating correction of the mutation with a CRISPER/Cas9 technology in order to correct the genetic mutation in the regenerated lens. Also, in the case of cataracts occurring as part of systemic disease, early conventional or genetic therapy of the underlying metabolic disease should mitigate the associated cataracts.
For age related cataracts, the recent identification of two families with recessive forms of congenital cataract caused by mutations in the gene coding for lanosterol synthase (LSS), a key enzyme in the cholesterol biosynthesis pathway, has suggested a possible topical eye-drop therapy for cataract (Zhao et al., 2015). Because of its amphipathic (water-lipid solubility) properties and enrichment in the lens, lanosterol was tested for its ability to solubilize the amyloid-like fibril aggregates of mutant and wild-type crystallins associated with inherited and age-related forms of cataract. Not only did lanosterol treatment reverse crystallin aggregation in vitro and in transfected cells but it also improved transparency of rabbit and dog lenses with naturally occurring cataract. Comparable results have been obtained with a variety of structurally similar steroids acting on α-crystallin aggregates (Makley et al., 2015). These anti-aggregation or chaperone approaches might be combined with inhibitors of the UPR to help mitigate the effects of any unstable protein resistant to small molecule chaperones. In addition, it has been demonstrated that topical administration of an aldose reductase inhibitor (Kinostat) delays onset and progression of cataracts in dogs with naturally occurring diabetes mellitus (Kador et al., 2010). While these preliminary results with small molecules are promising, it is noteworthy that several other animal-model studies of small molecules with anti-oxidant and/or anti-aggregation (chaperone) properties including; plant-flavonoids, aspirin, N-acetyl carnosine, curcumin, caffeine, and multivitamins have not resulted in universal clinical approval for use as anti-cataract drugs in humans. Nevertheless, continued investigation of small-molecule strategies that combat protein misfolding and aggregation and/or oxidative stress in the lens are anticipated to aid in the discovery of efficacious medical treatments for cataract and may have broader treatment implications for other protein-aggregation diseases including neurodegenerative conditions and diabetes.
Acknowledgments
This work was supported in part by National Institutes of Health/National Eye Institute (NIH/NEI) grants EY012284 and EY023549 (to AS) and EY02687 (Core Grant for Vision research), and by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness (RPB).
References
- Abplanalp J, Laczko E, Philp NJ, Neidhardt J, Zuercher J, Braun P, Schorderet DF, Munier FL, Verrey F, Berger W, Camargo SM, Kloeckener-Gruissem B. The cataract and glucosuria associated monocarboxylate transporter MCT12 is a new creatine transporter. Hum. Mol. Genet. 2013;22:3218–3226. doi: 10.1093/hmg/ddt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapure BV, Stull JK, Firtina Z, Duncan MK. The unfolded protein response is activated in connexin 50 mutant mouse lenses. Exp. Eye Res. 2012;102:28–37. doi: 10.1016/j.exer.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Goldman JW. Autophagy and UPR in alpha-crystallin mutant knock-in mouse models of hereditary cataracts. Biochim. Biophys. Acta. 2015;1860:234–239. doi: 10.1016/j.bbagen.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek GB. Theory of transparency of the eye. Appl. Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Bhagyalaxmi SG, Padma T, Reddy GB, Reddy KR. Association of G>A transition in exon-1 of alpha crystallin gene in age-related cataracts. Oman J. Ophthalmol. 2010;3:7–12. doi: 10.4103/0974-620X.60014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, Petrash JM, Bhanuprakash RG, Padma T. A novel mutation (F71L) in alphaA-Crystallin with defective chaperone-like function associated with age-related cataract. Biochimica Biophysica Acta. 2009;1792:974–981. doi: 10.1016/j.bbadis.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharat SV, Shekhtman A, Pande J. The cataract-associated V41M mutant of human gammaS-crystallin shows specific structural changes that directly enhance local surface hydrophobicity. Biochem. Biophys. Res. Commun. 2014;443:110–114. doi: 10.1016/j.bbrc.2013.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, McGreal RS, Kantorow M. Oxidative stress defense and repair systems of the ocular lens. Front. Biosci. 2012;4:141–155. doi: 10.2741/365. [DOI] [PubMed] [Google Scholar]
- Burdon KP, McKay JD, Sale MM, Russell-Eggitt IM, Mackey DA, Wirth MG, Elder JE, Nicoll A, Clarke MP, FitzGerald LM, Stankovich JM, Shaw MA, Sharma S, Gajovic S, Gruss P, Ross S, Thomas P, Voss AK, Thomas T, Gecz J, Craig JE. Mutations in a novel gene, NHS, cause the pleiotropic effects of nance-horan syndrome, including severe congenital cataract, dental anomalies, and mental retardation. Am. J. Hum. Genet. 2003;73:1120–1130. doi: 10.1086/379381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, Burette A, Weber M, Metlapally S, Gilliland KO, Fowler WC, Mohamed A, Johnsen S. Electron tomography of fiber cell cytoplasm and dense cores of multilamellar bodies from human age-related nuclear cataracts. Exp. Eye Res. 2012;101:72–81. doi: 10.1016/j.exer.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datiles MB, III, Ansari RR, Suh KI, Vitale S, Reed GF, Zigler JS, Jr, Ferris FL., III Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch. Ophthalmol. 2008;126:1687–1693. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Ferda PE, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, Kocak-Altintas A, Sowden JC, Traboulsi E, Sarfarazi M, McInnes RR. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat. Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- Firtina Z, Danysh BP, Bai X, Gould DB, Kobayashi T, Duncan MK. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J. Biol. Chem. 2009;284:35872–35884. doi: 10.1074/jbc.M109.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois J. Genetics of cataract. Ophthalmologica. 1982;184:61–71. doi: 10.1159/000309186. [DOI] [PubMed] [Google Scholar]
- Gorman AM, Healy SJ, Jager R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Gupta S, Cuffe L, Szegezdi E, Logue SE, Neary C, Healy S, Samali A. Mechanisms of ER stress-mediated mitochondrial membrane permeabilization. Int. J. Cell Biol. 2010;2010:170215. doi: 10.1155/2010/170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Read DE, Deepti A, Cawley K, Gupta A, Oommen D, Verfaillie T, Matus S, Smith MA, Mott JL, Agostinis P, Hetz C, Samali A. Perk-dependent repression of miR-106b-25 cluster is required for ER stress-induced apoptosis. Cell Death Dis. 2012;3:e333. doi: 10.1038/cddis.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haargaard B, Wohlfahrt J, Rosenberg T, Fledelius HC, Melbye M. Risk factors for idiopathic congenital/infantile cataract. Invest Ophthalmol. Vis. Sci. 2005;46:3067–3073. doi: 10.1167/iovs.04-0979. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Peschek J, Buchner J, Weinkauf S. Structure and function of alpha-crystallins: traversing from in vitro to in vivo. Biochim. Biophys. Acta. 2015;1860:149–166. doi: 10.1016/j.bbagen.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Hejtmancik JF, Kaiser-Kupfer MI, Piatigorsky J. Molecular biology and inherited disorders of the eye lens. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw Hill; New York: 2001. pp. 6033–6062. [Google Scholar]
- Hejtmancik JF, Kantorow M. Molecular genetics of age-related cataract. Exp. Eye Res. 2004;79:3–9. doi: 10.1016/j.exer.2004.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik JF, Piatigorsky J. Lens proteins and their molecular biology. In: Alpert DM, Jakobiec FA, Azar DT, Gragoudas ES, editors. Principles and Practice of Ophthalmology. W.B.Saunders Co; Philadelphia: 2000. pp. 1409–1428. [Google Scholar]
- Hejtmancik JF, Smaoui N. Molecular genetics of cataract. In: Wissinger B, Kohl S, Langenbeck U, editors. Genetics in Ophthalmology. S.Karger; Basel: 2003. pp. 67–82. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp. Eye Res. 2006;83:508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van HV, Donnai D, Munier F, Black GC. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum. Mol. Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhou J, Yao Y, Zhu R, Liang C, Jiang S, Yang M, Lu Y, Xing Q, Guan H. Copy number variations of DNA repair genes and the age-related cataract: jiangsu eye study. Invest Ophthalmol. Vis. Sci. 2013a;54:932–938. doi: 10.1167/iovs.12-10948. [DOI] [PubMed] [Google Scholar]
- Jiang S, Hu N, Zhou J, Zhang J, Gao R, Hu J, Guan H. Polymorphisms of the WRN gene and DNA damage of peripheral lymphocytes in age-related cataract in a Han Chinese population. Age (Dordr) 2013b;35:2435–2444. doi: 10.1007/s11357-013-9512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kador PF, Webb TR, Bras D, Ketring K, Wyman M. Topical KINOSTAT ameliorates the clinical development and progression of cataracts in dogs with diabetes mellitus. Vet. Ophthalmol. 2010;13:363–368. doi: 10.1111/j.1463-5224.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Liao J, Su X, Chen P, Wang X, Xu L, Li X, Thean L, Tan C, Tan AG, Tay WT, Jun G, Zheng Y, Chew M, Wang YX, Tan QS, Barathi VA, Klein BE, Saw SM, Vithana EN, Tai ES, Iyengar SK, Mitchell P, Khor CC, Aung T, Wang JJ, Jonas JB, Teo YY, Wong TY, Cheng CY. Meta-analysis of genome-wide association studies in multiethnic Asians identifies two loci for age-related nuclear cataract. Hum. Mol. Genet. 2014;23:6119–6128. doi: 10.1093/hmg/ddu315. [DOI] [PubMed] [Google Scholar]
- Liao RF, Ye MJ, Liu CY, Ye DQ. An updated meta-analysis: risk conferred by glutathione S-Transferases (GSTM1 and GSTT1) polymorphisms to age-related cataract. J. Ophthalmol. 2015;2015:103950. doi: 10.1155/2015/103950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RA, Xu Y, Chung C, Zhang Y, Lin D, Patel S, Wu F, Cai H, Hou J, Wen C, Jafari M, Liu X, Luo L, Zhu J, Qiu A, Hou R, Chen B, Chen J, Granet D, Heichel C, Shang F, Li X, Krawczyk M, Skowronska-Krawczyk D, Wang Y, Shi W, Chen D, Zhong Z, Zhong S, Zhang L, Chen S, Morrison SJ, Maas RL, Zhang K, Liu Y. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016;531:323–328. doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Jiao X, Ma Z, Hejtmancik JF. Polymorphism rs7278468 is associated with Age-related cataract through decreasing transcriptional activity of the CRYAA promoter. Sci. Rep. 2016a;6:23206. doi: 10.1038/srep23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Piszczek G, Wingfield PT, Sergeev YV, Hejtmancik JF. The G18V CRYGS mutation associated with human cataracts increases γS-crystallin sensitivity to thermal and chemical stress. Biochemistry. 2009;48:7334–7341. doi: 10.1021/bi900467a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yao W, Chan CC, Kannabiran C, Wawrousek E, Hejtmancik JF. Human betaA3/A1-crystallin splicing mutation causes cataracts by activating the unfolded protein response and inducing apoptosis in differentiating lens fiber cells. Biochim. Biophys. Acta. 2016b;1862:1214–1227. doi: 10.1016/j.bbadis.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yao W, Theendakara V, Chan CC, Wawrousek E, Hejtmancik JF. Overexpression of human γC-crystallin 5bp duplication disrupts lens morphology in transgenic mice. Invest Ophthalmol. Vis. Sci. 2011;52:5269–5375. doi: 10.1167/iovs.11-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, Klevit RE, Andley UP, Gestwicki JE. Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science. 2015;350:674–677. doi: 10.1126/science.aac9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Taylor HR. The genetics of cataract. Invest Ophthalmol. Vis. Sci. 2001;42:1677–1678. [PubMed] [Google Scholar]
- Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J. Biol. Chem. 2004;279:3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- Merin S. Inherited cataracts. In: Merin S, editor. Inherited Eye Diseases. Marcel Dekker, Inc; New York: 1991. pp. 86–120. [Google Scholar]
- Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012;18:273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol. Vis. Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- Okano Y, Asada M, Fujimoto A, Ohtake A, Murayama K, Hsiao KJ, Choeh K, Yang Y, Cao Q, Reichardt JK, Niihira S, Imamura T, Yamano T. A genetic factor for age-related cataract: identification and characterization of a novel galactokinase variant, “Osaka,” in Asians. Am. J. Hum. Genet. 2001;68:1036–1042. doi: 10.1086/319512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma G, Mamata M, Reddy KR, Padma T. Polymorphisms in two DNA repair genes (XPD and XRCC1) - association with age related cataracts. Mol. Vis. 2011;17:127–133. [PMC free article] [PubMed] [Google Scholar]
- Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 2000;279:C596–C602. doi: 10.1152/ajpcell.2000.279.3.C596. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T. Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim. Biophys. Acta. 2014;1842:1794–1805. doi: 10.1016/j.bbadis.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Annunziata O, Asherie N, Ogun O, Benedek GB, Pande J. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry. 2005;44:2491–2500. doi: 10.1021/bi0479611. [DOI] [PubMed] [Google Scholar]
- Pande A, Ghosh KS, Banerjee PR, Pande J. Increase in surface hydrophobicity of the cataract-associated P23T mutant of human gammaD-crystallin is responsible for its dramatically lower, retrograde solubility. Biochemistry. 2010;49:6122–6129. doi: 10.1021/bi100664s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis Int. J. Program. Cell Death. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- Ren Z, Li A, Shastry BS, Padma T, Ayyagari R, Scott MH, Parks MM, Kaiser-Kupfer M, Hejtmancik JF. A 5-base insertion in the γC-crystallin gene is associated with autosomal dominant variable zonular pulverulent cataract. Hum. Genet. 2000;106:531–537. doi: 10.1007/s004390000289. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Hutcheson AM, Long HA, Prescott AR, Vrensen G, Loster J, Klopp N, Lutz RB, Graw J, Masaki S, Dobson CM, MacPhee CE, Quinlan RA. Altered aggregation properties of mutant gamma-crystallins cause inherited cataract. EMBO J. 2002;21:6005–6014. doi: 10.1093/emboj/cdf609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. J. Biol. Chem. 2004;279:16425–16432. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- Scott MH, Hejtmancik JF, Wozencraft LA, Reuter LM, Parks MM, Kaiser-Kupfer MI. Autosomal dominant congenital cataract: interocular phenotypic heterogeneity. Ophthalmology. 1994;101:866–871. doi: 10.1016/s0161-6420(94)31246-2. [DOI] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann. Neurology. 2003;54:804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal- dominant cataracts and ASMD. Nat. Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol. Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 2008;14:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. Genetic origins of cataract. Arch. Ophthalmol. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. Genetics of age-related cataract. In: Dart DA, Besharse J, Dana R, editors. Encyclopedia of the Eye. Elsevier; New York: 2010. [Google Scholar]
- Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies auto-somal dominant “zonular pulverulent” cataract, on chromosome 1q. Am. J. Hum. Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol. Chem. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- Su S, Yao Y, Zhu R, Liang C, Jiang S, Hu N, Zhou J, Yang M, Xing Q, Guan H. The associations between single nucleotide polymorphisms of DNA repair genes, DNA damage, and age-related cataract: jiangsu eye study. Invest Ophthalmol. Vis. Sci. 2013;54:1201–1207. doi: 10.1167/iovs.12-10940. [DOI] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J. Med. Genet. 2005;42:706–710. doi: 10.1136/jmg.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xi B, Yu L, Gao XC, Shi DJ, Yan YK, Xu DJ, Han Q, Wang C. Association of glutathione S-transferases polymorphisms (GSTM1 and GSTT1) with senile cataract: a meta-analysis. Invest Ophthalmol. Vis. Sci. 2010;51:6381–6386. doi: 10.1167/iovs.10-5815. [DOI] [PubMed] [Google Scholar]
- Sundaresan P, Ravindran RD, Vashist P, Shanker A, Nitsch D, Talwar B, Maraini G, Camparini M, Nonyane BA, Smeeth L, Chakravarthy U, Hejtmancik JF, Fletcher AE. EPHA2 polymorphisms and age-related cataract in India. PLoS One. 2012;7:e33001. doi: 10.1371/journal.pone.0033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Herbert KR, Kavanagh ET, Samali A, Gorman AM. Nerve growth factor blocks thapsigargin-induced apoptosis at the level of the mitochondrion via regulation of Bim. J. Cell Mol. Med. 2008;12:2482–2496. doi: 10.1111/j.1582-4934.2008.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N. Engl. J. Med. 1988;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- Toh T, Morton J, Coxon J, Elder MJ. Medical treatment of cataract. Clin. Exp. Ophthalmol. 2007;35:664–671. doi: 10.1111/j.1442-9071.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Friedrich MG. The etiology of human age-related cataract. Proteins don’t last forever. Biochim. Biophys. Acta. 2016;1860:192–198. doi: 10.1016/j.bbagen.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanita V, Singh JR, Singh D, Varon R, Sperling K. Novel mutation in the gamma-S crystallin gene causing autosomal dominant cataract. Mol. Vis. 2009;15:476–481. [PMC free article] [PubMed] [Google Scholar]
- Vendra VP, Chandani S, Balasubramanian D. The mutation V42M distorts the compact packing of the human gamma-S-crystallin molecule, resulting in congenital cataract. PLoS One. 2012;7:e51401. doi: 10.1371/journal.pone.0051401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Garber C, Baburins A, Taylor A. Nutritional modulation of cataract. Nutr. Rev. 2014;72:30–47. doi: 10.1111/nure.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi YB, Chen XJ, Zhao WJ, Yan YB. Congenital cataract-causing mutation G129C in gammac-crystallin promotes the accumulation of two distinct unfolding intermediates that form highly toxic aggregates. J. Mol. Biol. 2015;427:2765–2781. doi: 10.1016/j.jmb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhou S, Gu J, Wang Y, Guo M, Liu Y. Differences in unfolded protein response pathway activation in the lenses of three types of cataracts. PLoS One. 2015;10:e0130705. doi: 10.1371/journal.pone.0130705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guo X, Xiao X, Yi J, Jia X, Hejtmancik JF. Clinical description and genome wide linkage study of Y-sutural cataract and myopia in a Chinese family. Mol. Vis. 2004;10:890–900. [PubMed] [Google Scholar]
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin D, Wu F, Flagg K, Cai H, Li G, Cao G, Lin Y, Chen D, Wen C, Chung C, Wang Y, Qiu A, Yeh E, Wang W, Hu X, Grob S, Abagyan R, Su Z, Tjondro HC, Zhao XJ, Luo H, Hou R, Perry JJ, Gao W, Kozak I, Granet D, Li Y, Sun X, Wang J, Zhang L, Liu Y, Yan YB, Zhang K. Lanosterol reverses protein aggregation in cataracts. Nature. 2015;523:607–611. doi: 10.1038/nature14650. [DOI] [PubMed] [Google Scholar]